Abstract
The hepatitis C virus envelope protein, E2, is an endoplasmic reticulum (ER)-bound protein that contains a region of sequence homology with the double-stranded RNA-activated protein kinase PKR and its substrate, the eukaryotic translation initiation factor 2 (eIF2). We previously reported that E2 modulates global translation through inhibition of the interferon-induced antiviral protein PKR through its PKR-eIF2α phosphorylation site homology domain (PePHD). Here we show that the PKR-like ER-resident kinase (PERK) binds to and is also inhibited by E2. At low expression levels, E2 induced ER stress, but at high expression levels, and in vitro, E2 inhibited PERK kinase activity. Mammalian cells that stably express E2 were refractory to the translation-inhibitory effects of ER stress inducers, and E2 relieved general translation inhibition induced by PERK. The PePHD of E2 was required for the rescue of translation that was inhibited by activated PERK, similar to our previous findings with PKR. Here we report the inhibition of a second eIF2α kinase by E2, and these results are consistent with a pseudosubstrate mechanism of inhibition of eIF2α kinases. These findings may also explain how the virus promotes persistent infection by overcoming the cellular ER stress response.
Hepatitis C virus (HCV) is a major public health problem, with at least 2.7 million persons infected in the United States and more than 170 million infected worldwide. Most HCV infections become persistent, which often leads to chronic liver disease, including cirrhosis and hepatocellular carcinoma. Viral persistence may be partially attributable to the ability of the virus to evade the host immune responses. Viral variants that escape innate or acquired immunity are likely to also be important in allowing the virus to establish persistence. For instance, many strains of HCV are resistant to alpha interferon (IFN) therapy and may also be resistant to endogenous IFN. Several mechanisms for IFN resistance have been proposed, including interactions of both the E2 envelope protein and NS5A with the IFN-induced double-stranded RNA-activated protein kinase PKR (7, 23). HCV E2 protein is one of two envelope glycoproteins and contains a region of sequence homology with a region of PKR that contains autophosphorylation sites that affect the activation of the kinase (23, 24). Adjacent to this region of PKR homology lies a sequence identical to the phosphorylation site in the alpha subunit of the eukaryotic translation initiation factor 2 (eIF2α) targeted by PKR for translational inhibition during virus infection. This region in E2, termed the PKR-eIF2α phosphorylation site homology domain (PePHD), is required for inhibition of PKR by E2 (23). HCV E2 containing PePHD sequences from IFN-resistant strains of virus inhibits PKR, confirming that these sequences are important for the interaction of E2 with PKR.
The presence of the PePHD in the E2 protein suggests that E2 may serve as a substrate or pseudosubstrate for PKR. Therefore, we set out to examine the possible effects of E2 on the activity of another eIF2α kinase. The PKR-like endoplasmic-reticulum (ER)-resident kinase (PERK) (8), also known as pancreatic eIF2 kinase (19), is a part of the host cell antiviral response (12). PERK may be involved in the down-regulation of protein synthesis during viral infection due to ER stress because the ER is overloaded with proteins (17). ER stress is characterized by cellular responses that attempt to reduce the accumulation of aggregated and misfolded proteins. Signal transduction that results in the upregulation of chaperone gene expression and an arrest in protein synthesis are two features that help the cell cope with ER stress.
PERK is a type I transmembrane protein that attenuates protein synthesis during ER stress by phosphorylating serine 51 on the alpha subunit of the heterotrimeric translation initiation factor eIF2. A ternary complex of eIF2, methionyl-tRNA, and GTP recruits the 40S ribosome to the mRNA and is required for general translation initiation. Phosphorylated eIF2 entraps the guanine nucleotide exchange factor, eIF2B, thus preventing subsequent rounds of translation initiation (10). In this way PERK acts to limit protein synthesis during ER stress events, such as virus infection.
Although the kinase domain of PERK is cytoplasmic, the lumenal portion contains the regulatory domain, comprised of an oligomerization domain and a chaperone-binding domain (15). PERK is held in an inactive, monomeric state by association with the chaperone GRP78 (BiP) and GRP94, which are released upon ER stress (1, 15). Release of BiP enables oligomerization, autophosphorylation, and activation of PERK, resulting in attenuation of translation (1). ER stress can be induced by misfolded E2, which binds to BiP, thus activating both grp78 and grp94 promoters (14), resulting in higher levels of BiP. BiP is thought to entrap stable HCV glycoprotein aggregates, and this complex is involved in a nonproductive folding pathway (2). Because overexpression of BiP leads to the inactivation of PERK, the two proteins are presumably related through a negative-feedback mechanism involving competitive binding (1). Here we show that E2 can serve as a pseudosubstrate inhibitor of PERK and a potential viral regulator of the ER stress response.
MATERIALS AND METHODS
Cells and transfections.
Human embryonic kidney (HEK 293) cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with fetal bovine serum (10%). Transfections were performed as described previously (22). Stable cell lines were constructed by transfecting pTre or pTre-E2 into a HeLa tetracycline (TET)-off (TET removed) cell line (Clontech) selected in DMEM with 100 mg of G418 (Invitrogen)/ml, 0.4 mg of hygromycin (Gibco-Invitrogen)/ml, and 1 μg of TET/ml. Stable cell lines were measured for growth by seeding 1.4 × 105 cells per 6-cm dish. At 24-h intervals, cells were trypsinized and counted (Beckman Coulter Inc.). Each values is expressed as the mean of three samples. E2 expression was induced by the removal of TET. Cells were treated with castanospermine (CST) at a concentration of 1 or 2 mM for 2 h or thapsigargin (Tg) at 1 μM or tunicamycin (Tu) at 2.5 μg/ml for 3 h at 37°C. Cells were metabolically labeled by depletion in DMEM minus methionine for 30 min and addition of [35S]methionine (30 μCi/ml) for an additional 30 min during CST treatment.
