Abstract
OBJECTIVE
Benzodiazepines are the mainstay of treatment for mild-to-moderate alcohol withdrawal in outpatient settings, but they can interact with alcohol, cause motor incoordination, or be abused. This study compared the therapeutic responses of the benzodiazepine lorazepam and the anticonvulsant carbamazepine for the outpatient treatment of acute alcohol withdrawal in terms of patients' previous detoxification histories, and compared the effects of these 2 medications on drinking behaviors in the immediate postdetoxification period.
DESIGN
This was a randomized double-blind trial comparing patient responses to carbamazepine and lorazepam across 2 levels of detoxification histories (0–1 or ≥2 previous medicated detoxifications).
SETTING
A university medical center substance abuse clinic in Charleston, SC.
PATIENTS
One hundred thirty-six patients in moderate alcohol withdrawal were randomized. Major exclusions were significant hepatic or hematologic abnormalities and use of medications that could alter withdrawal symptoms.
INTERVENTIONS
Patients received 600–800 mg of carbamazepine or 6–8 mg of lorazepam in divided doses on day 1 tapering to 200 mg of carbamazepine or 2 mg of lorazepam.
MAIN OUTCOME MEASURES
The Clinical Institute Withdrawal Assessment for Alcohol-Revised was used to assess alcohol withdrawal symptoms on days 1 through 5 and postmedication at days 7 and 12. Daily drinking was measured by patient report using a daily drinking log and a breath alcohol level with each visit. Side effects were recorded daily.
RESULTS
Carbamazepine and lorazepam were equally effective at decreasing the symptoms of alcohol withdrawal. In the post-treatment period, 89 patients drank on at least 1 day; on average, carbamazepine patients drank less than 1 drink per drinking day and lorazepam patients drank almost 3 drinks per drinking day (P = .003). Among those with multiple past detoxifications, the carbamazepine group drank less than 1 drink per day on average and the lorazepam group drank about 5 drinks per day on average (P = .033). Lorazepam-treated patients had a significant rebound of alcohol withdrawal symptoms post-treatment (P = .007) and the risk of having a first drink was 3 times greater (P = .04) than for carbamazepine-treated patients. Twenty percent of lorazepam-treated patients had dizziness, motor incoordination, or ataxia and did not recognize their impairment. Twenty percent of carbamazepine-treated patients reported pruritus but no rash.
CONCLUSIONS
Carbamazepine and lorazepam were both effective in decreasing the symptoms of alcohol withdrawal in relatively healthy, middle-aged outpatients. Carbamazepine, however, was superior to lorazepam in preventing rebound withdrawal symptoms and reducing post-treatment drinking, especially for those with a history of multiple treated withdrawals.
Keywords: alcohol, withdrawal, detox, carbamazepine, lorazepam, relapse, randomized trial
Clinical reviews and randomized prospective trials have found that in mild-to-moderate alcohol withdrawal, outpatient treatment as compared to inpatient treatment is equally efficacious, safe, and less expensive.1–7 These studies also indicate that attrition, drinking during treatment, and hospitalization may occur in a third to one half of patients having an outpatient detoxification. Although a single episode of alcohol withdrawal can be self-limited8 and may not require medication, reviews have concluded that benzodiazepines are the current treatment of choice for moderate to severe outpatient alcohol withdrawal.9–13 However, this approach has several limitations. Benzodiazepines may interact with alcohol, may cause motor incoordination, and may be abused.
The anticonvulsant carbamazepine has been used in northern Europe for over 25 years to treat alcohol withdrawal. Carbamazepine has been demonstrated to be superior to placebo14 and to nonbenzodiazepine sedative-hypnotics in suppressing alcohol withdrawal symptoms.15,16 Carbamazepine has been shown in 2 double-blind trials17,18 to be as effective as oxazepam in the inpatient treatment of alcohol withdrawal. Additionally, 2 small placebo-controlled trials19,20 suggested that carbamazepine reduced some measures of alcohol consumption in alcohol-dependent outpatients in the postwithdrawal period.
Patients with multiple treated withdrawals have more severe withdrawal symptoms and an increased risk of seizures21–27 compared with patients having a first withdrawal.28,29 Withdrawals may work in a way analogous to the effect of repeated brain electrical stimulations below the seizure threshold that eventually lead to recurrent generalized convulsions in animals.30 Laboratory animals experienced increased frequencies of seizures with repeated withdrawals.31–33 Therefore, carbamazepine may be especially efficacious among persons who have experienced multiple episodes of alcohol withdrawal.
