Abstract
Macrophages and CD4+ lymphocytes are the principal target cells for human immunodeficiency virus type 1 (HIV-1) infection, but the molecular details of infection may differ between these cell types. During studies to identify cellular molecules that could be involved in macrophage infection, we observed inhibition of HIV-1 infection of macrophages by monoclonal antibody (MAb) to the tetraspan transmembrane glycoprotein CD63. Pretreatment of primary macrophages with anti-CD63 MAb, but not MAbs to other macrophage cell surface tetraspanins (CD9, CD81, and CD82), was shown to inhibit infection by several R5 and dualtropic strains, but not by X4 isolates. The block to productive infection was postfusion, as assessed by macrophage cell-cell fusion assays, but was prior to reverse transcription, as determined by quantitative PCR assay for new viral DNA formation. The inhibitory effects of anti-CD63 in primary macrophages could not be explained by changes in the levels of CD4, CCR5, or β-chemokines. Infections of peripheral blood lymphocytes and certain cell lines were unaffected by treatment with anti-CD63, suggesting that the role of CD63 in HIV-1 infection may be specific for macrophages.
Human immunodeficiency virus (HIV-1) is known to infect several primary cell types, predominantly CD4+ T lymphocytes and macrophages. HIV-1 infection results in a gradual decline in the number of CD4+ T cells, leading to the development of AIDS. Macrophages are of particular importance for the pathogenesis of HIV-1, as these cells contribute to viral persistence and dissemination and are likely to be the major cell type involved in mucosal transmission of the virus (60, 61). Furthermore, HIV-1 infection of macrophages has been implicated as contributing to many of the clinical manifestations of AIDS (14, 17, 19, 22, 33, 40, 48, 53, 57, 60, 61). Due to the importance of macrophages in the pathogenesis of HIV-1, identification of molecular determinants of macrophage infection is relevant and may lead to novel therapies specific for this cell type. Identification of the β-chemokine receptor CCR5 as an HIV-1 coreceptor for macrophages and T cells has led to the development of specific inhibitors of these receptors, which block HIV-1 entry (1, 6, 7, 12, 13, 18, 50, 51). Several lines of evidence, however, indicate the possible involvement of additional factors in macrophage infection. For example, neither antibodies to CCR5 nor the ligands to CCR5 inhibit infection of macrophages as efficiently as they do T cells (10, 13), suggesting that CCR5 utilization may be different in macrophages or that cofactors in addition to CCR5 may be involved in macrophage tropism.
Moreover, although the α-chemokine receptor CXCR4 is expressed on macrophages and some atypical HIV-1 strains can utilize this coreceptor along with CD4 for entry into macrophages, viruses that use CD4 and CCR5 (R5 or macrophagetropic strains) typically enter macrophages far more efficiently than those using CD4 and CXCR4 (X4, T-tropic, or T-cell line-adapted [TCLA] strains) (3, 10). While primary X4 strains are capable of macrophage entry, TCLA strains are unable to replicate efficiently in macrophages. It has been proposed that aspects such as receptor or coreceptor density levels (43, 52), inadequate cell surface associations between CD4 and CXCR4 (11, 26, 58), and chemokine receptor signaling (28, 55) may be important for macrophage tropism. In addition, it has been shown that TCLA strains which enter macrophages but fail to replicate may be blocked at an early postentry step (47), suggesting that postentry factors may also be important for infection of macrophages. Collectively, these studies focus primarily on answering the significant question of why TCLA strains are unable to infect macrophages. There have been fewer studies, however, evaluating whether there may be unique factors, in addition to CD4 and CCR5, that could be involved specifically in R5-mediated macrophage infection.
Our laboratory has implicated the cell membrane glycoprotein CD63 as playing a potential role in HIV-1 infection of macrophages. CD63 belongs to the tetraspan transmembrane protein family (also known as the tetraspanins), whose members include CD9, CD37, CD81, CD82, CD53, and CD151. CD63 is structurally characterized by four membrane-spanning domains, resulting in two extracellular loops of unequal size and two short cytoplasmic domains which may be involved in signal transduction in some cell types (49). Although the precise function of CD63 remains unknown, it has been characterized as an activation or differentiation marker on a wide variety of cell types and is known to associate closely in the cell membrane with β1 integrins, major histocompatibility complex antigens, and other tetraspanin proteins (30, 34, 46). It is noteworthy that another tetraspanin, CD81, has been proposed as a receptor for hepatitis C virus (42), and CD82 and CD9 have been implicated in syncytium formation by human T-cell leukemia virus type 1 and infection by feline immunodeficiency virus, respectively (9, 16, 20). In this report, we show that CD63 is likely to be involved in HIV-1 entry into macrophages.
MATERIALS AND METHODS
Cells and plasmid constructs.
As described previously in Rich et al. (45), primary monocyte-derived macrophages were isolated from healthy HIV-1-negative blood donors by Ficoll-Hypaque centrifugation followed by adherence for 7 days to plastic petri dishes coated with human AB serum. By use of this methodology, the macrophage purity (CD14+ cells) was found to be >98%. Nonadherent cells were treated with phytohemagglutinin (PHA) for 72 h prior to propagation in RPMI 1640 supplemented with 20% fetal calf serum and 20 U of interleukin-2 per ml. During differentiation, macrophages were cultured in Iscove's modified Dulbecco's medium supplemented with 20% fetal calf serum. Cell lines were carried in Dulbecco's modified Eagle medium with 10% fetal calf serum. JC53BL cells were obtained through Genzyme for the experiments performed at the University of Alabama at Birmingham, and U373-MAGI-CCR5E cells were obtained through the NIH AIDS Research and Reference Reagent Program (contributed by Michael Emerman). CD63 expression construct was generated by reverse transcription-PCR of human peripheral blood mononuclear cell (PBMC) RNA, followed by cloning into the TA vector pcDNA3.1/V5/His-TOPO (Invitrogen). Clones were screened by restriction digestion, and positive clones were confirmed by sequencing. QT6 cells and expression plasmids encoding CD4, CCR5, and CXCR4 were a kind gift from Robert W. Doms (12). UGO21 and JRFL envelope plasmids have been described previously (59).
Viruses, antibodies, and reagents.
