Abstract
OBJECTIVE
To examine the association of socioeconomic barriers, familial barriers, and clinical variables with health-related quality of life (HRQL).
METHODS
A cross-sectional study was conducted of 186 African Americans with type 2 diabetes recruited from 2 primary care clinics in East Baltimore, Maryland. Physical functioning, social functioning, mental health, and general health were measured using the Medical Outcomes Study 36-item short form. Socioeconomic (money, housing, street crime) and familial (family problems, caretaker responsibilities) barriers were assessed by standardized interview. Insulin use, comorbid disease, and measured abnormalities in body mass index, hemoglobin A1c (HbA1c), blood pressure, lipids, and renal function were investigated.
RESULTS
Mean HRQL scores were: physical functioning, 61 ± 29; social functioning, 76 ± 26; mental health, 69 ± 21; and general health, 48 ± 21. Linear regression analyses revealed that each barrier to care was significantly associated with lower scores in 1 or more HRQL domain. As number of socioeconomic and familial barriers increased from 0 to 5, HRQL scores decreased by 18 for social functioning, 21 for general health, 23 for physical functioning, and 28 for mental health (all P for trend <.01). Clinical variables significantly associated with reduced HRQL were obesity, impaired renal function, insulin use, and comorbid disease. Blood pressure, lipids, and HbA1c were not significantly associated with HRQL.
CONCLUSIONS
An independent, graded relationship was found between socioeconomic and familial barriers to care and HRQL. This relationship was at least as strong as the association between HRQL and the clinical variables more likely to be perceived by participants as causing symptomatic distress or impacting lifestyle.
Keywords: health status, chronic illness, socioeconomic factors, urban health
Type 2 diabetes imposes a heavy public health burden on African Americans. This has been well documented with regard to diabetes-related morbidity and mortality.1 Despite growing attention to quality of life as a health outcome measure,2 much less is known about health-related quality of life (HRQL) in this population. HRQL in urban African Americans could be adversely affected by diabetes complications and by socioeconomic problems unique to or exaggerated within a poor, inner city environment. Previous studies of HRQL in persons with diabetes have included few African Americans and have not assessed urban socioeconomic problems.3 We, therefore, conducted a cross-sectional study to examine in a sample of urban African Americans with type 2 diabetes: 1) the relationship between HRQL and socioeconomic and familial barriers to care, including problems with money, housing, street crime, family, and caretaker responsibilities, and 2) the relationship between HRQL and clinical variables including insulin use, comorbid disease, and measured abnormalities in body mass index (BMI), hemoglobin A1c (HbA1c), blood pressure, lipids, and renal function. We hypothesized that socioeconomic problems would be associated with reduced physical functioning, social functioning, mental health, and general health in this inner city, African-American, type 2 diabetes sample.
RESEARCH DESIGN AND METHODS
Setting and Population
The study sample was comprised of 186 African Americans with type 2 diabetes who lived in East Baltimore, Maryland. The study was conducted as part of Project Sugar 1, a randomized controlled trial of the effectiveness of a multi-faceted behavioral intervention to improve metabolic control and health behaviors of urban African Americans with type 2 diabetes. To be eligible for the study, subjects had to meet the following criteria: age 35 to 75 years, African-American ancestry by self report, presence of type 2 diabetes as indicated by physician diagnosis, absence of comorbid conditions limiting probable lifespan to <4 years (e.g., cancer, AIDS), residence in 1 of 7 East Baltimore zip codes, attendance at either of 2 Johns Hopkins–affiliated primary care clinics within the previous year, and no indication of end-stage complications of diabetes (e.g., kidney dialysis or transplant, blindness, or lower extremity amputation). Following review of 3,800 medical charts, 822 individuals were identified as African Americans with type 2 diabetes. Telephone screenings revealed that 156 of these individuals did not meet eligibility criteria. An additional 241 refused participation, 78 did not show for an initial screening visit, and 161 persons were unable to be contacted. These 480 persons were considered nonresponders. One hundred eighty-six completed both of 2 required screening visits and were randomized into the study. Further details regarding sample selection are reported elsewhere.4 Comparisons of participants and nonparticipants revealed that the groups were similar with regard to age and sex, but HbA1c was lower in participants than nonparticipants.5 At baseline (1995–1997), detailed interview and clinical data were collected. The study was approved by the Joint Committee on Clinical Investigation of the Johns Hopkins University School of Medicine, and written informed consent was obtained for each subject.
