Abstract
CONTEXT
Prescribing errors involving medication dose formulations have been reported to occur frequently in hospitals. No systematic evaluations of the characteristics of errors related to medication dosage formulation have been performed.
OBJECTIVE
To quantify the characteristics, frequency, and potential adverse patient effects of prescribing errors involving medication dosage forms .
DESIGN
Evaluation of all detected medication prescribing errors involving or related to medication dosage forms in a 631-bed tertiary care teaching hospital.
MAIN OUTCOME MEASURES
Type, frequency, and potential for adverse effects of prescribing errors involving or related to medication dosage forms.
RESULTS
A total of 1,115 clinically significant prescribing errors involving medication dosage forms were detected during the 60-month study period. The annual number of detected errors increased throughout the study period. Detailed analysis of the 402 errors detected during the last 16 months of the study demonstrated the most common errors to be: failure to specify controlled release formulation (total of 280 cases; 69.7%) both when prescribing using the brand name (148 cases; 36.8%) and when prescribing using the generic name (132 cases; 32.8%); and prescribing controlled delivery formulations to be administered per tube (48 cases; 11.9%). The potential for adverse patient outcome was rated as potentially “fatal or severe” in 3 cases (0.7%), and “serious” in 49 cases (12.2%). Errors most commonly involved cardiovascular agents (208 cases; 51.7%).
CONCLUSIONS
Hospitalized patients are at risk for adverse outcomes due to prescribing errors related to inappropriate use of medication dosage forms. This information should be considered in the development of strategies to prevent adverse patient outcomes resulting from such errors.
Keywords: medication errors, error prevention strategies, medication dosage forms, adverse drug events
Medication prescribing deficiencies are the most common cause of actual and potential adverse drug events.1–3 Factors related to prescribing errors include: inadequate drug therapy knowledge; inadequate consideration of patient characteristics; dose calculations; nomenclature; and dosage formulation.4 Detailed understanding of these contributing factors is useful in designing and implementing improvements in the medication use system.4–6 Medication dosage formulation is an important method of improving the utility of pharmacologic agents.7,8 Common goals of medication dosage form design include: improving drug bioavailability; allowing administration via alternative routes; providing “delayed” or “sustained” drug delivery; improving patient convenience; facilitating use in different indications; facilitating use in special populations (such as pediatrics); and facilitating final dose preparation processes. As a result, many medications are available in a number of different dosage forms and dose sizes. While substantial patient benefit and convenience are achieved through the proper use of available medication dosage formulations, inappropriate use of dosage forms poses risk to the patient.9–45 We have previously reported that prescribing errors involving medication dosage forms accounted for more than 10% of all errors detected in a long-standing medication errors detection and prevention program.4–7,10,46,47 The risk of such prescribing errors is compounded by the substantial deficiency in the understanding of medication dosage formulation issues by many health care professionals.11–14 The ever-growing number and complexity of available medication dose formulations are likely to result in growing risk to patients.9
Considering the available evidence,4,6,9–47 it appears that the risk to patients from errors involving medication dosage forms is under-appreciated, under-reported, and poorly understood. Increased awareness and improved understanding of the nature of such errors will be useful in the design and implementation of error reduction initiatives. The purpose of this study was to characterize prescribing errors involving or related to medication dosage forms.
METHODS
Identification of Medication Prescribing Errors
The study was conducted in a 631-bed tertiary care teaching hospital located in northeastern New York State. Medication prescribing error data used for study analysis were collected over the period of January 1, 1996 to December 31, 2000, as previously described.4–6,46,47 All medication orders either written by or cosigned by a credentialed prescriber during the study period were included in the analysis. Medication orders were handwritten or in the form of preprinted order sets. Copies of the original orders were sent to the pharmacy via facsimile or via pneumatic tube. All medication orders were reviewed and entered into the pharmacy computer system by staff pharmacists prior to dispensing. Staff pharmacists routinely utilized all available information resources to evaluate medication orders for appropriateness. Following the identification of medication orders potentially in error, the pharmacist contacted the prescriber or a cross-covering provider to obtain additional information and to discuss the orders in question. Potential prescribing problems were defined as medication orders that involved: the wrong patient, drug, dose, dosing frequency, route of administration, or dosage form; inappropriate indication for use; inappropriate combinations of drugs; documented allergies to ordered medications; contraindicated therapy; missing critical information; and other miscellaneous problems. The medication order(s) in question were either confirmed as correct as written, or were clarified, changed, or discontinued following the discussion between the pharmacist and the physician. All identified problem orders that were jointly determined by the physician and the pharmacist to require a “correction” and that were subsequently changed were considered to be “confirmed problem orders.” All confirmed medication prescribing problems were further reviewed by a clinical pharmacist within 24 hours and by the author within 72 hours. This secondary review was to ensure that the proper actions were taken and to assure provision of appropriate therapy. Problem orders that were determined by the secondary review to be in error were then defined as “confirmed medication prescribing errors.”