HIV-1-SX is a chimeric R5 virus encoding the majority of the HIV-1-JRFL envelope protein (Envs) in an NL4-3 backbone and has been described previously (39). BL-4 and BL-6 are R5 HIV-1 primary isolates that were isolated from PBMC of infected individuals by using standard methods. The recombinant SP6 polymerase vaccinia virus, vSIMB (21), and recombinant T7 polymerase vaccinia virus, vTF1.1 (12), were gifts from Stuart Isaacs and Robert W. Doms, respectively. All other primary HIV-1 isolates and recombinant vaccinia viruses were obtained from the NIH AIDS Research and Reference Reagent Program. All antibodies used in infection assays were dialyzed before use to remove sodium azide. The MAb panel was generously provided by R. Todd (Myeloid Antigen Differentiation Workshop). Anti-CD63 (CLB-gran12) was obtained from Caltag Laboratories. Control antibodies anti-CD9 (M-L13), anti-CD53 (HI29), anti-CD81 (JS-81), anti-CD82 (50F11), anti-CD151 (14A2.H1), and anti-CCR5 (2D7) were obtained from BD Pharmingen. Anti-CD4 (Leu3a) was obtained from BD Biosciences and Immunocytometry Systems. Antibodies used for flow cytometry studies were obtained from Caltag (fluorescein isothiocyanate [FITC]/phycoerythrin [PE]-anti-CD63/CLB-gran12 and FITC/PE-mouse immunoglobulin G1 [IgG1] isotype), BD Immunocytometry Systems (PE/FITC-anti-CD4/Leu3a, FITC-anti-CD3/SK7, PE-anti-CD14/MφP9, and matching labeled isotypes), and BD Pharmingen (PE-anti-CCR5/2D7, PE-anti-CXCR4/12G5, and matching labeled isotypes). The CXCR4 inhibitor AMD3100 (8) was generously provided by Anormed (Langley, British Columbia, Canada). Enzyme-linked immunosorbent assays (ELISAs) for p24 (Beckman-Coulter) and β-chemokines MIP-1α, MIP-1β, or RANTES (R&D Systems) were performed according to the manufacturer's instructions.
Infection assays.
Macrophages adherent for 7 days or PBMC treated with PHA for 72 h were infected 1 day after plating at 100,000 cells per well in 96-well microtiter plates. Briefly, anti-CD63 (10 μg/ml), control antibodies (10 μg/ml), and/or AMD3100 (10 μg/ml) were preincubated with cells for 30 min at 37°C prior to addition of a small volume of virus at an approximate multiplicity of infection of 0.01. Following 2 h of incubation at 37°C, virus inoculum was aspirated and replaced with 200 μl of medium. Cells were fed with fresh medium 4 days later, and supernatants were harvested and diluted for p24 ELISA on day 7 postinfection. In infection experiments involving cross-linking of anti-CD63 and control antibodies, a second 30-min preincubation was performed with 5 μg of rat anti-mouse monoclonal antibody (MAb) (Pharmingen).
Quantitative PCR entry assays.
Macrophages were infected with DNase I-treated, 0.2 μm-filtered viral supernatants (or control supernatants that were heat inactivated at 60°C for 1 h) and then lysed 24 h later in gel lysis buffer (50 mM NaCl, 10 mM Tris [pH 8.3], 2.5 mM MgCl2, 0.1 mg of gelatin/ml, 0.45% NP-40, 0.45% Tween 20) and proteinase K (1 mg/ml). Purified cellular DNA and quantitation standards for HIV-1 copy number were subjected to PCR in a Perkin-Elmer thermocycler (94°C for 1 min and 65°C for 2 min for 25 cycles) using 32P-end-labeled HIV-1 R/U5 long terminal repeat-specific primers. The amount of input cellular DNA was assessed by coamplification with human β-globin primers.
Fusion assays.
Cell-cell fusion assays involving QT6 cells (12) and macrophages (21) have been described previously. For QT6 cell fusion assays, cells (200,000 cells/well) were plated in 24-well tissue culture trays and transfected with CD4, coreceptor, CD63, and T7 luciferase plasmids by calcium phosphate precipitation. A second set of cells was seeded in 6-well plates and infected with Env and T7 polymerase-expressing vaccinia viruses, followed by overnight incubation at 32°C in the presence of 100 μg of rifampin/ml to inhibit vaccinia virus replication. The following day, the two sets of cells were mixed and incubated at 37°C in the presence of 100 μg of rifampin/ml and 10 μM AraC to further inhibit vaccinia virus replication. After 6 h, the cells were lysed in 150 μl of 0.5% Triton X-100, and then 40 μl of lysate was mixed with 50 μl of luciferase assay substrate (Promega) and read in a luminometer. Macrophage fusion assays were performed in a similar manner. Briefly, 293T cells were infected with recombinant vaccinia virus expressing T7 polymerase, transfected with SP6-driven luciferase and T7-driven Env plasmids by using FUGENE, and then mixed with primary macrophages that had been infected with SP6 polymerase-expressing recombinant vaccinia virus. Prior to and during cell mixing, macrophages were incubated with 20 μg of anti-CD63/ml, 20 μg of negative control antibodies/ml, or 0.1 μg of anti-CD4/ml. In some cases, cross-linking with 5 μg of rat anti-mouse immunoglobulin/ml was performed.
Flow cytometry.
Cells were assessed by using an analytical flow cytometer (FACScan; Becton Dickinson). For detection of cell surface proteins on macrophages, background fluorescence was minimized by treatment with Fc receptor antibodies (1 μg/ml) prior to and during incubation with fluorochrome-conjugated antibody or isotype control.
RESULTS
MAb to CD63 inhibits macrophagetropic HIV-1 infection of primary monocyte-derived macrophages.
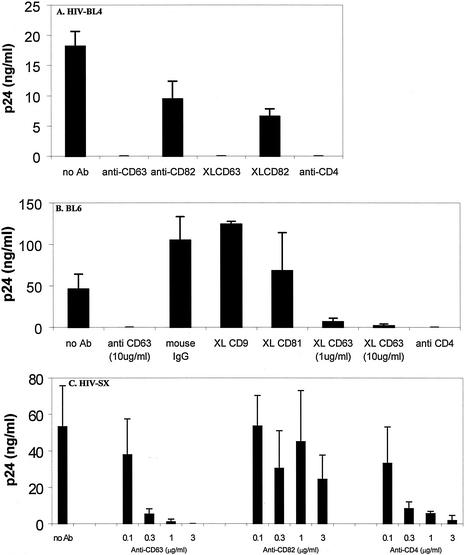
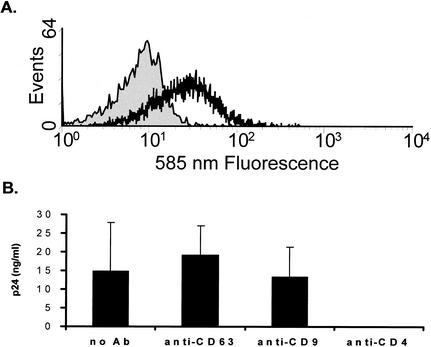
To identify potential cellular molecules which may be involved in HIV-1 infection of macrophages, we screened a myeloid MAb library (n = 120) for antibodies which showed increases in binding (i.e., increases in mean channel fluorescence of ≥30%) to primary macrophages at 6 versus 3 days of culture, as permissiveness to HIV-1 infection is greatly enhanced following 6 days of adherence. Fifteen MAbs were found which met these criteria, and of these 15 MAbs, 4 independently generated anti-CD63 MAbs were found to block replication (HIV-1 R/U5 DNA formation and p24 production) of macrophage-tropic HIV-1 in macrophages (data not shown). In subsequent infection assays (Fig. 1), which measured virus production by p24 ELISA in the presence or absence of a commercially available anti-CD63 MAb, we sought to generalize this phenomenon by using various primary R5 strains of HIV-1. In these experiments, infection of macrophages by seven different R5 strains was inhibited by MAb to CD63. Figure 1 shows the results of representative experiments utilizing the R5 strains HIV-1-BL4 (Fig. 1A), HIV-1-BL6 (Fig. 1B), and HIV-1-SX (Fig. 1C). In these assays, MAb to CD63, but not control antibodies to other tetraspanin proteins (CD9, CD81, and CD82), inhibited HIV-1 infection of macrophages. In some experiments, treatment of cells with anti-CD63 or control MAb was followed by a second incubation with anti-mouse antibody (known as receptor cross-linking). This treatment has previously been used to enhance MAb-mediated effects, and while unnecessary for the inhibition of infection by anti-CD63 (see Fig. 1A and B, where both methodologies were used), anti-CD9 and anti-CD82 MAbs did not inhibit HIV-1 infection even with cross-linking treatment. In addition, increasing amounts of anti-CD63 resulted in progressively increased inhibition, as demonstrated by the dose dependence shown in Fig. 1B and C.