Data Collection
Socioeconomic and Familial Barriers to Care
Participants were asked the following questions to assess presence of 5 types of socioeconomic and familial barriers in relation to diabetes self-care: “Does lack of money make it hard for you to take care of your diabetes?”“Do housing problems make it hard for you to take care of your diabetes?”“Does being concerned about street crime make it hard for you to take care of your diabetes?”“Do family problems make it hard for you to take care of your diabetes?”“Does having to take care of someone who depends on you make it hard for you to take care of your diabetes?” Number of socioeconomic and familial barriers to care was determined by adding the number of barriers reported, ranging from 0 (none) through 5 (all).
Clinical Variables
Blood samples after a 10- to 12-hour fast were analyzed for: HbA1c (using high-pressure liquid chromatography), plasma lipids, and serum creatinine (using Jaffé reaction). A first morning urine sample was analyzed using a urine dipstick. Blood pressure was measured 3 times at each of 2 visits by a trained technician using a random-zero sphygmomanometer. The mean of 6 readings was used to determine elevated blood pressure. Height and weight were measured during clinical examination, and values were converted to BMI for use in analyses.
These physiological data were used to create dichotomous definitions of the following conditions: elevated HbA1c was defined as >9.0%6; impaired renal function was defined as ≥1+ proteinuria or serum creatinine >1.5 mg/dL7; abnormal lipids was defined as HDL <40 mg/dL and/or LDL >130 mg/dL8; and elevated blood pressure was defined as mean systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg.9 Abnormal BMI was separated into 2 categories of obesity, BMI 30 to 35 kg/m2 and BMI >35 kg/m2.10
Diabetes treatment was assessed on structured interview. The Charlson Comorbidity Index11 was used to classify comorbid disease, based on a baseline review of the participants' medical records. This weighted index is based upon the number and severity of comorbid disease, with higher scores indicating greater comorbidity. Validation data report lowest cumulative mortality attributable to comorbid disease in persons with a score of 0 and highest in persons with a score ≥5.11
HRQL
The Medical Outcomes Study (MOS) Short Form-36 Health Survey (SF-36), a multidimensional measure of health status designed for self or interviewer administration,12 was used. The SF-36 has demonstrated reliability and validity12,13 and is widely used in health outcomes and policy research.2 Four SF-36 health domains were selected for the present study in order to represent most effectively the physical and mental components of both functional limitations and well-being.12,14 Physical functioning, designated as the most valid measure of the physical component of health status,14 assesses limitations in physical activities because of health problems. Mental health, the most valid measure of the mental and emotional component of health status,13 measures psychological well-being and distress. Social functioning measures limitations in social activities because of physical or emotional problems. General health measures personal evaluation of health, influenced by both physical and emotional limitations and well-being. SF-36 responses are recorded on 5-point scales. Scores for each health domain scale range from 0 to 100, with higher scores indicating better functioning or well-being.12
Statistical Analyses
Student's t tests and analysis of variance (ANOVA) were used to determine differences in HRQL on the basis of gender, age, and income. Associations of socioeconomic and familial barriers with HRQL and clinical abnormalities with HRQL were analyzed using linear regression models adjusted for age and sex. Beta-coefficients (β) were used in interpretation of the associations because in most cases the socioeconomic and familial barriers and the clinical abnormalities were dichotomized. β indicates, on average, the difference in the HRQL score for participants who did report the barrier as compared to the participants who did not report the barrier. Likewise, it represents the difference in HRQL score for those who had a clinical abnormality versus those who did not. Linear regression analyses adjusted for age and sex were also used to model number of socioeconomic problems on HRQL. X2 analyses were used to examine interrelationships among the socioeconomic and familial barriers and relationships between income/insurance and the socioeconomic and familial barriers. All analyses were conducted using Stata statistical software, (Release 5.0; Stata Corp., College Station, Tex).