The significance of each error was determined based on the general potential of the error to be carried out, and if carried out as ordered, to result in adverse consequences, either an increased risk of adverse effects or an inadequate therapeutic response. Orders that were unlikely to be carried out because of product characteristics, physical and mechanical factors, or the drug distribution and preparation processes of the hospital, etc., were not considered significant. Assessment of the potential adverse outcome of each error was based on available patient and pharmacologic information regarding the risk for adverse events. The potential significance of errant orders was evaluated using a previously described rating scale, and rated as either “Potentially Fatal or Severe,” Potentially Serious,” or “Potentially Significant.”4–6,46,47 (See Appendix A). Consistency and reproducibility of assigning an error severity classification to specific errors has been previously validated.4,9,46–48 Examples of error ratings are as follows. Potentially fatal/severe: ordering amphotericin B at the dose for amphotericin B lipid complex; potentially serious: ordering “Humalog 70 units q am” instead of Humulin N; and potentially significant: ordering “verapamil SR 240mg per tube q 24h.”
Errors related to dosage forms were defined as those in which there was an order for the inappropriate use of a specific dosage form, an order for the wrong dosage form (errors of commission), or the failure to specify the correct dosage form when more than 1 dose form is commonly available (error of omission). A classification schema for dosage form errors was developed based on evaluation of errors prior to September 1, 1999 (Table 1). All prescribing errors involving dosage formulation issues classified as at least “clinically significant,” i.e., either “potentially fatal/severe,”“potentially serious,” or “potentially significant” were included in this study.
Table 1.
Types and Frequency of Dosage Form Prescribing Errors
| Error Description | Example | n | % | n (%) Error Types Rated Fatal/Severe or Serious (Total Fatal/Serious/Severe Errors = 52) |
|---|---|---|---|---|
| Failure to specify controlled delivery dosage form; prescribed by brand name | Cardizem 240 mg PO daily | 148 | 36.8 | 27 (51.9) |
| Failure to specify correct controlled delivery form; prescribed by generic name | Nifedipine 90 mg PO daily | 132 | 32.8 | 5 (9.6) |
| Controlled delivery formulation ordered to be given per tube | Imdur 30 mg per tube once daily | 48 | 11.9 | 0 |
| Controlled delivery formulation prescribed on an “as needed” basis | OxyContin 20 mg PO every 8 h as needed for pain | 30 | 7.5 | 0 |
| Failure to specify unique formulation with significant bioavailability differences | Cyclosporine 100 mg PO daily when cyclosporine modified microemulsion intended | 12 | 3 | 12 (23.1) |
| Inappropriate dose frequency for controlled-delivery dosage form | Fentanyl transdermal patch every 7 days | 10 | 2.5 | 0 (0) |
| Oral dose amount ordered to be given IV | Colchicine 0.5 mg IV every 6 h | 4 | 1 | 2 (3.8) |
| Failure to specify unique dosage form with significant pharmacodynamic differences | Amphotericin B 250 mg IV daily instead of amphotericin B lipid complex | 3 | 0.7 | 3 (5.8) |
| Order to crush or otherwise disrupt a controlled-release formulation | Imdur 60 mg daily; crush and sprinkle in applesauce | 2 | 0.5 | 0 |
| Order for IM administration of formulation designed to be given SC | NPH insulin 20 units IM | 2 | 0.5 | 0 |
| Order for IV administration of formulation designed to be given IM | Betamethasone 12 mg IV every 12 h | 2 | 0.5 | 2 (3.8) |
| Order for oral administration at dose used for IM formulation | Fluphenazine 50 mg PO daily | 2 | 0.5 | 0 |
| Order to give topical paste at transdermal patch frequency | Nitrogycerine paste 0.2% daily | 2 | 0.5 | 0 |
| Order to give IV dose PO | Vancomycin 1 g PO every 12 h | 1 | 0.2 | 1 (1.9) |
| IM formulation to be given SC | Testosterone enanthate SC | 1 | 0.2 | 0 |
| Order to give controlled delivery formulation sublingually | Nifedipine 30 mg SL daily | 1 | 0.2 | 0 |
| Order to give rectal dosage form orally | Mesalamine rectal suspension PO every 6 h | 1 | 0.2 | 0 |
| Wrong indication for dosage form | Divalproex for acute oral loading dose of valproic acid instead of valproiuc acid liquid | 1 | 0.2 | 0 |
| Total | 402 | 52 |
PO, by mouth; IV, intravenously; IM, intramuscularly; SC, subcutaneously; SL, sublingually.
All “clinically significant” medication prescribing errors involving medication dosage forms detected between January 1, 1996 and December 31, 2000 were used to determine trends in annual number of detected dosage form–related prescribing errors. Errors detected between September 1, 1999 and December 31, 2000 were evaluated in detail to provide a current assessment of medications involved, type of error, and the nature and severity of potential adverse effects had the order been carried out.