FIG. 1.
Anti-CD63 treatment of primary macrophages inhibits infection by R5 HIV-1 isolates. Cells were preincubated with anti-CD63 (mouse IgG1 isotype, 10 μg/ml) or control antibodies (mouse IgG1, 10 μg/ml) and then infected with the blood-derived R5 isolate HIV-1-BL4 (A) or BL6 (B). For the R5 virus HIV-1-SX (C), anti-CD63, anti-CD82, or anti-CD4 MAb at concentrations ranging from 0 to 3 μg/ml was used. Virus production was measured in culture supernatants at 7 days postinfection by p24 ELISA. In some experiments, cross-linking (XL) of mouse anti-CD63 with a secondary rat anti-mouse MAb was used. Anti-CD4 (Leu3a, 0.5 μg/ml) was used as a positive control, and mouse IgG1, CD9, CD81, and CD82 (all tetraspanin proteins) were used as negative controls (10 μg/ml). Data shown are representative of results with seven different donors and seven different R5 virus isolates.
Isotype control antibodies are directed against cell surface molecules expressed at levels similar to or higher than that of CD63 on primary macrophages.
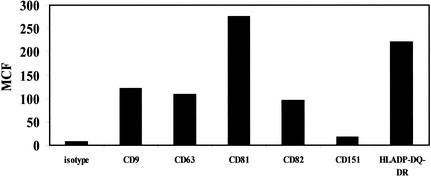
One possible explanation for the effects of anti-CD63 on HIV-1 infection of macrophages is simple steric hindrance by antibody, which prevents access of the virus to the cell surface. In particular, cross-linking of cell surface receptors with secondary antibody could potentially cause a large, nonspecific physical barrier to infection. Therefore, macrophage cell surface molecules used as controls for antibody inhibition experiments were assessed by flow cytometry and found to be expressed at levels comparable to that of CD63 on the surface of macrophages (Fig. 2). Due to the similar expression levels of these proteins, it is likely that similar levels of nonspecific steric hindrance would be produced by antibodies to these proteins. A lack of inhibitory effect when control MAbs are cross-linked (Fig. 1A and B) further supports the specificity of anti-CD63-mediated inhibition. Since antibodies directed against these molecules do not inhibit HIV-1 infection of macrophages, it is likely that steric hindrance is not the key factor in the inhibition resulting from anti-CD63.
FIG. 2.
Expression of macrophage surface antigens. Primary macrophages were incubated with the indicated anti-tetraspanin, anti-HLA-DP/DQ/DR, or isotype control MAb for 30 min at 4°C, followed by a 30-min incubation with FITC-labeled secondary antibody. Cells were then fixed and assessed by flow cytometry. Data are representative of results with four different donors. MCF, mean channel fluorescence.
Inhibition of macrophage infection by anti-CD63 occurs at an early step of the virus life cycle, which includes viral entry.
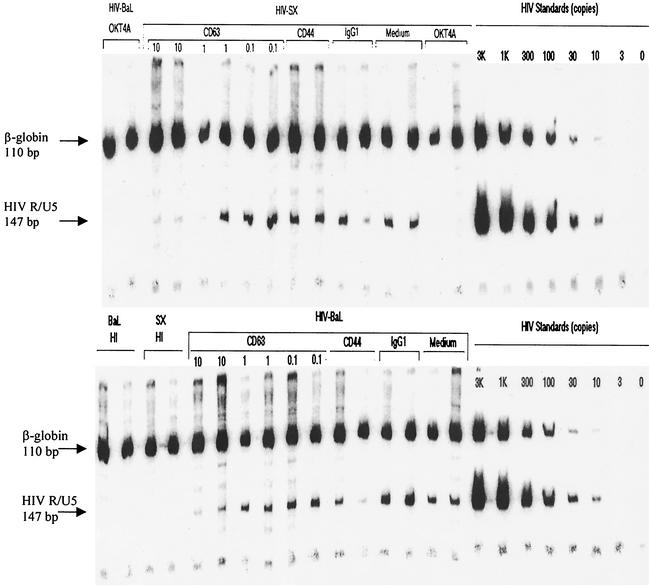
The experiments in Fig. 1 demonstrate inhibition of macrophage infection as measured by virus (p24) production in culture supernatants. To assess early aspects of HIV-1 infection, new viral DNA formation was quantitated as a measure of viral entry (Fig. 3). In this assay, primary macrophages were treated with 0, 0.1, 1, or 10 μg of anti-CD63 or control MAb/ml and then infected with DNase-treated and filtered virus supernatants. Heat-inactivated virus was included as a control to demonstrate that amplified products were not due to DNA contamination from input viral supernatants. Cells were lysed 24 h following infection, and DNA was purified for PCR amplification by using 32P-end-labeled R/U5 long terminal repeat-specific primers (lower bands) as well as β-globin primers (upper bands) to ensure that comparable numbers of cells were analyzed for each condition. Quantitation standards for HIV-1 copy number were amplified in parallel. As shown in Fig. 3, anti-CD63 treatment of macrophages resulted in a dose-dependent inhibition of new viral DNA formation for two separate R5 strains, SX and BaL, indicating that the block occurs early in the virus life cycle, prior to reverse transcription. While some variability in the amount of R/U5 PCR product was observed between replicate samples, anti-CD4 and anti-CD63 were the only conditions that resulted in concordant inhibition of p24 production (data not shown).