RESULTS
Sample Characteristics
Sociodemographic Characteristics
Selected characteristics of the study sample are presented in Table 1. The sample was 76% female, with a mean age of 59 ± 9 years. Mean education was 10 ± 3 years. Fifty-three percent of the sample had an annual household income below $7,500. Participants represented a low socioeconomic group, with 90% of the total sample living in poverty. Poverty status was determined from 1996 Federal Register guidelines based upon income and size of family unit. Eighty-six percent of participants had either Medicaid or private insurance, with 24% having no insurance.
Table 1.
Selected Characteristics of 186 African Americans with Diabetes by Gender
| Characteristic | Female (N = 141) | Male (N = 45) |
|---|---|---|
| Sociodemographic characteristic | ||
| Mean age, y, ±SD | 59 ± 8 | 57 ± 10 |
| Education <12 y, n (%) | 100 (71) | 31 (69) |
| Monthly household income, n (%) | ||
| <$420 | 30 (21) | 7 (16) |
| $420–$624 | 49 (35) | 13 (29) |
| $625–$833 | 24 (17) | 10 (22) |
| $834–$1,249 | 26 (19) | 5 (11) |
| ≥$1,250 | 10 (7) | 9 (20) |
| Persons in poverty, n (%) | 129 (93) | 35 (80) |
| Insurance status, n (%) | ||
| No insurance | 34 (24) | 11 (24) |
| Medicaid | 59 (42) | 19 (42) |
| Private insurance | 48 (34) | 15 (33) |
| Socioeconomic and familial barriers to care, n (%) | ||
| Money problems | 57 (40) | 21 (47) |
| Housing problems | 17 (12) | 5 (11) |
| Street crime | 22 (15) | 5 (11) |
| Family problems | 23 (16) | 4 (9) |
| Caretaker responsibilities | 13 (9) | 9 (20) |
| Clinical variables | ||
| Measured clinical abnormalities, n (%) | ||
| BMI (kg/m2) | ||
| 30–35 | 46 (32) | 10 (23) |
| >35 | 52 (37) | 8 (18) |
| Elevated HbA1c* | 45 (32) | 19 (42) |
| Elevated blood pressure† | 36 (26) | 9 (20) |
| Impaired renal function‡ | 20 (14) | 5 (11) |
| Abnormal lipids§ | 100 (71) | 33 (73) |
| Uses insulin, n (%) | 74 (52) | 17 (38) |
| Charlson Comorbidity Index‖, mean ±SD | 1.7 ± 1.0 | 2.5 ± 1.4 |
Defined as >9.0%.
Defined as systolic ≥140 mmHg and/or diastolic ≥90 mmHg.
Defined as ≥1+ proteinuria and/or serum creatinine >1.5 mg/dL.
Defined as HDL <40 mg/dL and/or LDL >130 mg/dL.
N = 172 (131 women, 41 men).
Socioeconomic and Familial Barriers
Forty-two percent of the sample reported having money problems that interfered with diabetes self-care, 12% reported housing problems, 14% reported problems with street crime, 14% reported family problems, and 12% reported problems associated with caretaker role and responsibilities. With regard to number of barriers, 70 participants (38%) reported 1 or 2 barriers, and 21(11%) reported 3 or more barriers.
Clinical Variables
Of the measured clinical abnormalities, 69% of the women and 40% of the men had BMI consistent with obesity classifications of moderate (BMI 30 to 35) or severe (BMI >35). HbA1c was elevated in 34% of the total sample, blood pressure was elevated in 24% of the sample, and impaired renal function was found in 13%. Abnormal lipids was common (72%). The mean duration of diabetes was 10 years. Forty-four percent of the sample reported using insulin, and 49% reported using oral hypoglycemic agents. Seventy-two percent reported use of blood pressure medication and 23% reported cholesterol medication. With regard to number and severity of comorbid disease, the majority of the sample (75%) had Charlson Comorbidity Index scores of 1 to 2, 21% had scores of 3 to 4, and 4% had the highest severity, ≥5.
HRQL
Mean SF-36 scale scores for the sample were: physical functioning, 61 ± 29; social functioning, 76 ± 26; mental health, 69 ± 21; and general health, 48 ± 21. Analyses revealed no significant differences in SF-36 scale scores based on gender, age, income, or insurance status.