RESULTS
Frequency of Errors
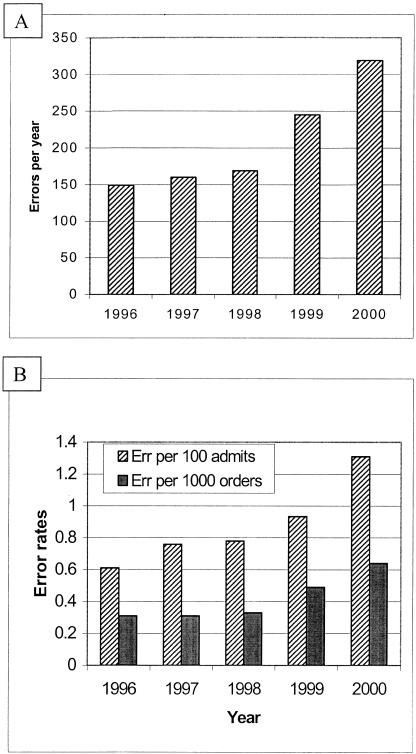
A total of 1,115 confirmed “clinically significant” medication prescribing errors involving or related to medication dosage formulation were detected during the years 1996 to 2000. The total number of detected errors, and number of errors per 100 admissions and number per 1,000 new medication orders detected increased annually over this period (Fig. 1). Four hundred two confirmed “clinically significant” medication prescribing errors involving medication dosage forms were detected and averted from September 1, 1999 to December 31, 2000. These 402 errors were further evaluated for current determination of error characteristics, medications involved and potential for adverse outcomes. During these 16 months, errors were detected at a rate of 1.23 per 100 admissions, 1.84 per 1,000 patient days and 0.61 per 1,000 new medication orders.
FIGURE 1.
Number of errors per year (A) and frequency of prescribing errors per 100 admissions and per 1,000 new orders (B) from 1996 through to 2000.
Error Types and Medications Involved
Of the 402 errors detected between September 1, 1999 and December 31, 2000, the most common types of errors were: failure to specify controlled release formulation (total 280 cases; 69.7%), which included prescribing using the brand name (148 cases; 36.8%) and using the generic name (132 cases; 32.8%); prescribing controlled delivery formulations to be administered per tube (48 cases; 11.9%); and prescribing controlled delivery dosage forms to be administered “as needed” when either a continuous “around the clock” effect was indicated or the more rapid onset of effect from a noncontrolled delivery formulation was appropriate (30 cases; 7.5%). Table 1 lists the frequency of each type of error detected.
Errors most commonly involved cardiovascular agents (208 cases; 51.7%), antidiabetic agents (39 cases; 9.7%), narcotic analgesics (37 cases; 9.2%), psychiatric medications (34 cases; 8.5%), and xanthines (29 cases; 7.2%). Table 2 lists the number of errors detected by specific medication involved and by medication class.
Table 2.
Medications Involved in Dosage Form Prescribing Errors
| Drug Class/Drug | n | % | n (%) Error Types Rated as Fatal/Severe or Serious (N = 52) |
|---|---|---|---|
| Cardiovascular agents | 208 | 51.7 | 2 (3.8) |
| Clonidine | 2 | 0.5 | 0 |
| Diltiazem | 58 | 14.4 | 0 |
| Disopyramide | 1 | 0.2 | 1 (1.9) |
| Isosorbide dinitrate | 8 | 2.0 | 0 |
| Isosorbide mononitrate | 48 | 11.9 | 0 |
| Metoprolol | 6 | 1.5 | 0 |
| Nicardipine | 1 | 0.2 | 0 |
| Nifedipine | 45 | 11.2 | 0 |
| Nimodipine | 1 | 0.2 | 0 |
| Nitroglycerine | 3 | 0.7 | 0 |
| Procainamide | 2 | 0.5 | 1 (1.9) |
| Propranolol | 5 | 1.2 | 0 |
| Verapamil | 28 | 7.0 | 0 |
| Anti-diabetic agents | 39 | 9.7 | 30 (57.7) |
| Glipizide | 2 | 0.5 | 0 |
| Insulin | 37 | 9.2 | 30 (57.7) |
| Narcotic analgesic agents | 37 | 9.2 | 0 |
| Fentanyl | 2 | 0.5 | 0 |
| Morphine | 18 | 4.5 | 0 |
| Oxycodone | 17 | 4.2 | 0 |
| Psychiatric agents | 34 | 8.5 | 2 (3.8) |
| Bupropion | 14 | 3.5 | 0 |
| Fluphenazine | 3 | 0.7 | 1 (1.9) |
| Haloperidol | 1 | 0.2 | 1 (1.9) |
| Lithium | 1 | 0.2 | 0 |
| Venlafaxine | 15 | 3.7 | 0 |
| Xanthines (theophylline) | 29 | 7.2 | 0 |
| Anti-epileptic agents | 13 | 3.2 | 0 |
| Carbamazepine | 8 | 2.0 | 0 |
| Valproate/valproic acid | 5 | 1.2 | 0 |
| Antimicrobials | 10 | 2.5 | 6 (11.5) |
| Amphotericin B | 3 | 0.7 | 3 (5.8) |
| Penicillin | 2 | 0.5 | 0 |
| Saquinavir | 3 | 0.7 | 3 (5.8) |
| Vancomycin | 2 | 0.5 | 0 |
| Immunosuppressants (cyclosporine) | 8 | 2.0 | 8 (15.4) |
| Anti-parkinsonian agents (levodopa/carbidopa) | 7 | 1.7 | 0 |
| Gastrointestinal agents | 4 | 1.0 | 0 |
| Bisacodyl | 1 | 0.2 | 0 |
| Hyoscyamine | 1 | 0.2 | 0 |
| Mesalamine | 2 | 0.5 | 0 |
| Hormonal agents | 2 | 0.5 | 1 (1.9) |
| Betamethasone | 1 | 0.2 | 1 (1.9) |
| Testosterone | 1 | 0.2 | 0 |
| Ophthalmics (timolol) | 3 | 0.7 | 0 |
| Antihistamines (fexofenadine) | 2 | 0.5 | 0 |
| Colchicine | 2 | 0.5 | 2 (3.8) |
| Rhimmune globulin | 1 | 0.2 | 1 (1.9) |
| Guaifenesin | 1 | 0.2 | 0 |
| Aspirin | 1 | 0.2 | 0 |
| Sumatriptan | 1 | 0.2 | 0 |
| Total | 402 | 52 |
Potential Adverse Events
The most common potential adverse events, had the orders been carried out, were increased expected drug effects or side effects early in a dosing interval (shortly after drug administration) and/or ineffective therapy at the end of a dosing interval (316 of 402 cases; 78.5%). In 60 cases (15%), the error had the potential to result in ineffective or less-effective therapy only, and in 26 cases (6.5%), a potential for “non–dose-related” toxic effects existed.