In the present study, carbamazepine was compared to lorazepam for the treatment of outpatient alcohol withdrawal, focusing on withdrawal symptoms and drinking behaviors in the immediate 7 days post-treatment. We hypothesized that both agents would be effective in suppressing alcohol withdrawal, but that carbamazepine might be more effective in ameliorating alcohol withdrawal in the group with a history of multiple episodes of treated alcohol withdrawal and more effective in reducing post-treatment drinking.
METHODS
Subjects
Participants were treatment-seeking patients recruited via newspaper ads and clinical referral. Assessments of the number of previous treated detoxifications were made by clinicians blinded to treatment assignment. Subjects were asked if they had ever been treated with medications (other than vitamins) when they had stopped drinking abruptly in the past. Eligibility requirements for study entry included: satisfaction of Diagnostic and Statistical Manual Version Four criteria for alcohol dependence and alcohol withdrawal, blood alcohol level ≤0.1 g/dL, residence within 50 miles of the study site, a Mini-Mental State Exam score34 ≥26, and admission score on the Clinical Institute Withdrawal Assessment for Alcohol-Revised35 (CIWA-Ar) ≥10. Individuals were excluded from participation for the following: all substance abuse syndromes other than alcohol dependence, nicotine dependence, or cannabis abuse; major Axis I psychiatric disorder; use of medication in the preceding thirty days that could alter the withdrawal process such as benzodiazepines, β blockers, calcium channel antagonists, or antipsychotics; history of head injury or other neurologic illness including idiopathic epilepsy; medical instability; electroencephalogram abnormalities; or grossly abnormal laboratory values (liver enzymes up to 3 times above normal allowed). Patients who had a history of alcohol withdrawal seizures were not excluded. All participants who met criteria for acceptance into the study signed an Institutional Review Board approved informed consent form prior to admission to the study. Given safety concerns, no placebo arm was included.
Treatment Assessment
Patients were stratified into 2 groups based on the number of prior medical detoxifications and were randomized to 5 days of fixed-dose taper of carbamazepine or lorazepam. Subject randomization was based on a computer-generated schedule administered by a research pharmacist not involved in data collection. Patients received 600–800 mg of carbamazepine on day 1 of detoxification, tapering to 200 mg as a single dose on day 5. Patients randomized to lorazepam took 6–8 mg in divided doses on day 1, tapering to a single 2 mg dose on day 5. The lorazepam/carbamazepine dosage equivalency was extrapolated from studies comparing oxazepam to carbamazepine. Prior to study initiation, a CIWA-Ar response curve was generated by titrating the lorazepam daily dose until lorazepam pilot subjects achieved CIWA-Ar score reductions each day that approximated the oxazepam results of the previous studies.17,18 All patients received 100 mg of thiamine orally for 12 days.
Patients were asked to report type and frequency of side effects of treatment medication with each visit. Sedation and ataxia/incoordination were assessed independently of subjects' complaints. Intensity of side effects and attribution to study medication (not related, possibly related, definitely related) were rated by a blinded Master's-level research assistant. Reported side effects were categorized by a physician rater naive of group assignment into 1 of 7 systems: gastrointestinal, central nervous system, cardiovascular, dermatologic, neuromuscular, autonomic, and other.
Measures
Upon admission to the study, but prior to medication treatment, patients were administered the CIWA-Ar, a validated 10-item scale used to monitor the clinical course of alcohol withdrawal symptoms. The CIWA-Ar total score relates to aggregate withdrawal severity, and individual items include evaluation of nausea, tremor, sweating, anxiety, agitation, perceptual disturbances, and clouding of sensorium. CIWA-Ar scores of 6 or lower are considered to be very mild withdrawal. Scores of 7 to 12 are in the moderate category. Scores higher than 12 represent marked withdrawal. Scores of 18 to 20 represent severe withdrawal, and these patients should be hospitalized for withdrawal treatment. Patients also completed the Alcohol Dependence Scale36 (a 29-item self-report scale that allows for quantification of the severity of alcohol dependence) and a Daily Drinking Log.37 We assessed alcohol use during the 14 days prior to study entry and daily use during the detoxification treatment phase and during follow up (days 6 to 12). Alcohol consumption was converted to standard drinks per drinking day. Heavy drinking (i.e., relapse) was defined as 5 or more standard drinks per day for males and 4 or more for females. Patients were administered the CIWA-Ar daily by a Master's level research assistant for 5 days at approximately the same time each day during the treatment phase and on days 7 and 12 (2 and 7 days post-treatment, respectively). Breath alcohol levels were measured at each assessment point.