FIG. 3.
Quantitative PCR analysis of new HIV-1 DNA formation. Primary macrophages were pretreated with anti-CD63 (0, 0.1, 1, and 10 μg/ml) or control antibodies, followed by infection with filtered, DNase-treated HIV-1-SX and HIV-1-BaL. After 24 h, cells were lysed and DNA was extracted for PCR amplification with HIV-1 R/U5 primers (lower bands) and with β-globin primers (upper bands) as an internal control. HIV-1 quantitation standards were amplified in parallel. HI, heat-inactivated virus; OKT4A, positive control anti-CD4 MAb (0.5 μg/ml); Medium, no MAb treatment; CD44 and IgG1, negative isotype control MAb (10 μg/ml). Data are representative of five experiments (five donors).
MAb to CD63 also inhibits dualtropic (R5/X4) but not X4-mediated HIV-1 infection of primary monocyte-derived macrophages.
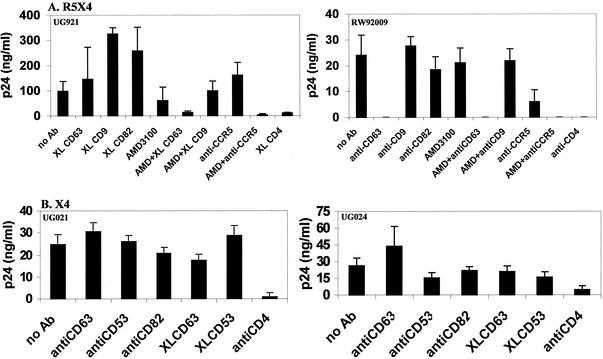
Since it was found that infection of macrophages by R5 HIV-1 was inhibited by anti-CD63 MAb (Fig. 1), we extended this observation by assessing the effects of anti-CD63 MAb on R5X4 strains of HIV-1. To examine possible coreceptor dependence of anti-CD63 inhibition, these experiments were performed in the presence or absence of the CXCR4 inhibitor AMD3100 as well as the CCR5-specific MAb 2D7. As shown in Fig. 4A, R5X4 strains UG921 and RW92009 were susceptible to the inhibitory effects of anti-CD63, but not control antibodies against tetraspanin proteins CD9 and CD82. While infection of macrophages by RW92009 was inhibited completely by anti-CD63, macrophage infection by UG921 required AMD3100 treatment combined with anti-CD63 MAb treatment for complete inhibition (similar to the effects of treatment with AMD3100 and 2D7 combined). Similar effects were observed with the prototypic R5X4 strain 89.6 (data not shown). These results suggest that anti-CD63 effects may be linked to CCR5 usage. Further support for CCR5 dependence was shown with two unusual primary X4 isolates that replicate in macrophages through use of CXCR4, UG021, and UG024 (49)59, which were not inhibited by anti-CD63 pretreatment (Fig. 4B).
FIG. 4.
Anti-CD63 MAb inhibits infection of macrophages by R5X4 strains, but not X4 strains, of HIV-1. Primary macrophages were incubated with anti-CD63 or negative control MAb (10 μg/ml), anti-CD4 (Leu3A, 0.5 μg/ml), anti-CCR5 (2D7, 10 μg/ml), AMD3100 (10 μg/ml), or a combination of anti-CD63-AMD3100 (5 μg/ml each) or anti-CCR5-AMD3100 (5 μg/ml each) and then infected with the dualtropic strain UG921 or RW92009 (A) or the macrophagetropic X4 strain UG021 or UG024 (B). Following a 7-day incubation, supernatants were harvested for p24 ELISA. Data are representative of four experiments.
MAb to CD63 does not inhibit infection of PBL.
Since CD63 is expressed on both macrophages (Fig. 2) and CD4+ lymphocytes (Fig. 5A), we next asked whether inhibition of HIV-1 infection by anti-CD63 could also be observed in lymphocytes. A representative experiment is shown in Fig. 5B, where peripheral blood lymphocytes (PBL) were treated with anti-CD63 or control antibodies followed by infection with the R5 isolate HIV-1-SX. Since PBL were found to be resistant to the inhibitory effect of anti-CD63, macrophages may be infected by HIV-1 in a manner distinct from that for T lymphocytes.
FIG. 5.
(A) CD63 is expressed on CD4+ lymphocytes. PHA-stimulated CD8-, CD19-, and macrophage-depleted PBL (>90% CD4+) were treated with PE-anti-CD63, FITC-anti-CD4, or matching fluorochrome-conjugated isotype control prior to flow cytometry assessment. The histogram shown is gated on CD4 (FITC)-positive cells. The solid histogram shows the isotype control, and the open histogram shows CD63 expression. Data are representative of results with three donors. (B) Anti-CD63 treatment does not inhibit infection of primary PBL. Cells were treated with anti-CD63 (10 μg/ml) or with control MAb anti-CD9 (10 μg/ml) or anti-CD4 (Leu3A, 0.5 μg/ml) and then infected with HIV-1-SX. Following a 7-day incubation, supernatants were harvested and analyzed for p24 by ELISA. Data are representative of five experiments.
A cell line expressing high levels of CD4 and CCR5 is also resistant to anti-CD63-mediated inhibition.
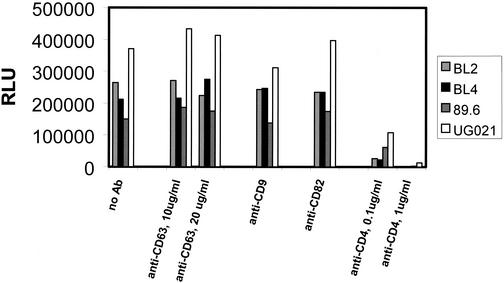
Since infection of PBL was not affected by anti-CD63, we next asked whether this phenomenon is specific for macrophages. Therefore, we tested the effects of anti-CD63 on infection of the cell lines JC53-BL (56) and U373-MAGI-CCR5E (54) with various R5, R5X4, and X4 isolates (Fig. 6 and data not shown). Since infection of these cells was unaffected by anti-CD63 treatment, similar to that of PBL (Fig. 5), it is possible that the involvement of CD63 in HIV-1 infection may be restricted to macrophages.
FIG. 6.
Anti-CD63 treatment does not inhibit infection of JC53-BL cells. Cells were treated with anti-CD63 (10 μg/ml), negative control anti-CD82 or anti-CD9 MAb (10 μg/ml), or anti-CD4 (Leu3A, 0.5 μg/ml) and then infected with the primary R5 strains BL2 and BL4, the dualtropic strain 89.6, or the primary X4 virus UG021. Following a 48-h incubation, the cells were lysed and analyzed by luminometry. RLU, relative light units.