The potential for adverse patient outcome was rated as potentially “fatal/severe” in 3 cases (0.7%), “serious” in 49 cases (12.2%), and “significant” in 350 cases (87.1%). Potentially fatal/severe or serious errors were most commonly caused by failure to specify appropriate dosage form (total of 32 of 52 cases; 61.5%), primarily when the brand name was used (27 cases; 51.9%), and failure to specify unique dosage form with significant bioavailability differences (12 cases 23.1%). Table 1 lists the number of errors rated as severe or serious for each type of dosage form prescribing error. Table 2 lists the number of severe or serious errors for each medication class and specific medication. The most common medications involved in errors rated as fatal/severe or serious were insulin (30 of 52 cases; 57.7%) and cyclosporine (8 cases; 15.4%).
DISCUSSION
Errors and deficiencies in prescribing have been reported to be the most common cause of preventable adverse drug events in hospitals.1–3 A limited number of factors, such as lack of knowledge or information regarding therapeutics, inadequate availability and use of patient information, confusing prescribing and drug nomenclature, need for dose calculations, and inappropriate use of dosage formulation contribute to the majority of prescribing errors.2–6 Medications are available in multiple or special dose “formulations” for a number of different reasons related to improving the utility of the agent. However, the resulting availability of multiple dosage formulations, lack of caregiver appreciation for the uses and properties of various preparations, as well as high potential for adverse event if dose formulations are used improperly, create a significant risk of adverse patient events. The results of this study suggest that hospitalized patients are at significant and increasing risk for adverse events from prescribing errors involving medication dose formulations. Based on our own experience, and available published error reports, risk for dosage form–related errors exist not only in prescribing, but also in the medication order review/checking, preparation, dispensing, and administration steps of the medication use system.4,6,9–47 By defining how specific factors, such as dosage formulation, contribute to error, effective risk reduction strategies can be implemented.4–6 A limited number of “types” of errors accounted for the majority of prescribing errors involving dosage forms, suggesting that a limited number of safety improvements could provide substantial risk reduction.
A large number of reports of significant adverse patient outcomes resulting from the inappropriate use of dosage formulations are available.9–45 The large dose units, altered bioavailability, or unique physico-chemical properties of special dosage forms present inherent risk for adverse events if misused. A limitation of this study is the determination of the severity of an error based on the prediction of harm had the drug been administered as ordered. Prediction of potential harm was based on consideration of pharmacologic, disease state, and individual patient characteristics. Clearly, the same error may produce a serious adverse effect in one patent but have minimal effects in another, and the error severity classifications should be considered as “generalized” and not absolute. Despite these limitations, the classification system used has been found to be reliable and reproducible.4,6,46–48 Most commonly, an adverse event from inappropriate use of a dosage form is a result of the delivery of excess or inadequate amounts of drug to the site of action, delivery to the wrong site, or toxicity from the dosage form itself. For most of the errors reported, only mild to modest adverse events are likely, although serious events may occur in some patients.11,34 Serious adverse outcomes may be expected when errors involve highly toxic drugs22–28,36,37,40,41 or medications used in serious illnesses.6,39 The characteristics of a specific dose formulation may further complicate events when other types of errors occur simultaneously. An example of a fatal adverse outcome related to inappropriate administration of a dosage formulation combined with dispensing and administration error was reported by Smetzer and Cohen.14 In this tragic case, a misinterpreted prescription for benzathine penicillin led to a 10-fold error in preparation and dispensing, which led to the erroneous intravenous administration of a large volume of benzathine penicillin suspension to a newborn child.