Data Analyses
The study was designed as a 2 × 2 × 7 split-plot factorial with carbamazepine versus lorazepam groups and number of previous detoxifications (0–1 vs ≥2) comprising the 2 between-patient factors. Study day served as the within-patient factor with 7 levels (days 1–5, 7, and 12). A mixed-model analysis of covariance (ANCOVA)38 was used to analyze CIWA-Ar scores during the 12-day study period. CIWA-Ar scores were adjusted with respect to both the time since last drink as a covariate and the imbalances caused by missing data under the assumption that such data were uninformative. The ANCOVA model included all main effects and interactions involving single/multiple previous detoxifications, carbamazepine/lorazepam group, and study day, and covaried for the number of hours since last drinking prior to each CIWA-Ar rating. The mixed-model approach allowed for missing data (under the assumption that such data was uninformative, given the terms in the model) and an unstructured variance-covariance matrix. The Type I error rate associated with the statistical test of each ANCOVA effect was held at 0.05 for a given dependent variable. Statistically significant interactions were further analyzed using analyses of simple main effects. The Type I error rate in these subsequent analyses was controlled across the sources of variation contributing to the sample main effect as described by Kirk.39 An ANCOVA was used to analyze these data, with prestudy drinks per drinking day as the sole covariate and treatment group and single versus multiple previous detoxifications as between-subject factors. Cox regression analyses were used to assess the main effects and interaction of single/multiple previous detoxifications and carbamazepine/lorazepam groups on the survival time to first drinking day and the survival time to first heavy drinking day, respectively.
RESULTS
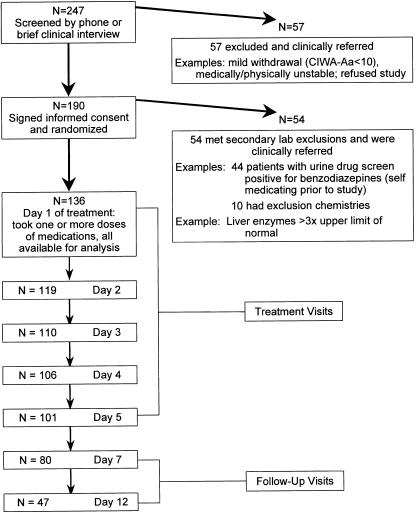
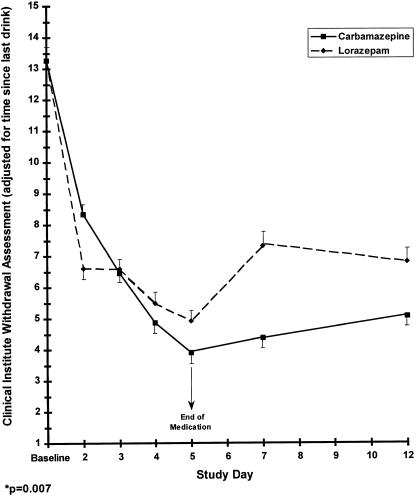
There were no significant differences in demographic or clinical characteristics between the 4 groups defined by treatment medication and number of previous detoxifications (Table 1). Retention rates did not differ between the carbamazepine and lorazepam groups or between the single versus multiple previous detoxification patients. Three patients in the carbamazepine group and 2 patients in the lorazepam group had a history of alcohol withdrawal seizures, while none of the patients had a history of delirium tremens. The number of subjects available for analysis each day in the study is shown in Figure 1. We analyzed the CIWA-Ar score data in 3 ways and found: 1) no significant difference by treatment group in CIWA-Ar scores when all twelve study days were considered (P = .23); 2) a difference by treatment group in CIWA-Ar scores over time (P = .007); and 3) a difference in CIWA-Ar score on day 7 (P = .01) (see Fig. 2).
Table 1.