Socioeconomic and Familial Barriers to Care and HRQL
To determine association of socioeconomic and familial problems with HRQL independent of age and sex, we conducted a series of linear regression analyses (Table 2). Each of the barriers was significantly associated with reduced HRQL on 1 or more of the SF-36 dimensions assessed (physical functioning, social functioning, mental health, general health). The β coefficients presented in Table 2 represent, on average, the difference in HRQL score between persons who did and did not report the barrier to diabetes care. Caretaker responsibilities was significantly associated with reduced HRQL on each dimension (all P < .05). Compared to persons who did not report caretaker responsibilities as a barrier, persons who did report the caretaker barrier had HRQL scores that were 23 points lower in physical functioning (P < .0001), 14 points lower in social functioning (P < .05), and about 17 points lower in both mental health (P <. 001) and general health (P < .001). In addition to caretaker responsibilities, each of the remaining socioeconomic and familial barriers was significantly associated with reduced mental health scores; compared with persons not reporting barriers, persons who did report barriers had mental health scores that were lower by 9 points for money problems (P < .01), 14 points for housing problems (P < .01), 15 points for problems with street crime (P < .001), and 14 points for family problems (P < .01). Both housing and street crime were significantly associated with lowered social functioning and general health as well (all P < .05).
Table 2.
Association of Socioeconomic and Familial Barriers to Care with HRQL in 186 African Americans
| Type of Barrier | Physical Functioning | Social Functioning | Mental Health | General Health |
|---|---|---|---|---|
| Money | −7.0 | −3.3 | −9.6* | −2.8 |
| Housing | −11.0 | −18.6* | −14.1* | −11.7† |
| Street crime | −10.6 | −13.7† | −15.5‡ | −15.6‡ |
| Family | −7.4 | −9.3 | −13.9* | −7.0 |
| Caretaker responsibilities | −23.5‡ | −14.4† | −16.6‡ | −16.8‡ |
P < .01.
P < .05.
P < .001.
Data presented are age and sex-adjusted β coefficients from linear regression models. Each socioeconomic and familial barrier was analyzed in a distinct model.
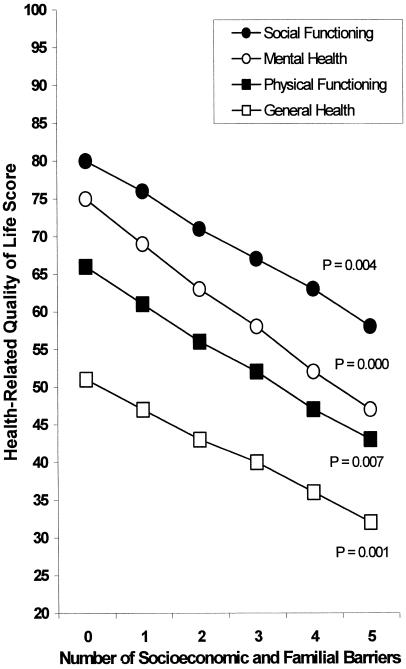
The relationship between number of socioeconomic problems and HRQL was modeled using linear regression analyses adjusted for age and sex. For each HRQL domain, there was a graded relationship between greater number of problems and reduced HRQL, as shown in Figure 1. As number of problems increased from none to 5, mean SF-36 scale scores decreased by 18 for social functioning, 21 for general health, 23 for physical functioning, and 28 for mental health (all P for trend <.01).
FIGURE 1.
Relationship of number of socioeconomic and familial barriers to health-related quality of life. Linear regression models adjusted for age and sex. P for the linear trend is presented for each scale. n = 94 (51%) for no barriers, 46 (25%) for one barrier, 24 (13%) for two barriers, 7 (4%) for three barriers, 9 (5%) for four barriers, and 5 (3%) for five barriers.
Clinical Variables and HRQL
Of the measured clinical abnormalities, only BMI and impaired renal functioning were significantly associated with reduced HRQL (Table 3). Physical functioning in persons with BMI of 30 to 35 kg/m2 was 11 points lower than in persons with BMI <30 (P < .05). And, in persons with BMI >35 kg/m2, physical functioning was 17 points lower (P < .01), social functioning was 11 points lower (P < .05), and general health was almost 10 points lower (P < .05) than persons with BMI <30. Persons with impaired renal function scored significantly lower than those without impaired renal functioning in physical functioning and general health (both P < .05). Elevated HbA1c, elevated blood pressure, and abnormal lipids were not significantly associated with reduced HRQL. Comorbid disease index was significantly associated with reduced HRQL in each domain except mental health (all P < .01). Insulin use was significantly associated with reduced physical functioning (P < .05).