An additional possible limitation to the generalizability of this study is that long term pharmacy-based error prevention programs, such as that used, may actually increase the number and/or alter the type of errors detected, as prescribers rely on the system to catch errors and therefore are less concerned about the correctness of their prescriptions. However, the practices at the study hospital are similar to those in many U.S. hospitals, and the errors detected by our processes are consistent with published case reports9–45 and with those reported to the Institute for Safe Medication Practices (available at: www.ismp.org.org). Thus, the errors reported in this study are likely to be representative of errors occurring in other teaching hospitals without prescriber computer order entry.
The underlying factors related to dosage form errors include: inadequate caregiver and patient knowledge and understanding; availability of and difficulty differentiating between multiple dose formulations; confusing and inconsistent nomenclature; inadequate attention to safety concerns in drug preparation and packaging design; product marketing; and inadequate health care system processes to safeguard patients. Given the many contributors to these errors, systems implemented to protect patients from potential risks must be multifaceted as well.
Formal education of health care providers and patients regarding the properties, availability, and proper use of the various medication dosage forms marketed is inadequate. Cohen15 reported that 35% of nurse anesthetists were unable to name the proper route of administration of long-acting injectable penicillin preparations. The inadequacy of training and resulting lack of appreciation for the importance of dosage formulation appeared to contribute to a number of detected errors, such as ordering controlled delivery agents on an “as needed” basis, ordering controlled delivery forms to be crushed or given per tube, and failing to specify the appropriate dosage form when prescribing. Inclusion of basic information regarding the role of dose formulation issues in pharmacology and therapeutics courses for active and in-training prescribers, nurses, and pharmacists is necessary. As new agents or new dosage forms of previously marketed agents become available, health care organizations, “pharmacy and therapeutics” committees, and providers must consider the role, value, and potential risks of such products.
The nomenclature used for dosage forms is inconsistent and confusing. Terminology varies for individual drugs, within drug classes, or from one manufacturer to another. Adding suffixes to established names of medications (e.g., Cardizem CD, Depakote ER, Wellbutrin SR) is common. This practice results in a lack of clear definition of different formulation properties, and the common problem of omitting the suffix when prescribing. Interestingly, some medications were more commonly found to be prescribed by their brand name, omitting the suffix (bupropion, 13 of 13 occurrences; insulin, 32 of 35 occurrences; nifedipine, 37 of 39 occurrences; venlafaxine, 13 of 15 occurrences), whereas others were found to be more commonly prescribed as generic drugs without specifying the appropriate dosage form (cyclosporine, 12 of 12 occurrences; isosorbide mononitrate, 26 of 30 occurrences; theophylline, 30 of 30 occurrences; verapamil, 22 of 27 occurrences). It is likely that this reflects the frequency with which these drugs are referred to or thought of by brand or generic name, and the impact of marketing of specific agents. Compounding this problem is the failure of pharmaceutical firms to adequately address safety issues related to dose formulation in packaging and marketing of products. For example, controlled-delivery formulations are often available in dose sizes that are simple multiples of the immediate-release form. This practice increases the likelihood that standard-release forms could be used to provide a controlled-release–sized dose (e.g., nifedipine is available in 10- and 20-mg standard-release forms, and 30-, 60-, and 90-mg sustained-release forms). Considering the large number of agents now available for which suffixes denote special dose forms, any value this practice has in terms of convenience, simplification, and product identification, recognition, and differentiation, is minimal. Use of suffixes in the naming of medications may now be causing so much confusion, and possibly patient harm, that the pharmaceutical industry and FDA should consider a moratorium on the practice until a full risk assessment can be performed. At a minimum, to reduce risk, the simple practice of providing sustained-release forms in dose amounts that are not simple multiples of other dosage forms should be strongly considered. The use of unique names for special formulations (i.e., Neoral, Tiazac, Covera HS) might also reduce risk for error in prescribing by brand name. However, since specific “brands” of an agent may not be available in all health care settings, a strong chance for confusion still exists. Establishing an improved method of selecting and approving drug names and formulation designations may reduce the frequency of dose formulation–related errors and confusion. Pharmaceutical firms and the U.S. Food and Drug Administration are urged to formally and systematically address dosage form issues in their drug naming and design processes.49,50
Improving the medication safety processes within health care generally and, where appropriate, those specifically related to dose formulation issues is necessary to the reduction of risk for adverse drug events from these types of errors.1–4,6,51,52 Automated processes including prescriber computer order entry, computer-generated medication administration records, and bar code reconciliation of medication dispensing and administration could significantly reduce errors related to dose formulation.53 Control of access to medications through safe hospital formulary, purchasing, dispensing, distribution, storage, and drug stock removal processes can prevent errors simply by making a medication unavailable or available only in limited circumstances.51,52 This study also demonstrates the critical importance pharmacist review of medication orders prior to administration of medication, as stipulated by the Joint Commission on Accreditation of Healthcare Organization standards.54 Further, improved pharmaceutical decision support through incorporation of pharmacists in patient care has been identified as an effective method to improve prescribing and reduce risk for adverse drug events in general, and would likely improve the prescribing and use of dose formulations.3,51,55
CONCLUSIONS
Prescribing errors involving dosage formulations are common, and without appropriate safety processes such as pharmacist order review in place, present significant risk to patients. The increasing use and availability of unique dosage forms may account for the increasing number of errors detected over the 5-year study period. The finding of recurring errors of similar type supports the concept that prescribing errors are associated with identifiable factors, which provides opportunity for targeted improvements in the process of medication use. Improvements in health care provider knowledge, dosage form safety design, improved nomenclature, and improvements in medication use system processes are necessary for safeguarding patients from errors involving medication dosage formulation.