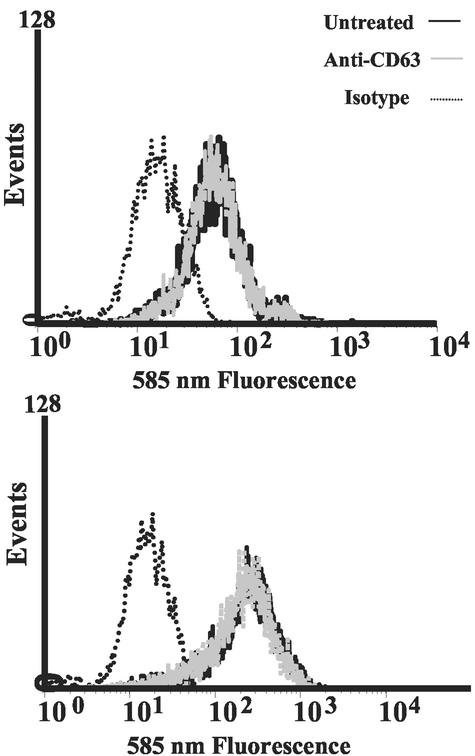
CD4 and CCR5 expression levels are unaltered upon anti-CD63 MAb treatment.
To explore the possibility that inhibition of macrophage infection by anti-CD63 may involve antibody-mediated alterations in cell surface receptor or coreceptor levels, CD4 and CCR5 expression levels were assessed with or without anti-CD63 treatment. In Fig. 7, primary macrophages were treated for 30 min with anti-CD63 MAb or medium alone and then labeled with PE-anti-CD4 (upper graph) or PE-anti-CCR5 (lower graph). Following flow cytometry analysis, anti-CD63-treated samples were found to express levels of cell surface CD4 and CCR5 similar to those of the untreated controls. Therefore, it is unlikely that the inhibitory effects of anti-CD63 are due to downregulation of CD4 or CCR5.
FIG. 7.
Anti-CD63 MAb treatment does not affect CD4 or CCR5 expression on macrophages. Cells were pretreated with anti-CD63 MAb and then labeled with an irrelevant PE-isotype control antibody, PE-anti-CD4 (upper graph), or PE-anti-CCR5 (lower graph), followed by flow cytometry assessment.
Another potential mechanism that could explain anti-CD63-mediated inhibition of R5 but not X4 HIV-1 strains is the induction of β-chemokine secretion in response to anti-CD63 MAb treatment. Therefore, this possibility was assessed by measuring β-chemokine production in both macrophages and PBL at various time points posttreatment by ELISA. While low levels (50 to 100 pg/ml) of MIP-1α and MIP-1β (but not RANTES) were secreted by both macrophages and PBL by 1 h posttreatment with anti-CD63 (data not shown), this is unlikely to explain the inhibitory effects of anti-CD63, since concentrations over 1,000 times in excess of these levels have been shown to result in only partial and low-level inhibition of HIV-1 replication in macrophages, even when all three of these β-chemokines are used together (13). Moreover, there was no detectable difference in the levels of anti-CD63-induced β-chemokine secretion between macrophages and PBL.
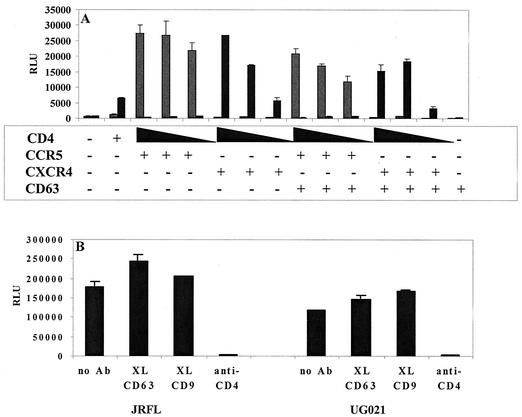
CD63 does not enhance Env-mediated cell-cell fusion.
HIV-1-infected cells characteristically express HIV-1 Env gp120-gp41 in the cell membrane and therefore have the ability to form syncytia with other CD4- and chemokine receptor-expressing cells. Support for a role of CD63 in HIV-1 entry would be provided by the demonstration of increased fusion with the introduction of exogenous CD63 into cells expressing low levels of CD63. Therefore, in order to provide further support for the involvement of CD63 specifically at the level of Env-mediated fusion, we assessed cell-to-cell fusion by using the quail fibrosarcoma line QT6 (Fig. 8), which expresses low levels of endogenous CD63 and is therefore useful for CD63 transfection experiments. Briefly, QT6 cells transduced with various HIV-1 Env-encoding vaccinia virus expression constructs and the T7 RNA polymerase-encoding vaccinia virus vTF1.1 (provided by Robert W. Doms) were mixed and allowed to form syncytia with T7 luciferase and receptor-, coreceptor-, and CD63-transfected QT6 cells, followed by lysis and measurement of luciferase activity by luminometry. As shown in Fig. 8A, CD63 transfection did not affect Env-mediated cell-cell fusion in QT6 cells, even with cells expressing lower levels of CD4, which were used in an attempt to more closely mimic the macrophage membrane environment. In a modification of this assay, primary macrophages were transduced with an SP6 polymerase-encoding vaccinia virus and used to assess cell-cell fusion with 293T cells expressing HIV-1 Env and SP6-luciferase (Fig. 8B). Prior to cell mixing, macrophages were treated with anti-CD63 or control antibodies, and this treatment was shown to have little effect on the levels of cell-cell fusion. Together, these studies suggest that the anti-CD63-mediated block to macrophage infection is postfusion or that there may be compensatory factors contributed by the cell lines used in these assays.
FIG. 8.
CD63 is not involved in Env-mediated cell-cell fusion. (A) Cell-cell fusion in QT6 cells. The indicated expression constructs were transfected into QT6 cells and allowed to fuse with QT6 cells expressing ADA Env (striped bars) or HXB Env (black bars). Relative levels of fusion were determined by luciferase production at 6 h, as described in Materials and Methods. Decreasing amounts of transfected CD4 plasmid are shown by decrescendo bars. (B) Macrophage fusion assay. Macrophages were treated with anti-CD63 or control MAb followed by cross-linking (XL) with anti-mouse secondary antibody, and the relative levels of fusion with JRFL or UG021 Env-transfected 293T cells were measured at 6 h, as described in Materials and Methods. RLU, relative light units.
DISCUSSION
In the present study, we provide evidence for the involvement of the tetraspan transmembrane glycoprotein CD63 in the infection of primary macrophages by HIV-1. Inhibition of macrophage infection is observed when cells are pretreated with anti-CD63 MAb, similar to the inhibition seen upon pretreatment with MAb specific for the primary receptor CD4. This inhibition is not observed in PBL, indicating specific relevance to macrophage tropism. In addition, inhibition of macrophage infection prior to reverse transcription suggests that the anti-CD63-mediated block occurs early in the virus life cycle, affecting events associated with virus entry.