Subject Characteristics by Experimental Group
| 0–1 Previous Detoxifications (n = 103) | Multiple Detoxifications (n = 33) | |||
|---|---|---|---|---|
| Lorazepam | Carbamazepine | Lorazepam | Carbamazepine | |
| n | 58 | 45 | 17 | 16 |
| Male, % | 75.9 | 80.0 | 70.6 | 62.5 |
| Mean age, y (±SD) | 39.3 (9.2) | 37.7 (9.9) | 38.0 (6.4) | 37.7 (7.4) |
| White, % | 81.0 | 80.0 | 94.1 | 93.8 |
| Mean income (past 30 d) in U.S. dollars (±SD) | 1,329 (1,251) | 1,425 (1,682) | 1,137 (1,011) | 1,634 (1,609) |
| Mean years of education (±SD) | 12.8 (2.3) | 12.9 (2.5) | 12.6 (2.2) | 12.4 (1.6) |
| Standard drinks in 14 days prior to detoxification i (±SD) | 181.7 (137.4) | 177.6 (149.1) | 164.2 (98.8) | 182.3 (73.7) |
| Mean alcohol dependence scale score (±SD) | 18.6 (6.9) | 21.7 (7.7) | 23.4 (7.9) | 24.4 (6.7) |
| Mean number of years drinking (±SD) | 21.6 (8.2) | 19.6 (10.0) | 22.2 (7.5) | 19.4 (7.8) |
| Mean drinks per drinking day (days 6–12) (±SD) | 1.9 (3.7) | 1.1 (2.4) | 4.8 (5.6) | 0.1 (0.4) |
FIGURE 1.
Study subjects disposition.
FIGURE 2.
Clinical institute withdrawal assessment as a function of carbamazepine or lorazepam and treatment day.
Patients who had multiple previously treated withdrawals generally had higher CIWA-Ar scores throughout treatment and during the post-treatment follow-up than did the individuals who had 0 to 1 previous withdrawals (P = .009). The individuals in the multiple detoxification group had an upward rebound of CIWA-Ar withdrawal scores on days 7 and 12 that was about 50% higher than it was on day 5.
Drinking Behaviors Post-Treatment
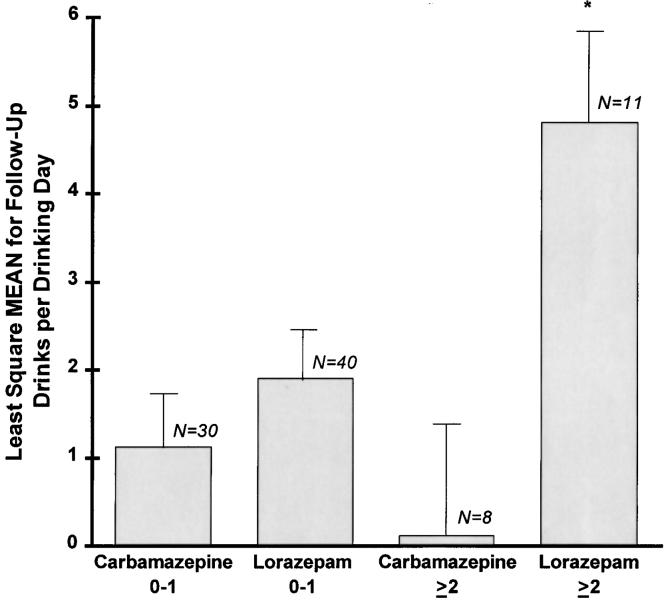
Eighty-nine individuals had at least 1 day of post-treatment drinking data, and thus post-treatment drinks per drinking day (based on data from day 6 through day 12) was analyzed for these subjects. There was no main effect of single versus multiple previous detoxifications on post-treatment drinking. However, there was a statistically significant effect of treatment group (P = .003) and the treatment group interacted with single versus multiple previous detoxifications (P = .033). Both of these effects favored carbamazepine (see Fig. 3). The mean drinks per drinking day were similar for both carbamazepine- and lorazepam-treated patients who had 0 to 1 previous detoxifications. Those with multiple detoxifications receiving lorazepam drank about 5 drinks per day on average compared to less than 1 drink a day on average for those receiving carbamazepine (P = .004). An additional analysis using baseline and within-treatment drinks per drinking day as covariates produced similar results.
FIGURE 3.
Drinks per drinking day, day 6–day 12.
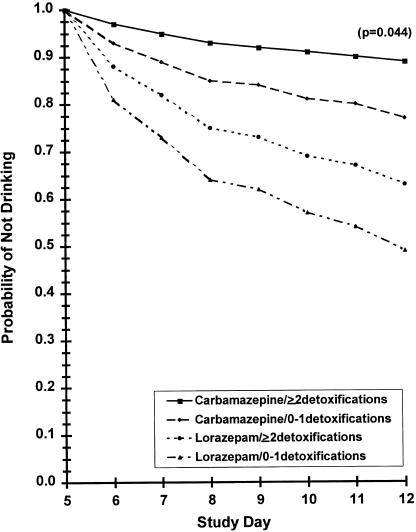
A Cox regression model was used to examine the effects of treatment group, single versus multiple previous detoxifications, and their interaction over time to first drinking day (Fig. 4). The analysis was run separately for within-treatment (days 1 to 5) and follow-up (days 6 to 12) periods. The analysis of drinking during treatment failed to reveal any statistically significant effect of drug group. However, there was a statistically significant effect of treatment group in the time to first drink during the follow-up period (P = .044.) Specifically, the relative risk of having a first drink for the lorazepam group was over 3 times more likely than for the carbamazepine group.