Table 3.
Association of clinical variables with HRQL in 186 African Americans
| Clinical Variable | Physical Functioning | Social Functioning | Mental Health | General Health |
|---|---|---|---|---|
| Measured clinical abnormality | ||||
| BMI (kg/m2) | ||||
| 30–35 | −11.6* | −2.8 | −5.3 | −7.0 |
| >35 | −17.4† | −11.3* | −3.7 | −9.7* |
| Elevated HbA1c§ | 6.2 | 0.8 | −3.4 | 0.2 |
| Elevated blood pressure‖ | 7.8 | 5.2 | 1.3 | −1.9 |
| Impaired renal function¶ | −12.5* | −6.4 | −0.3 | −8.6* |
| Abnormal lipids# | 0.5 | −4.1 | −0.9 | −6.2 |
| Uses insulin | −9.2* | −2.2 | −1.7 | −1.1 |
| Charlson Comorbidity Index** | −7.7‡ | −9.4‡ | −2.9 | −3.9† |
P < .05.
P < .01.
P < .001.
Defined as >9.0%.
Defined as systolic ≥140 mmHg and/or diastolic ≥90 mmHg.
Defined as ≥1+ proteinuria and/or serum creatinine >1.5 mg/dL.
Defined as HDL <40 mg/dL or LDL >130 mg/dL.
N = 171 (131 women, 41 men).
Data presented are age- and sex-adjusted β coefficients from linear regression models. Each clinical variable was analyzed in a distinct model.
DISCUSSION
The following conclusions are supported by our data. First, socioeconomic and familial barriers to care were significantly associated with reduced HRQL, and there was a strong, graded relationship of number of barriers to reduced HRQL. Second, the clinical variables of obesity, impaired renal function, insulin use, and comorbid disease were significantly associated with reduced HRQL. Third, the barriers to care and the clinical variables had somewhat different patterns of association with HRQL. In general, socioeconomic and familial barriers were most consistently associated with mental health, general health, and social functioning, while the clinical variables that were related to HRQL were more consistently associated with physical functioning and general health.
The MOS revealed persons with type 2 diabetes to have lower HRQL than the general population but generally better HRQL than people with chronic conditions such as cardiac problems, clinical depression, gastrointestinal disorders, and chronic lung problems.12 Comparison of HRQL scores for the MOS normative type 2 diabetes sample12 and our Project Sugar sample, respectively, revealed a trend toward lower scores for our sample (physical functioning 68 vs 61; social functioning 82 vs 76; mental health 77 vs 69; general health 56 vs 48). It is likely that the lower HRQL scores in our sample are at least in part reflective of the differences in socioeconomic characteristics of the samples. Although the MOS and Project Sugar samples were similar with regard to gender, the MOS groups represent a very different sample from that of the present study with regard to other sociodemographic characteristics (76% white, 82% with ≥12 years of education, and 17% poverty in MOS). The reported 5 most prevalent comorbidities in the MOS type 2 diabetes group (hypertension, back pain/sciatica, musculoskeletal complaints, recent angina, and dermatitis)12 suggest that, similar to our Project Sugar sample, the MOS diabetes group did not represent a group with multiple advanced or end-stage complications of diabetes.
We found that, as number of barriers increased from none to 5, the decrease in mean individual SF-36 scale scores ranged from 18 to 28 points. If the socioeconomic and familial barriers examined in this study are viewed in the context of life stressors that impact both psychological and physical well-being,15 then the cumulative nature of the relationship of these barriers to reduced HRQL, on each dimension assessed, would not be unexpected. There are no specific guidelines for interpreting clinical significance of point differences on the individual SF-36 scales. However, McHorney et al.,13 in a construct validation study of the SF-36, reported the mean difference in scores between patients with minor medical conditions and those with serious medical conditions as 23 points for physical functioning and 18 points for general health. Also, the mean difference in scores between psychiatric patients and patients with minor medical conditions was 27 points for social functioning and 28 points for mental health. On the basis of these findings, we may conclude that the reduction we observed on individual HRQL scales as number of problems increased is likely clinically relevant.