The inhibitory effect of anti-CD63 on infection of macrophages by HIV-1 is limited to CCR5-utilizing isolates (R5 and R5X4 strains) and is not seen with viruses that enter macrophages via CXCR4 exclusively (Fig. 4B). This specificity of anti-CD63-mediated effects for R5 and R5X4 strains suggests the possible linkage of these effects to CCR5 utilization. R5X4 strains exhibit different patterns of inhibition of infection in macrophages (Fig. 4A), with some strains requiring blockade of CXCR4 interactions as well as anti-CD63 treatment (i.e., RW92009, which is completely inhibited by anti-CD63 alone) while others require inhibition of CXCR4 in addition to CD63 (i.e., UG921). This may relate to whether a dualtropic strain has a preference for CCR5- or CXCR4-mediated entry in macrophages.
While the precise mechanism of anti-CD63-mediated inhibition is not yet resolved, secondary effects on receptor expression or receptor ligand induction are unlikely. Inhibitory levels of secreted MIP-1α, MIP-1β, or RANTES were not detected upon treatment of macrophages with anti-CD63, and changes in CD4 or CCR5 expression in response to anti-CD63 were not observed. Indeed, if CD63 involvement in HIV-1 infection were linked to effects on CD4 expression, then inhibition of infection by anti-CD63 would be expected for X4 strains as well as R5 and R5X4 strains.
While a decrease in mean channel fluorescence of CD4 or CCR5 on primary macrophages was not observed upon treatment with anti-CD63 (Fig. 7), it is possible that anti-CD63-induced CD4 or CCR5 alterations may be conformational in nature and therefore not detected by the antibodies used for flow cytometry. For example, an antibody-induced change in CD4-CCR5 complex formation may theoretically affect the efficiency of infection without affecting the actual levels of these receptors on the cell surface. Differential involvement of CD63 in infection by R5 but not X4 viruses could be explained in this manner, as it is possible that CD63 could be involved in the stabilization of critical cell surface receptor associations, specifically for CD4-CCR5 rather than CD4-CXCR4. Previous studies have shown that specific and interdependent cell surface concentrations of CD4 and CCR5 are important for infection by macrophage-tropic isolates (2, 43). It is possible that these associations may be more important in the limiting CD4 environment present on the macrophage surface, thus explaining the differential effects of anti-CD63 on macrophages rather than T cells, which have a high density of surface CD4.
The importance of particular CD4-coreceptor associations in HIV-1 infection has been demonstrated by others (11, 24-26, 58). For example, Lapham et al. (25) reported constitutive CD4-CCR5 complex formation in macrophages and proposed that inadequate CD4-CXCR4 complex formations may contribute to inefficient macrophage entry by X4 strains. It is possible that CD63 could be involved in the stabilization of CD4-CCR5 associations important for infection and that treatment with anti-CD63 MAb may disturb these associations, resulting in inhibition of macrophage infection by R5 HIV-1 strains. Indeed, the tetraspanins in general exist in large multimolecular complexes and have been proposed to act as molecular facilitators, grouping together and stabilizing specific membrane protein associations required for signaling events or other cellular functions (29).
Specialized membrane structures also proposed to function in this same manner are the lipid rafts, which are ordered membrane microdomains enriched in cholesterol and glycosphingolipids. In addition to their function in viral egress (27, 35), it is likely that the structural integrity of lipid rafts may be required for HIV-1 binding or entry events (23, 38, 44), as well as for the conformational integrity and function of chemokine receptors (36, 37). It is possible that CD63 may colocalize to lipid rafts, perhaps participating in differential signaling events in macrophages versus T cells or in response to R5 versus X4 virus strains.
The results of cell-cell fusion assays indicated no change in QT6 cell fusion upon CD63, CD4, and coreceptor cotransfection (Fig. 8A). Since macrophages express relatively low levels of CD4 (32), we attempted to mimic macrophage membrane organization in these experiments by transfecting progressively lower amounts of CD4-encoding plasmid. As expected, there were decreasing levels of fusion, which correlated with decreasing amounts of transfected CD4 plasmid; however, no CD63-induced increase in fusion was observed. Cell-cell fusion assay results with QT6 cells were consistent with fusion assay results with a primary macrophage-293T cell fusion system (Fig. 8B), in which anti-CD63 MAb did not inhibit fusion. This lack of CD63-mediated effect on cell-cell fusion combined with the results of PCR entry assays (Fig. 3) suggest that the involvement of CD63 may be postfusion but prior to reverse transcription. Little is known about this portion of the viral life cycle, and further investigation into the role of CD63 in HIV-1 infection may lead to the delineation of important late events of HIV-1 entry.
Alternatively, there may be aspects of cell-cell fusion that differ from virus-cell fusion, and if such is the case, then CD63 involvement at the level of viral binding or fusion may still occur. It is possible that important infectivity factors that are provided in a cell-to-cell context may be lacking in the context of cell-free virus infection. Furthermore, rather than being solely a cellular molecule, CD63 is present on the surface of the virus (31, 41) and may also function as a virion-associated attachment factor, similar to the role described for virion-associated ICAM-1 (4, 15). However, nonspecific MAb-mediated inhibition due to antibody binding to virions is unlikely, as antibodies to other molecules (besides CD63) that are present in abundant quantities on the virion surface, such as HLA-DR or other tetraspanins (5, 41), do not inhibit macrophage infection (Fig. 1 and data not shown). In addition, if CD63 MAb-mediated inhibition were nonspecific or related solely to antibody-mediated inhibition of virion-associated CD63, then it would be expected that infection of other cell types, such as PBL or JC53-BL, would also be inhibited. Anti-CD63 MAb, however, was not found to inhibit infection of these cell types (Fig. 5 and 6).
In conclusion, we have demonstrated the potential importance of a new cofactor, CD63, in HIV-1 infection of macrophages. We have shown that infection of macrophages, but not PBL, can be inhibited by anti-CD63 MAb and that this observation is specific for HIV-1 strains which utilize CCR5 but not CXCR4. Furthermore, we have shown that inhibition of infection occurs prior to reverse transcription and that cell-cell fusion is unaffected by anti-CD63 MAb. Finally, we have ruled out several possible secondary mechanisms for anti-CD63-mediated inhibition. Taken together, these findings indicate that CD63 may be important for HIV-1 infection of macrophages. Identification of a novel cellular factor involved in HIV-1 entry, such as CD63, may represent an important new therapeutic target for the development of antiretroviral drugs.