FIGURE 4.
Time to the first drinking day (day 6–day 12).
Medication Side Effects
Information on side effects was available from 133 subjects. Two patients dropped out on day 2 prior to reporting side effects, and data were unavailable for 1 other subject. The overall frequency of side effects did not differ between carbamazepine- and lorazepam-treated patients (Fisher's Exact Test, 2-tailed, P = .599). Pruritus occurred in 18.9% of carbamazepine patients and 1.3% of lorazepam patients (Fisher's Exact Test, 2-tailed, P = .004). Patients in the lorazepam and carbamazepine groups did not report central nervous system side effects commonly (about 5% for both groups). However, the clinician rated central nervous system side effects of dizziness, incoordination, light-headedness, and drowsiness as probably being caused by study medication 6.9% of the time for those taking carbamazepine and 22.7% of the time for those taking lorazepam (Fisher's Exact Test, 2-tailed, P = .02). Hepatic transaminases (alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl aminotransferase) and serum sodium did not differ for the 2 medication groups at day 5 of treatment.
DISCUSSION
Carbamazepine appeared as effective as lorazepam in decreasing the acute symptoms of alcohol withdrawal in this outpatient study. This is not surprising, since we sought dosage equivalence through literature review and our own pilot work with these two drugs. Carbamazepine appeared to have some potentially important advantages over lorazepam in the immediate postdetoxification period. Carbamazepine-treated patients were less likely to have a first drink, and when they did drink, drank less than lorazepam-treated patients. The differential effect of medication drinking behaviors was particularly evident in the group with a history of multiple treated alcohol withdrawal. At the conclusion of treatment, alcohol withdrawal signs and symptoms rebounded in the lorazepam-treated patients but not in the carbamazepine-treated patients. No patients in the study developed alcohol withdrawal seizures or delirium tremens.
Lorazepam-treated patients in the present sample drank sooner and drank more post-treatment, particularly those in the multiple previous detoxification group. Alcohol-dependent rodents during a period of alcohol withdrawal do not usually self-administer alcohol. However, diazepam administration during alcohol withdrawal reinstated alcohol self-administration.40 In addition, alcohol-dependent rodents who receive diazepam during involuntary alcohol deprivation, when re-exposed to alcohol, drink at equal or greater intensity to predeprivation (abstinence) levels.41 In the present study, the rebound of alcohol withdrawal symptoms, and the propensity for benzodiazepines to enhance reinstatement of alcohol use, could possibly explain the greater amounts of alcohol consumption in the post-treatment period for the lorazepam-treated patients. Kranzler et al.19 used carbamazepine or placebo to treat a group of cocaine-dependent patients who were also alcohol dependent. At a 3-month follow-up, although there was no effect on cocaine use, alcohol use was significantly decreased in the carbamazepine group. In a small trial, Mueller et al.20 demonstrated less relapse drinking in carbamazepine-treated compared to placebo-treated alcoholics. O'Connor et al.42 have reported that postdetoxification relapse to alcohol can be predicted in part by the intensity of alcohol withdrawal symptoms at the end of treatment. It is of interest that the group that had the most alcohol withdrawal rebound in the present study (lorazepam-treated multiple detoxification patients) also had the most drinking during this period. Rebound symptoms with benzodiazepines have been reported in other conditions, such as the short-term treatment of insomnia43 and anxiety disorders.44 It could be argued that the use of a longer-acting benzodiazepine for alcohol withdrawal might well prevent the problem of rebound symptoms. However, withdrawal phenomena from long-acting benzodiazepines can occur as well.45 Furthermore, the use of a long-acting benzodiazepine in the outpatient setting could lead to drug accumulation and higher blood levels of the benzodiazepine. This might result in an increased risk of impaired motor coordination, a liability we noted with lorazepam in the present trial. About 1 in 5 patients on lorazepam experienced clinically significant dizziness, ataxia, sleepiness, and incoordination. Patients did not perceive these limitations. Coordination and motor impairment from carbamazepine was not common. Thus, driving, operating machinery, or climbing might well be impaired in a significant number of patients who take lorazepam during the outpatient treatment of alcohol withdrawal. For those working, these effects could lead to decreased productivity and increased job-related accidents.