With regard to the cumulative nature of the observed relationship between barriers and HRQL, the issue of possible homogeneity of the socioeconomic and familial variables is important to discuss. In selecting the questions regarding barriers to care, we intended to characterize different aspects of socioeconomic and familial barriers that might relate differently to dimensions of HRQL. When we examined interrelationships among the 5 barriers, we found that the variables were moderately associated. Based on this, it is plausible to think that income may be the underlying factor of all the barriers. Therefore, we examined the relationship between income and the barriers, but we found no statistically significant associations (all P > .05). The limited range of income probably attenuated associations. Evaluating insurance status as another socioeconomic marker, we found that insurance status was associated with money problems (P = .001), housing problems (P = .02), and street crime (P = .03), but insurance status was not associated with family problems or caretaker responsibilities (both P = .24). This suggests that the barriers, although related, did assess somewhat different aspects of social issues. Moreover, the finding that the 5 barriers each had different patterns of relationships with the HRQL dimensions supports some distinction among what the items assessed.
To examine whether access to care explained relationships between the barriers/socioeconomic status and HRQL, we compared the HRQL scores for participants who had insurance versus those who did not have insurance. We found no differences between participants who did and did not have insurance in any dimension of HRQL (P = .35 to .97). Because our inclusion criteria required participants to have attended an affiliated primary care clinic within the year prior to recruitment, our sample represents persons who had at least a minimal utilization of non-emergency health care services over a previous year.
Our findings do not suggest that the relationship between socioeconomic and familial barriers and HRQL is unique to diabetes. It has been established that lower socioeconomic status is associated with lower health status16,17 and that African Americans, who disproportionately reside in urban areas, have increased vulnerabilities to poor health status as a result of problems in the urban environment.18 However, because our inquiry was specific to the influence of these barriers on taking care of diabetes, our data represent the relationship of these socioeconomic and familial barriers to diabetes care. The question of why problems with money, housing, street crime and family might be associated with difficulty caring for diabetes in our sample has been discussed in part elsewhere.19 Data were collected from Project Sugar 1 community health worker and nurse case manager visits in this community-based intervention. These data revealed that 77% of intervention visits addressed a non–diabetes-specific issue, with 58% of all visits addressing problems including social issues and health insurance issues.19 It may be that when individuals are concerned about socioeconomic and social issues, focus on diabetes care may become less of a priority, as it was even in the context of diabetes-specific visits.
Our finding that a larger percentage of the men than the women in our sample reported caretaker responsibilities as a barrier to diabetes care was unexpected. To explore this, we looked at percentages of men and women who reported having a dependent child, elder, or ill person at home who depends on them for care 5 days or more a week. Between 12% and 39% of women reported these caretaker responsibilities, which can be perceived as physical caretaking. We found that 9% to 13% of men reported these physical caretaking responsibilities, but 20% of men reported caretaker responsibilities as a barrier. Therefore, we suggest that some of these men may have responded to the question on the basis of financial caretaking responsibilities.
Of the clinical variables, our findings are consistent with previous research with regard to the associations of insulin use20,21 and obesity22 with lower HRQL in persons with type 2 diabetes. Similarly, previous studies have consistently reported a relationship between increased disease severity and reduced HRQL.21,23–26 In our sample, comorbid disease and impaired renal function were significantly associated with lower HRQL. The reason we did not find significant associations between HRQL and HbA1c, blood pressure, and lipids is likely that these clinical abnormalities, which were assessed by clinical screening or laboratory data, may not have resulted in marked symptom awareness or distress for participants. When examining perceived health status and quality of life, disease-related complications or clinical abnormalities that have overt or severe symptomatology and lifestyle sequelae are more likely to be associated with self-reported HRQL.27 Our exclusion of participants with advanced diabetes complications and comorbidities in this sample, therefore, likely contributes to an underestimation of the degree to which physical functioning, social functioning, mental health, and general health may be lower in persons with more severe complications.
Several limitations of the study deserve comment. First, our sample was drawn from primary care clinics in an inner city and represented a largely impoverished minority population. Therefore, generalizability of these findings to other populations should be done with caution. It is important to note, however, that the sociodemographic characteristics of our sample (e.g., race, gender, income, education, poverty status) are comparable to those reported in other studies of low-income primary care populations.15,28 Although participants in our study did not differ from nonparticipants with regard to age and gender, participants did have lower HbA1c than nonparticipants. Bias inherent to the self-selection of those eligible participants who chose to participate resulted in our sample representing persons in relatively better glycemic control.5 Consequently, our findings may underestimate an association that may exist between poorer glycemic control and HRQL in the wider population from which our sample was drawn.