Acknowledgments
This work was supported in part by Public Health Service grants AI46250 and AI52041 and the UTMB Sealy Foundation (W.A.O.); Public Health Service grants AI35502 and NS27405 (R.G.C.); and Public Health Service grant T32/AI07388, grant F98-LA-134 from the University of California Universitywide AIDS Research Program, and Veterans Affairs Medical Research Funds (K.G.-F.). J.J.V.L. is supported by the UTMB McLaughlin Predoctoral Fellowship in Infection and Immunity.
We thank Yolanda Houston for technical assistance, Robert W. Doms and Bridget Puffer (University of Pennsylvania) for provision of reagents and assistance with fusion studies, and Mark Griffin and Joan Nichols (University of Texas Medical Branch) for flow cytometry assistance.
REFERENCES
- 1.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 2.Bannert, N., D. Schenten, S. Craig, and J. Sodroski. 2000. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J. Virol. 74:10984-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazan, H. A., G. Alkhatib, C. C. Broder, and E. A. Berger. 1998. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J. Virol. 72:4485-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantin, R., J. F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. [DOI] [PubMed] [Google Scholar]
- 6.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, and C. Gerard. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 7.Clapham, P. R., D. Blanc, and R. A. Weiss. 1991. Specific cell surface requirements for the infection of CD4− positive cells by human immunodeficiency virus types 1 and 2 and by simian immunodeficiency virus. Virology 181:703-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Clercq, E., and D. Schols. 2001. Inhibition of HIV infection by CXCR4 and CCR5 chemokine receptor antagonists. Antivir. Chem. Chemother. 12(Suppl. 1):19-31. [PubMed] [Google Scholar]
- 9.deParseval, A., D. L. Lerner, P. Borrow, B. J. Willett, and J. H. Elder. 2001. Blocking of feline immunodeficiency virus infection by a monoclonal antibody to CD9 is via inhibition of virus release rather than interference with receptor binding. J. Virol. 71:5742-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Marzio, P., J. Tse, and N. R. Landau. 1998. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res. Hum. Retrovir. 14:129-138. [DOI] [PubMed] [Google Scholar]
- 11.Dimitrov, D. S., D. Norwood, T. S. Stantchev, Y. Feng, X. Xiao, and C. C. Broder. 1999. A mechanism of resistance to HIV-1 entry: inefficient interactions of CXCR4 with CD4 and gp120 in macrophages. Virology 259:1-6. [DOI] [PubMed] [Google Scholar]
- 12.Doranz, B. J., J. Rucker, Y. Yi, R. J. Smyth, M. Samson, S. C. Peiper, M. Parmentier, R. G. Collman, and R. W. Doms. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149-1158. [DOI] [PubMed] [Google Scholar]
- 13.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 14.Eilbott, D. J., N. Peress, H. Burger, D. Laneve, J. Orenstein, H. E. Gendelman, R. Seidman, and B. Weiser. 1989. Human immunodeficiency virus type 1 in spinal cords of acquired immunodeficiency syndrome in patients with myelopathy: expression and replication in macrophages. Proc. Natl. Acad. Sci. USA 86:3337-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukudome, K., M. Furuse, T. Imai, M. Nishimura, S. Takagi, Y. Hinuma, and O. Yoshie. 1992. Identification of membrane antigen C33 recognized by monoclonal antibody T cell leukemia virus type-1 (HTLV-1)-induced syncytium formation: altered antigen in HTLV-1-positive T cells. J. Virol. 66:1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gartner, S., P. Markovits, D. M. Markovitz, M. H. Kaplan, R. C. Gallo, and M. Popovic. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215-219. [DOI] [PubMed] [Google Scholar]
- 18.Hwang, S. S., T. J. Boyle, H. K. Lyerly, and B. R. Cullen. 1991. Identification of envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science 253:71-74. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi, T., C. R. Brown, Y. Endo, A. Buckler-White, Plishka, N. Bischofberger, V. V. Hirsch, and M. A. Martin. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 98:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai, T., K. Fukudome, S. Takagi, M. Nagira, M. Furuse, N. Fukuhara, M. Nishimura, Y. Hinuma, and O. Yoshie. 1992. C33 antigen recognized by monoclonal antibodies inhibitory to human T cell leukemia virus type 1-induced syncytium formation is a member of a new family of transmembrane proteins including CD9, CD37, CD53, and CD63. J. Immunol. 149:2879-2886. [PubMed] [Google Scholar]
- 21.Isaacs, S. N., Y. Yi, A. Singh, and R. G. Collman. 1999. A macrophage fusion assay for rapid screening of cloned HIV-1 Env using dual recombinant vaccinia viruses expressing distinct RNA polymerases. J. Virol. Methods 81:55-61. [DOI] [PubMed] [Google Scholar]
- 22.Koenig, S., H. E. Gendelman, J. M. Orenstein, M. C. Dal Canto, G. H. Pezeshkpour, M. Yungbluth, F. Janotta, A. Aksamit, M. Martin, and A. S. Fauci. 1986. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. J. Acquir. Immune Defic. Syndr. 5:1089-1093. [DOI] [PubMed] [Google Scholar]
- 23.Kozak, S. L., J. M. Heard, and D. Kabat. 2002. Segregation of CD4 and CXCR4 into distinct lipid microdomains in T lymphocytes suggests a mechanism for membrane destabilization by human immunodeficiency virus. J. Virol. 76:1802-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapham, C. K., J. Ouyang, B. Chandrasekhar, N. Y. Nguyen, D. S. Dimitrov, and H. Golding. 1996. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science 274:602-605. [DOI] [PubMed] [Google Scholar]
- 25.Lapham, C. K., M. B. Zaitseva, S. Lee, T. Romanstseva, and H. Golding. 1999. Fusion of monocytes and macrophages with HIV-1 correlates with biochemical properties of CXCR4 and CCR5. Nat. Med. 5:303-308. [DOI] [PubMed] [Google Scholar]
- 26.Lee, S., C. K. Lapham, H. Chen, L. King, J. Manischewitz, T. Romantseva, H. Mostowski, T. S. Stantchev, C. C. Broder, and H. Golding. 2000. Coreceptor competition for association with CD4 may change the susceptibility of human cells to infection with T-tropic and macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 74:5016-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Q. H., D. A. Williams, C. McManus, F. Baribaud, R. W. Doms, D. Schols, E. De Clercq, M. I. Kotlikoff, R. G. Collman, and B. D. Freedman. 2000. HIV-1 gp120 and chemokines activate ion channels in primary macrophages through CCR5 and CXCR4 stimulation. Proc. Natl. Acad. Sci. USA 97:4832-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maecker, H. T., S. C. Todd, and S. Levy. 1997. The tetraspanin superfamily: molecular facilitators. FASEB J. 11:428-442. [PubMed] [Google Scholar]