Acknowledgments
The author wishes to acknowledge the effort of the Albany Medical Center Pharmacists in the detection of errors and collection of the data reported in this manuscript
APPENDIX A
Potential Severity Classifications for Order Errors (Adapted from reference 46)
| Note: Classification system provides for assigning a generalized, population-based assessment of risk. It is recognized that errors rated as “fatal or severe” may produce little or no adverse effects in some patients, just as it is recognized that errors rated as “significant” may produce life-threatening adverse effects in some patients. |
| A. Potentially Fatal or Severe Adverse Outcomes (Examples: cardiovascular arrest, serious arrhythmia, stroke, life-threatening metabolic abnormality, therapeutic failure in life-threatening illness, reaction following administration of a large volume of oral suspension IV) |
| 1. A dose or dose delivery ordered for 10-fold overdose of medication with low therapeutic index. |
| 2. A dose or dose delivery ordered for a medication with a very low therapeutic index that would potentially produce severe or fatal adverse effects in a substantial proportion of patients. |
| 3. A drug, dose, or dose delivery ordered that would produce severe or fatal toxicity in a substantial proportion of patients with similar medical characteristics. |
| 4. A drug, dose, or dose delivery ordered for a medication for a life-threatening illness or severe disorder that would potentially result in therapeutic failure in a substantial proportion of patients. |
| 5. Drug or dose form ordered to be administered by route or method that would potentially result in fatal or severe toxicity in a substantial proportion of patients. |
| B. Potentially Serious Outcomes (Example: significant cardiovascular decompensation, metabolic disorder requiring urgent treatment, inadequate, incomplete or significantly delayed therapeutic response in serious or severe illness.) |
| 1. A dose or dose delivery ordered for 4- to 10-fold overdose of medication with low therapeutic index. |
| 2. A dose or dose delivery ordered for a medication with a very low therapeutic index that would potentially produce serious adverse effects in a substantial proportion of patients. |
| 3. A drug, dose, or dose delivery ordered that would produce serious toxicity in a substantial proportion of patients with similar medical characteristics. |
| 4. A drug, dose, or dose delivery ordered for a medication for a serious illness that would potentially result in therapeutic failure or suboptimal response in a substantial proportion of patients. |
| 5. Drug or dose form ordered to be administered by route or method that would potentially result in serious adverse events in a substantial proportion of patients. |
| C. Potentially Significant Adverse Outcomes (examples: symptomatic hypotension, metabolic abnormality requiring treatment, suboptimal therapeutic response, gastrointestinal upset, dizziness). |
| 1. A dose or dose delivery ordered for 1.5- to 4-fold overdose of medication with low therapeutic index. |
| 2. A dose or dose delivery ordered for a medication that would potentially produce some adverse effects in some proportion of patients. |
| 3. A drug, dose, or dose delivery ordered that would produce adverse effects in some proportion of patients with similar medical characteristics. |
| 4. A drug, dose, or dose delivery ordered for a medication that would potentially result in reduced, incomplete, or delayed therapeutic response in some proportion of patients. |
| 5. Drug or dose form ordered to be administered by route or method that would potentially result in adverse events in some proportion of patients. |
REFERENCES
- 1.Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. JAMA. 1995;274:35–43. [PubMed] [Google Scholar]
- 2.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 3.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285:2114–20. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 4.Lesar TS, Briceland LL, Stein DL. Factors related to errors in medication prescribing. JAMA. 1997;277:312–7. [PubMed] [Google Scholar]
- 5.Lesar TS. Errors in the use of medication dosage equations. Arch Pediatr Adolesc Med. 1998;152:340–4. doi: 10.1001/archpedi.152.4.340. [DOI] [PubMed] [Google Scholar]
- 6.Purdy B, Raymond A, Lesar T. Antiretroviral prescribing errors in a teaching hospital. Ann Pharmacother. 2000;34:833–8. doi: 10.1345/aph.19399. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg RJ, Kaniecki DL. Selection of oral controlled release drugs: a critical decision for the physician. S Med J. 1993;86:208–14. doi: 10.1097/00007611-199302000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Casteneda-Hernandez G, Caille G, duSouich P. Influence of drug formulation on drug concentration-effect relationships. Clin Pharmacokinet. 1994;26:135–43. doi: 10.2165/00003088-199426020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lesar TS. Medication errors involving medication dosage formulations. Medscape Pharmacist. 2001. Available at: http://www.medscape.com/Medscape/pharmacists/journal/2001/v02.n04/mph7675.lesa/mph7675.lesa-01.html. Accessed September, 2001.