Second, the relatively small number of subjects used in the analyses of socioeconomic and familial barriers limits the stability of those estimates. This was particularly true for the analyses regarding number of barriers and HRQL. Moreover, the small sample size contributes to low statistical power and may have contributed to marginal and negative findings with regard to the relationship between clinical abnormalities and HRQL. The limited range in income, a defining characteristic of a low-income population, probably attenuated statistical significance of relationships between income and socioeconomic and HRQL variables.
Third, our finding that less-symptomatic diabetes-related clinical abnormalities were not significantly associated with HRQL may be related to the use of a general, albeit well-established, HRQL measure. Studies that have reported glycemic control as associated with HRQL, for example, have found this relationship most consistently with diabetes-specific measures of quality of life or symptom distress rather than with general measures.3 Both diabetes-specific and general measures of HRQL contribute to an understanding of the perceived health status and well-being of persons with diabetes; however, the distinction in utility of these types of instruments appears to be in whether the primary focus of investigation is functioning and general well-being or detection of perhaps less-overt, diabetes-specific lifestyle issues.21,25 Incorporation of a disease-specific quality-of-life measure in future studies may help to determine if the lack of association between less-symptomatic clinical abnormalities and HRQL is an issue of insensitivity of the general measure versus perception of lesser distress associated with diabetes in this urban minority population.
Nonetheless, this study is unique in its focus on identifying types of socioeconomic problems influencing diabetes self-care in urban African Americans with type 2 diabetes, and it contributes valuable information regarding the relationship of these socioeconomic and familial barriers to HRQL. The main implication is that, in inner city, low-income, minority populations, diabetes intervention programs that target only clinical management of diabetes may result in only partial improvements in HRQL. Interventions that are tailored to address coping skills and resources for these salient socioeconomic and familial barriers to care may result in enhanced diabetes management and improvements in HRQL.
The findings presented here represent baseline assessment of the relationship of these barriers to HRQL in Project Sugar 1, a randomized controlled trial of primary care and community-based intervention. In other analyses, we found that community-based diabetes interventions that utilize a community health worker/nurse case manager can address social problems encountered by individuals, and that addressing these problems may in fact be unavoidable.19 We are currently implementing an expanded, community-based diabetes intervention that is designed to address the socioeconomic problems, environmental problems, and familial barriers, as well as to provide education and case management for improved clinical management of diabetes (Project Sugar 2). Through this unique clinical trial to improve coordination of care and health outcomes in this high-risk, urban African-American population, Project Sugar 2 will allow us to analyze both the successfulness and the cost-effectiveness of such an intervention. Future studies should also examine the effectiveness of multifaceted interventions designed to address, at least in part, the adverse role of social problems in health status and quality of life in urban samples. In addition, pathways underlying the graded relationship of socioeconomic and familial barriers to HRQL (e.g., prioritizing behaviors, adherence behaviors, direct effect of stress on health) warrant further investigation.
Acknowledgments
The authors would like to thank the Project Sugar staff and the Johns Hopkins Outpatient General Clinical Research Center staff for their support with data collection. We would also like to thank the Project Sugar participants, whose participation and cooperation made this research possible.
This work was supported by grants from the National Institutes of Health (R01-DK48117-04 and R01-DK48117-03S1) and the Johns Hopkins University Outpatient Department General Clinical Research Center (R0052).