- 30.Mannion, B. A., F. Berditchevski, S. K. Kraeft, L. B. Chen, and M. E. Hemler. 1996. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin α4β1 (CD49d/CD29). J. Immunol. 157:2039-2047. [PubMed] [Google Scholar]
- 31.Meerloo, T., M. A. Sheikh, A. C. Bloem, A. de Ronde, M. Schutten, C. A. van Els, P. J. Roholl, P. Joling, J. Goudsmit, and H. J. Schuurman. 1993. Host cell membrane proteins on human immunodeficiency virus type 1 after in vitro infection of H9 cells and blood mononuclear cells. An immuno-electron microscopic study. J. Gen. Virol. 74:129-135. [DOI] [PubMed] [Google Scholar]
- 32.Meltzer, M. S., and H. E. Gendelman. 1992. Mononuclear phagocytes as targets, tissue reservoirs, and immunoregulatory cells in human immunodeficiency virus disease. Curr. Top. Microbiol. Immunol. 181:239-263. [DOI] [PubMed] [Google Scholar]
- 33.Meltzer, M. S., D. R. Skillman, D. L. Hoover, B. D. Hanson, J. A. Turpin, D. C. Kalter, and H. E. Gendelman. 1990. Macrophages and the human immunodeficiency virus. Immunol. Today 11:217-223. [DOI] [PubMed] [Google Scholar]
- 34.Metzelaar, M. J., P. L. J. Wijngaard, P. J. Peters, J. J. Sixma, H. K. Nieuwenhuis, and H. C. Clevers. 1991. CD63 antigen. J. Biol. Chem. 266:3239-3245. [PubMed] [Google Scholar]
- 35.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen, D. H., and D. Taub. 2002. Cholesterol is essential for macrophage inflammatory protein 1 beta binding and conformational integrity of CC chemokine receptor 5. Blood 99:4298-4306. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen, D. H., and D. Taub. 2002. CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168:4121-4126. [DOI] [PubMed] [Google Scholar]
- 38.Nisole, S., B. Krust, and A. G. Hovanessian. 2002. Anchorage of HIV on permissive cells leads to coaggregation of viral particles with surface nucleolin at membrane raft microdomains. Exp. Cell Res. 276:155-173. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien, W. A., Y. Koyanagi, A. Namazie, J.-Q. Zhao, A. Diagne, K. Idler, J. A. Zack, and I. S. Y. Chen. 1990. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature (London) 348:69-73. [DOI] [PubMed] [Google Scholar]
- 40.Orenstein, J. M., C. Fox, and S. M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science 276:1857-1861. [DOI] [PubMed] [Google Scholar]
- 41.Orentas, R. J., and J. E. Hildreth. 1993. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res. Hum. Retrovir. 9:1157-1165. [DOI] [PubMed] [Google Scholar]
- 42.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 43.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rich, E. A., I. S. Y. Chen, J. A. Zack, M. L. Leonard, and W. A. O'Brien. 1992. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus expression. J. Clin. Investig. 89:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubinstein, E., F. Le Naour, C. Lagaudriere-Gesbert, M. Billard, H. Conjeaud, and C. Boucheix. 1996. CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. Eur. J. Immunol. 26:2657-2665. [DOI] [PubMed] [Google Scholar]
- 47.Schmidtmayerova, H., M. Alfano, G. Nuovo, and M. Bukrinsky. 1998. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J. Virol. 72:4633-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw, G. M., M. E. Harper, B. H. Hahn, L. G. Epstein, D. C. Gajdusek, R. W. Price, B. A. Navia, C. K. Petito, C. J. O'Hara, J. E. Groopman, E.-S. Cho, J. M. Oleske, F. Wong-Stall, and R. C. Gallo. 1985. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science 227:177-181. [DOI] [PubMed] [Google Scholar]
- 49.Skubitz, K. M., K. D. Campbell, J. Iida, and A. P. Skubitz. 1996. CD63 associates with tyrosine kinase activity and CD11/CD18, and transmits an activation signal in neutrophils. J. Immunol. 157:3617-3626. [PubMed] [Google Scholar]
- 50.Strizki J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C. C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takashima, K., H. Miyake, R. A. Furuta, J. I. Fujisawa, Y. Iizawa, N. Kanzaki, M. Shiraishi, K. Okonogi, and M. Baba. 2001. Inhibitory effects of small-molecule CCR5 antagonists on human immunodeficiency virus type 1 envelope-mediated membrane fusion and viral replication. Antimicrob. Agents Chemother. 45:3538-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tokunaga, K., M. L. Greenberg, M. A. Morse, R. I. Cumming, H. K. Lyerly, and B. R. Cullen. 2001. Molecular basis for cell tropism of CXCR4-dependent human immunodeficiency virus type 1 isolates. J. Virol. 75:6776-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tschachler, E., V. Groh, M. Popovic, D. L. Mann, S. Konrad, B. Safai, L. Eron, F. diMarzo Veronese, K. Wolff, and G. Stingl. 1987. Epidermal Langerhans cells—a target for HTLV-III/LAV infection. J. Investig. Dermatol. 88:233-237. [DOI] [PubMed] [Google Scholar]
- 54.Vodicka, M. A., W. C. Goh, L. I. Wu, M. E. Rogel, S. R. Bartz, V. L. Schweickart, C. J. Raport, and M. Emerman. 1997. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology 233:193-198. [DOI] [PubMed] [Google Scholar]
- 55.Wang, J. M., and J. J. Oppenheim. 1999. Interference with the signaling capacity of CC chemokine receptor 5 can compromise its role as an HIV-1 entry coreceptor in primary T lymphocytes. J. Exp. Med. 190:591-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiley, C. A., R. R. Schrier, J. A. Nelson, P. W. Lampert, and M. B. A. Oldstone. 1986. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. USA 83:7089-7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao, X., D. Norwood, Y. R. Feng, M. Moriuchi, A. Jones-Trower, T. S. Stantchev, H. Moriuchi, C. C. Broder, and D. S. Dimitrov. 2000. Inefficient formation of a complex among CXCR4, CD4 and gp120 in U937 clones resistant to X4 gp120-gp41-mediated fusion. Exp. Mol. Pathol. 68:139-146. [DOI] [PubMed] [Google Scholar]
- 59.Yi, Y., S. N. Isaacs, D. A. Williams, I. Frank, D. Schols, E. De Clercq, D. L. Kolson, and R. G. Collman. 1999. Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism. J. Virol. 73:7117-7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, H., G. Dornaldula, M. Beumont, L. Livornese, Jr., B. Van Uitert, K. Henning, and R. J. Pomerantz. 1998. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. New Engl. J. Med. 339:1803-1816. [DOI] [PubMed] [Google Scholar]
- 61.Zhu, T., H. Mo, N. Wang, D. Nam, Y. Cao, R. Koup, and D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]