This study has several limitations. The design of the study is partially reliant on patient self-report of previously medically treated alcohol withdrawal episodes. Our sample was composed of primarily middle-aged, lower middle-class, relatively healthy Caucasian males who had about 2 decades of heavy alcohol consumption but minimal polysubstance abuse. A similarly designed study of patients seeking treatment in an emergency room setting might yield different results. In addition, the results are not generalizable to individuals with other major substance abuse syndromes, psychiatric disorders, and medications that could alter the withdrawal process. Carbamazepine interacts with multiple medications and, therefore, may not be an ideal choice among an older or sicker population.46 Considering the morbidity and mortality associated with alcohol dependence, it is likely that the short-term use of carbamazepine, particularly in patients with a history of multiple withdrawals, outweighs the risk of rare, but potentially fatal, side effects. In our study, carbamazepine appears to be a useful drug, particularly in individuals who have been treated multiple times for previous alcohol withdrawal. However, a single dose of lorazepam has been shown to be effective in reducing the incidence of a second alcohol withdrawal seizure, reducing hospitalization rates and second emergency room visits.47 This has not been evaluated with carbamazepine. Another concern is the dosage equivalency between carbamazepine and lorazepam. If the dosages were not equivalent, the differential results may simply be due to unequal dosing. However, we believe that dosage equivalency was achieved, since both drugs were similar in suppressing CIWA-Ar scores during the 5 days of treatment. If lorazepam doses had been increased, it is likely that there would have been more ataxia and sedation. If lorazepam doses had been decreased, it is possible that withdrawal symptoms would have been greater than in the carbamazepine group.
In summary, we found that in our outpatient setting among generally healthy individuals with mild-to-moderate alcohol withdrawal, carbamazepine appeared as effective as lorazepam in relieving the acute symptoms of alcohol withdrawal and was more effective than lorazepam in preventing rebound alcohol withdrawal symptoms and relapse to alcohol use in the immediate post-treatment period. Several newer anticonvulsants have minimal interactions with other pharmaceuticals, do not have potential serious side effects, and in preliminary work with animals and humans, suppress alcohol withdrawal symptoms.48 The present work should be replicated with these medications.
Acknowledgments
The authors wish to gratefully thank and acknowledge Melissa Michel, Kristi Cochran, and Wanda Smalls-Smith for assistance with data collection, Cynthia Dominick for laboratory assistance, and Vicki Brumbelow, Ann Blanton, and Gretchen Rivers for manuscript preparation. Reference review was by Wesley Pye.
This research was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism Center Grant no. AA10761.
REFERENCES
- 1.Stinnett J. Outpatient detoxification of the alcoholic. Intrl J Addictions. 1982;17:1031–46. doi: 10.3109/10826088209057773. [DOI] [PubMed] [Google Scholar]
- 2.Miller W, Hester R. Inpatient alcoholism treatment: who benefits. Am Psychol. 1986;41:794–805. doi: 10.1037//0003-066x.41.7.794. [DOI] [PubMed] [Google Scholar]
- 3.Webb M, Unwin A. The outcome of outpatient withdrawal from alcohol. Br J Addiction. 1988;83:929–34. doi: 10.1111/j.1360-0443.1988.tb01585.x. [DOI] [PubMed] [Google Scholar]
- 4.Alterman A, Hayasida M, O'Brien C. Treatment response and safety of ambulatory medical detoxification. J Stud Alcohol. 1988;49:160–6. doi: 10.15288/jsa.1988.49.160. [DOI] [PubMed] [Google Scholar]
- 5.Hayashida M, Alterman A, McLellan T, et al. Comparative effectiveness and costs of inpatient and outpatient detoxification of patients with mild-to-moderate alcohol withdrawal syndrome. N Engl J Med. 1989;320:358–65. doi: 10.1056/NEJM198902093200605. [DOI] [PubMed] [Google Scholar]