- 10.Lesar TS. Common prescribing errors. Ann Intern Med. 1992;117:537–8. doi: 10.7326/0003-4819-117-6-537_2. [DOI] [PubMed] [Google Scholar]
- 11.Grunewald RA, Mack CJ. Medical errors. Different formulations of drugs often look confusingly similar. BMJ. 2001;322:1423. [PubMed] [Google Scholar]
- 12.Mack CJ, Kuc S, Grunewald RA. Errors in prescribing, dispensing and administration of carbamazepine: a case report and analysis. Pharm J. 2000;265:756–9. [Google Scholar]
- 13.Cohen M, Davis N. Drug name suffixes can cause confusion. Am Pharm. 1992;32:301–2. doi: 10.1016/s0160-3450(15)31167-3. [DOI] [PubMed] [Google Scholar]
- 14.Smetzer JL, Cohen M. Lesson from the Denver medication error/criminal negligence case: look beyond blaming individuals. Hosp Pharm. 1998;33:640–57. [Google Scholar]
- 15.Cohen M. Many wrongly believe long-acting parenteral penicillins are for intravenous injection. ISMP Medication Safety Alert! 1999. p. 1. Available at: http://www.ismp.org/MSArtices/ParenPCN.html. Accessed September, 2001.
- 16.Pharmacopeia USP. Summary of the 1999 Information submitted to MedMARx. Available at: www.usp.org/medmarx. Accessed September, 2001.
- 17.Cohen M. Extra caution needed with U-500 insulin. ISMP Medication Safety Alert! January 29, 1997. Available at: www.ismp.org/mSArticles/U500ins.html. Accessed September, 2001.
- 18.Cohen M. Inadequate treatment of syphilis with BICILLIN C-R. ISMP Medication Safety Alert! September 22, 1999. Available at: http://www.ismp.org/MSArticles/Calendar/Sept99.html. Accessed September, 2001.
- 19.Cohen M. New strategies required to prevent esmolol related accidental deaths. ISMP Medication Safety Alert! September 25, 1996. Available at: www.ismp.org/mSArticles/esmolol.html. Accessed September, 2001.
- 20.Vissers RJ, Purssell R. Iatrogenic magnesium overdose: two case reports. J Emerg Med. 1996;14:187–91. doi: 10.1016/0736-4679(95)02115-9. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman RS, Smilkstein MJ, Rubenstein F. An ‘amp’ by any other name: the hazards of intravenous magnesium dosing. JAMA. 1989;261:557. [PubMed] [Google Scholar]
- 22.Cohen M. Willingness of staff to give oral meds IV is disconcerting. ISMP Medication Safety Alert! May 20, 1998. Available at: www.ismp.org/mSArticles/Oral2.html. Accessed September, 2001.
- 23.Krichbaum DW, Malone PM. Subcutaneous administration of nifedipine. Drug Intell Clin Pharm. 1988;22:891–2. doi: 10.1177/106002808802201113. [DOI] [PubMed] [Google Scholar]
- 24.Cohen M. Another ampho-terrible mix-up. ISMP Medication Safety Alert! July 15, 1998. Available at: http://www.ismp.org/MSAarticles/ampho-terrible.html. Accessed September, 2001.
- 25.Cohen M. Medication errors with certain lipid based products. ISMP Medication Safety Alert! August 18, 1998. Available at: http://www.ismp.org/MSAarticles/Ampho-alert.html. Accessed September, 2001.
- 26.Cohen M. FDA approval for an amphotericin B labeling supplement. ISMP Medication Safety Alert! Available at http://www.ismp.org/MSAarticles/Calendar/Jan99.html. Accessed September, 2001.
- 27.Cohen M. Safety Briefs. (Lipid based Amphotericin B) ISMP Medication Safety Alert! 1998. Available at: www.ismp.org. Accessed December, 2001.
- 28.Cohen M. Don't confuse new liposomal doxorubicin with conventional doxorubicin. ISMP Medication Safety Alert! October 9, 1996. Available at: www.ismp.org/MSArticles/doxorub.html. Accessed September, 2001.
- 29.Cohen M. Hazard Alert! Action needed to avert fatal errors from concomitant use of heparin products. ISMP Medication safety Alert! February 21, 2001. Available at: www.ismp.org/MSArticles/heparinalert.html. Accessed September, 2001.