REFERENCES
- 1.Tull ES, Roseman JM. Diabetes in African Americans. In: National Diabetes Data Group, ed. Diabetes in America. Bethesda, Md: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 1995. pp. 613–30. [Google Scholar]
- 2.McDowell I, Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires. 2nd ed. New York: Oxford University Press; 1996. [Google Scholar]
- 3.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15:205–18. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Gary TL, Crum RM, Cooper-Patrick L, Ford D, Brancati FL. Depressive symptoms and metabolic control in African-Americans with type 2 diabetes. Diabetes Care. 2000;23:23–9. doi: 10.2337/diacare.23.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Gary TL, Symonette V, Brancati FL. Assembly of a representative study sample for a “real world” effectiveness trial in African Americans with type 2 diabetes mellitus. Diabetes. 2001;50(suppl 1):A478. [Google Scholar]
- 6.Goldstein DE, Little RR, Lorenz R, Malone JI, Nathan D, Peterson CM. Tests of glycemia in diabetes. Diabetes Care. 1995;18:896–909. doi: 10.2337/diacare.18.6.896. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA. Diabetic nephropathy: etiologic and therapeutic considerations. Diabetes Rev. 1995;3:510–64. [Google Scholar]
- 8.American Diabetes Association. Medical Management of Type 2 Diabetes. Alexandria, Va: American Diabetes Association; 1998. Detection and Treatment of Complications; pp. 100–34. [Google Scholar]
- 9.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2000;23(suppl 1):32–42. [PubMed] [Google Scholar]
- 10.Kuczmarski R, Carrol M, Flegal K, Troiano R. Varying body mass index cutoff points to describe overweight prevalence among U.S. adults: NHANES III (1988 to 1994) Obes Res. 1997;5:542–8. doi: 10.1002/j.1550-8528.1997.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 13.McHorney CA, Ware JE, Raczek A. The MOS 36-item short form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–81. [PubMed] [Google Scholar]
- 15.Ames SC, Jones GN, Howe JT, Brantley PJ. A prospective study of the impact of stress on quality of life: an investigation of low-income individuals with hypertension. Ann Behav Med. 2001;23:112–9. doi: 10.1207/S15324796ABM2302_5. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan GA. Socioeconomic considerations in the health of urban areas. J Urban Health. 1998;75:228–35. doi: 10.1007/BF02345090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan GA, Haan MN, Syme SL, Minkler M, Winkleby M. Socioeconomic status and health. In: Amler RW, Dull HB, editors. Closing the Gap: The Burden of Unnecessary Illness. New York: Oxford University Press; 1987. pp. 125–9. [Google Scholar]
- 18.Williams DR. African-American health:the role of the social environment. J Urban Health. 1998;75:300–21. doi: 10.1007/BF02345099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batts ML, Gary TL, Huss K, Hill MN, Bone L, Brancati FL. Patient priorities and needs for diabetes care among urban African Americans adults. Diabetes Educ. 2001;27:405–12. doi: 10.1177/014572170102700310. [DOI] [PubMed] [Google Scholar]
- 20.Glasgow RE, Ruggerio L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care. 1997;20:562–7. doi: 10.2337/diacare.20.4.562. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson AM, De Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care. 1994;17:267–74. doi: 10.2337/diacare.17.4.267. [DOI] [PubMed] [Google Scholar]
- 22.Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med. 2000;15:789–96. doi: 10.1046/j.1525-1497.2000.90906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberger M, Kirkman MS, Samsa GP, et al. The relationship between glycemic control and health-related quality of life in patients with non-insulin-dependent diabetes. Med Care. 1994;32:1173–81. doi: 10.1097/00005650-199412000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Ahroni JH, Boyko EJ, Davignon DR, Pecoraro RE. The health and functional status of veterans with diabetes. Diabetes Care. 1994;17:318–21. doi: 10.2337/diacare.17.4.318. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RM, Fitzgerald JT, Wisdom K, Davis WK, Hiss RG. A comparison of global versus disease-specific quality-of-life measures in patients with NIDDM. Diabetes Care. 1997;20:299–305. doi: 10.2337/diacare.20.3.299. [DOI] [PubMed] [Google Scholar]
- 26.Klein BE, Klein R, Moss SE. Self-rated health and diabetes of long duration: the Wisconsin epidemiologic study of diabetic retinopathy. Diabetes Care. 1998;21:236–40. doi: 10.2337/diacare.21.2.236. [DOI] [PubMed] [Google Scholar]
- 27.Testa MA, Simonson DC. Assessment of quality-of-life outcomes. N Engl J Med. 1996;334:835–40. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- 28.Von Korff M, Shapiro S, Burke JD. Anxiety and depression in a primary care clinic. Arch Gen Psychiatry. 1987;44:152–6. doi: 10.1001/archpsyc.1987.01800140058008. [DOI] [PubMed] [Google Scholar]