- 6.Klijnsma M, Cameron M, Burns T, McGuigan S. Outpatient alcohol detoxification — outcome after two months. Alcohol Alcohol. 1995;30:669–73. [PubMed] [Google Scholar]
- 7.Wiseman EJ, Henderson KL, Briggs MJ. Individualized treatment for outpatients withdrawing from alcohol. J Clin Psychiatry. 1998;59:289–93. doi: 10.4088/jcp.v59n0603. [DOI] [PubMed] [Google Scholar]
- 8.Whitfield CL, Thompson G, Lamb A, Spencer V, Pfeifer M, Browning-Ferrando M. Detoxification of 1,024 alcoholic patients without psychoactive drugs. JAMA. 1978;239:1409–10. [PubMed] [Google Scholar]
- 9.Hall W, Zador D. The alcohol withdrawal syndrome. Lancet. 1997;349:1897–900. doi: 10.1016/S0140-6736(97)04572-8. [DOI] [PubMed] [Google Scholar]
- 10.Mayo-Smith MF. Pharmacological management of alcohol withdrawal: a meta-analysis and evidence-based practice guideline. JAMA. 1997;278:144–51. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- 11.Saitz R, O'Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81:881–907. doi: 10.1016/s0025-7125(05)70554-x. [DOI] [PubMed] [Google Scholar]
- 12.Prater CD, Miller KE, Zylstra RG. Outpatient detoxification of the addicted or alcoholic patient. Am Fam Physician. 1999;60:1175–83. [PubMed] [Google Scholar]
- 13.Koch-Weser J, Sellers EM, Kalant H. Alcohol intoxication and withdrawal. N Engl J Med. 1976;294:757–62. doi: 10.1056/NEJM197604012941405. [DOI] [PubMed] [Google Scholar]
- 14.Björkqvist S, Isohanni M, Makela R, Malinen L. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicentre double-blind comparison with placebo. Acta Psychiatr Scand. 1976;53:333–42. doi: 10.1111/j.1600-0447.1976.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 15.Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiat Scand. 1981;64:254–9. doi: 10.1111/j.1600-0447.1981.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 16.Flygenring J, Hansen J, Holst B, Petersen E, Sorensen A. Treatment of alcohol withdrawal symptoms in hospitalized patients: a randomized, double-blind comparison of carbamazepine (Tegretol) and barbital (Diemal) Acta Psychiat Scand. 1984;69:398–408. doi: 10.1111/j.1600-0447.1984.tb02511.x. [DOI] [PubMed] [Google Scholar]
- 17.Malcolm R, Ballenger JC, Sturgis ET, Anton R. Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. Am J Psychiatry. 1989;146:617–21. doi: 10.1176/ajp.146.5.617. [DOI] [PubMed] [Google Scholar]
- 18.Stuppaeck C, Pycha R, Miller C, Whitworth A, Oberbauer H, Fleischhacker W. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27:153–8. [PubMed] [Google Scholar]
- 19.Kranzler HR, Bauer LO, Hersh D, Klingerhoffer V. Carbamazepine treatment of cocaine dependence: a placebo-controlled trial. Drug Alcohol Depend. 1995;38:203–11. doi: 10.1016/0376-8716(95)01100-d. [DOI] [PubMed] [Google Scholar]
- 20.Mueller TI, Stout RL, Rudden S, et al. A double-blind, placebo-controlled pilot study of carbamazepine for the treatment of alcohol dependence. Alcohol Clin Exp Res. 1997;21:86–92. [PubMed] [Google Scholar]
- 21.Brown ME, Anton RF, Malcolm R, Ballenger JC. Alcoholic detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biol Psychiatry. 1988;23:507–14. doi: 10.1016/0006-3223(88)90023-6. [DOI] [PubMed] [Google Scholar]
- 22.Lechtenburg R, Worner TM. Seizure risk with recurrent alcohol detoxification. Arch Neurol. 1990;47:535–8. doi: 10.1001/archneur.1990.00530050055012. [DOI] [PubMed] [Google Scholar]
- 23.Lectenburg R, Worner TM. Relative kindling effect of detoxification and non-detoxification admissions in alcoholics. Alcohol Alcohol. 1991;26:221–5. doi: 10.1093/oxfordjournals.alcalc.a045104. [DOI] [PubMed] [Google Scholar]
- 24.Lechtenburg R, Worner TM. Total ethanol consumption as a seizure risk factor in alcoholics. Acta Neurol Scand. 1992;85:90–4. doi: 10.1111/j.1600-0404.1992.tb04004.x. [DOI] [PubMed] [Google Scholar]
- 25.Booth B, Blow F. The kindling hypothesis: further evidence from a U.S. national survey of alcoholic men. Alcohol Alcohol. 1993;28:593–8. [PubMed] [Google Scholar]