- 30.Davis N. How easy it is for an error to get through the system. Hosp Pharm. 1992;27:944. [Google Scholar]
- 31.Proulx SM, Melchiorre HA. New dosage forms lead to confusion. US Pharm. 2001. pp. 68–9.
- 32.Cohen M. Prevention of adverse events caused by potassium phosphate injection. ISMP Medication Safety Alert! March 13, 1996. Available at: www.ismp.org/MSArticles/96Phosphate.html. Accessed September, 2001.
- 33.Cohen M. ISMP Quarterly action agenda: January–March 2000. Procan SR dispensed for ProcanBID. ISMP Medication Safety Alert! April 5, 2000. Available at: http://www.ismp.org/MSArticles/2Q00Action.html. Accessed September, 2001.
- 34.Anon. Neurology/psychiatry: potential for mix-ups between the new Depakote ER and the original Depakote tablets. Pharm Lett. 2001;17:2. [Google Scholar]
- 35.Cohen M. Depakote ER—easy to confuse with Depakote (delayed release) ISMP Medication Safety Alert! February 7, 2001. Available at: www.ismp.org. Accessed September, 2001.
- 36.Laberge P, Martineau P, Sebajang H, Lalonde G. Verapamil intoxication after substitution of immediate-release for extended-release verapamil. Am J Health Syst Pharm. 2001;58:402–5. [PubMed] [Google Scholar]
- 37.Cohen M. Medication errors with oxycodone products. ISMP Medication Safety Alert! 1998. Available at: www.ismp.org. Accessed September, 2001.
- 38.Cohen M. Action may be necessary to prevent confusion between Roxanne's oral liquid opiate products. ISMP Medication Safety Alert! December 18, 1996. Available at: www.ismp.org/mSArticles/Roxanne.html. Accessed September, 2001.
- 39.Cohen M. Ongoing confusion: paregoric and opium tincture. ISMP Medication Safety Alert! June 19, 1996. Available at: www.ismp.org/mSArticles/Oral.html. Accessed September, 2001.
- 40.Cohen M. Zydis: a new dosage form can be confused with a drug name. Pharm Today. 2001;7:13. [Google Scholar]
- 41.Anon. What happens when critical information is left open to interpretation? (Neoral Advertisement) New York: Novartis Pharmaceuticals; 1998. [Google Scholar]
- 42.Kearns GL, Leeder JS, Wasserman GS. Acetaminophen overdose with therapeutic intent. J Pediatr. 1998;132:5–8. doi: 10.1016/s0022-3476(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 43.Cohen M. Safety Briefs. (liquid acetaminophen) ISMP Medication Safety Alert! 1999. Available at: www.ismp.org. Accessed September, 2001.
- 44.Cohen M. Using oral syringes won't necessarily protect against inadvertent IV injections of oral liquids. ISMP Medication Safety Alert! August 14, 1996. Available at: www.ismp.org/mSArticles/Oral.html. Accessed September, 2001.
- 45.Cohen M. Oral liquid medications may be more vulnerable to errors than previously recognized. ISMP Medication Safety Alert! June 28, 2000. Available at: www.ismp.org/mSArticles/oralliquids.html. Accessed September, 2001.
- 46.Lesar TS, Briceland LL, Delcoure K, Crilly Parmalee J, Masta-Gornic V, Pohl H. Medication prescribing errors in a teaching hospital. JAMA. 1990;263:2329–34. [PubMed] [Google Scholar]
- 47.Lesar TS, Lomaestro BM, Pohl H. Medication prescribing errors in a teaching hospital: a nine-year experience. Arch Intern Med. 1997;157:1569–76. [PubMed] [Google Scholar]
- 48.Overhage JM, Lukes A. Practical, reliable, comprehensive method for characterizing pharmacists' clinical activities. Am J Health Syst Pharm. 1999;56:2444–50. doi: 10.1093/ajhp/56.23.2444. [DOI] [PubMed] [Google Scholar]
- 49.Holquist C, Phillips J. How FDA reviews drug names. Drug Topics. 2001. p. 36. April 2.
- 50.Phillips J. FDA Efforts at minimizing the risks of preventable adverse drug events. Medscape Pharmacist. July, 2000. Available at: http://www.medscape.com/medscape/pharmacists/journal/2000/v01.n04/mph7174.phil/mph7174.phil-01.html. Accessed September, 2001.
- 51.Lesar TS. Recommendations for reducing medication errors. Medscape Pharmacist. 2000. Available at: www.medscape.com/medscape/pharmacist/journal/2000/v01.n04/mph7175.lesa-01.html. Accessed September, 2001.
- 52.Cohen M, editor. Medication Errors. Washington, DC: American Pharmaceutical Association; 1999. [Google Scholar]
- 53.Bates DW, Leape LL, Cullen DW, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;80:1311–6. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 54.Rich D. Ask the Joint Commission. Surveying medication use in 2001. Hosp Pharm. 2001;36:444–6. [Google Scholar]
- 55.Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267–70. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]