- 26.Worner TM. Relative kindling effect of readmissions in alcoholics. Alcohol Alcohol. 1996;31:375–80. doi: 10.1093/oxfordjournals.alcalc.a008164. [DOI] [PubMed] [Google Scholar]
- 27.Shaw GK, Waller S, Latham CJ, Dunn G, Thomson AD. The detoxification experience of alcoholic inpatients and predictors of outcome. Alcohol Alcohol. 1998;33:291–303. doi: 10.1093/oxfordjournals.alcalc.a008393. [DOI] [PubMed] [Google Scholar]
- 28.Gross MM, Rosendlatt SM, Lewis E, Chartoff S, Malenowski B. Acute alcohol psychoses in related syndromes: psychosocial and clinical characteristics and their implications. Br J Addict. 1972;67:15–31. doi: 10.1111/j.1360-0443.1972.tb01163.x. [DOI] [PubMed] [Google Scholar]
- 29.Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndrome. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- 31.Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling.”. Alcohol Clin Exp Res. 1993;17:94–8. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 32.McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–9. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 33.Veatch LM, Gonzales LP. Repeated ethanol withdrawal produces site-dependent increases in EEG spiking. Alcohol Clin Exp Res. 1996;20:262–7. doi: 10.1111/j.1530-0277.1996.tb01638.x. [DOI] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SW, McHugh PR. Mini-Mental State: A practical method of grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan J, Swift R, Lewis D. Benzodiazepine requirements during alcohol withdrawal syndrome: clinical implications of using a standardized withdrawal scale. J Clin Psychopharmacol. 1991;11:291–5. [PubMed] [Google Scholar]
- 36.Skinner HA, Allen BA. Alcohol dependent syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- 37.Sobell MB, Sobell LC. Behavioral Treatment of Alcohol Problems. New York: Plenum Press; 1978. [Google Scholar]
- 38.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS Systems for Mixed Models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- 39.Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Monterey, Calif: Brooks/Cole; 1982. [Google Scholar]
- 40.Deutsch JA, Walton NY. Diazepam maintenance of alcohol preference during alcohol withdrawal. Science. 1977;198:307–9. doi: 10.1126/science.561997. [DOI] [PubMed] [Google Scholar]
- 41.Hedlund L, Walstrom G. The effect of diazepam on voluntary alcohol intake in a rat model of alcoholism. Alcohol Alcohol. 1998;33:207–19. doi: 10.1093/oxfordjournals.alcalc.a008384. [DOI] [PubMed] [Google Scholar]
- 42.O'Conner PG, Gottlieb LD, Kraus ML, Segal SR, Horwitz RI. Social and clinical features as predictors of outcome in outpatient alcohol withdrawal. J Gen Intern Med. 1991;6:312–6. doi: 10.1007/BF02597427. [DOI] [PubMed] [Google Scholar]
- 43.Kales A, Manfredi RL, Vgontzas AN, Bixler EO, Vela-Bueno A, Fee EC. Rebound insomnia after only brief and intermittent use of rapidly eliminated benzodiazepines. Clin Pharmacol Ther. 1991;49:468–76. doi: 10.1038/clpt.1991.55. [DOI] [PubMed] [Google Scholar]
- 44.Fontaine R, Chouinard G, Annable L. Rebound anxiety in anxious patients after abrupt withdrawal of benzodiazepine treatment. Am J Psychiatry. 1984;141:848–52. doi: 10.1176/ajp.141.7.848. [DOI] [PubMed] [Google Scholar]
- 45.MacKinnon GL, Parker WA. Benzodiazepine withdrawal syndrome: a literature review and evaluation. Am J Drug Alcohol Abuse. 1982;9:19–33. doi: 10.3109/00952998209002608. [DOI] [PubMed] [Google Scholar]
- 46.DeVane CL, Nemeroff CB. Psychotropic drug interactions: 1999. Primary Psychiatry. 1999;6:39–88. [Google Scholar]
- 47.D'Onotrio G, Rathlev NK, Ulrich AS, Fish SS, Freeland ES. Lorazepam for the prevention of recurrent seizures related to alcohol. N Engl J Med. 1999;340:915–9. doi: 10.1056/NEJM199903253401203. [DOI] [PubMed] [Google Scholar]
- 48.Myrick H, Malcolm R, Brady KT. Gabapentin treatment of alcohol withdrawal. Am J Psychiatry. 1998;155:1632. [PubMed] [Google Scholar]