Plasmids and mutagenesis.
The E2 gene was cloned by PCR from the HCV genotype 1a cDNA (HCV-1; E2L-pcDNA3) or HCV genotype 1b (pcDNAEF-E2) and included the signal leader peptide as described previously (18, 23). The eIF2α homology region in the PePHD was mutated in E2L-pcDNA3 by the PCR fusion technique as described previously (23) to generate the following changes: nucleotides 2319 to 2330 (Ser-Glu-Leu-Ser) were changed to Gly-Gln-Gln-His, generating the mutant E2-clu1. E2-ΔC was constructed by deletion of nucleotides 1910 to 2526 in E2L-pcDNA3 as described previously (23). The construction of GST-E2, E2-flag, and hnRNPK has been described by Taylor et al. (23). GST-PERK consists of the kinase domain of PERK fused to glutathione S-transferase (GST). The construction of the GST-PERK, PERK-c-myc-pcDNA1, and PERK K/A plasmids has been described by Harding et al. (8). To create pTre-E2, a 900-bp EcoRI fragment containing the E2 coding sequence was isolated from pE2 (23) and ligated into the EcoRI polylinker site of plasmid pTre (Clontech).
Binding assay.
The expression of GST fusion proteins was induced by isopropyl-β-d-thiogalactopyranoside in BL21(DE3) bacterial cells. Proteins were purified from sonicated lysates by incubation with glutathione-coated beads at 4°C for 2 h. The beads were washed with phosphate-buffered saline containing 0.3% NP-40. The in vitro-translated, 35S-labeled E2 or hnRNP K proteins were then incubated with the beads for 2 h at 4°C in binding buffer (0.04 M HEPES-K [pH 7.5], 0.1 M KCl, 0.1% NP-40, 0.02 M β-mercaptoethanol). The beads were washed five times with binding buffer at 0.3% NP-40. Radiolabeled, bound proteins were eluted with sodium dodecyl sulfate (SDS) sample buffer and boiled before SDS-polyacrylamide gel electrophoresis (PAGE) analysis.
Immunoprecipitation.
HEK 293 cells were transfected with plasmids containing a wild-type PERK-pcDNA1 (8) and/or E2 (E2L-pcDNA3 or pcDNAEF-E2) by the calcium phosphate method as described previously (22) or with Fugene 6 (Roche) as per the manufacturer's specifications. c-Myc-tagged PERK was immunoprecipitated from cytoplasmic extracts with monoclonal anti-c-Myc antibody (Zymed) and protein A Sepharose as described previously (22).
Western blotting.
HeLa cells were stably transfected with pTRE, pTre-E2, or pTre-E2ΔC and treated with Tg or Tu as appropriate. Cell lysates were resolved on 8 to 16% polyacrylamide-SDS gels. Proteins were transferred to nitrocellulose by electrophoresis and probed with anti-eIF2α or anti-phospho-eIF2α antibodies (Cell Signaling).
Kinase assay.
GST and GST-E2 were purified from isopropyl-β-d-thiogalactopyranoside-induced BL21(DE3) cells with glutathione Sepharose 4B beads (Pharmacia Biotech). c-Myc-tagged PERK was immunoprecipitated with anti-c-Myc antibody bound to protein-A Sepharose beads, which were incubated with purified GST or GST-E2 protein for 30 min at 30°C. Kinase reactions were conducted by adding [γ-32P]ATP in kinase buffer according to the method of Taylor et al. (22) followed by incubation for 20 min at 30°C. Histone H2a (3 μg; Sigma) or eIF2α (3 μg) was then added for another 15 min at 30°C.
CAT assay.
Chloramphenicol acetyltransferase (CAT) enzyme activity was measured from cytoplasmic fractions of transfected HEK 293 cells as described previously (23).
RESULTS
E2 and PERK associate in vitro and in cells.
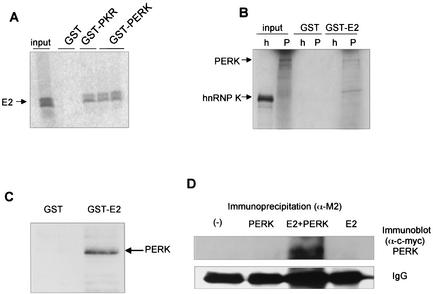
To determine whether E2 protein interacts with PERK in vitro, we performed an in vitro binding assay with a purified fusion protein expressed in Escherichia coli containing the C-terminal, kinase domain of PERK fused to GST. GST-PKR and GST protein were included as controls for specificity of binding (Fig. 1A). Radiolabeled unglycosylated E2 protein bound specifically to wild-type GST-PKR and to GST-PERK in vitro, but not to GST alone. Radiolabeled, in vitro translated full-length PERK or hnRNPK were incubated with GST or GST-E2 fusion proteins, bound to glutathione beads. Only PERK bound to GST-E2, suggesting that PERK binds specifically to E2 in vitro (Fig. 1B). HEK 293 cells were transfected with PERK-c-myc-pcDNA1, and lysates were incubated with GST or GST-E2 bound to glutathione Sepharose beads. After probing with anti-c-Myc antibodies, PERK was detected in the suspension that contained GST-E2 (Fig. 1C). In a coimmunoprecipitation assay (Fig. 1D), where Flag-tagged E2 and c-Myc-tagged PERK were overexpressed in HEK 293 cells, PERK coimmunopurified with E2 anti-Flag antibodies (anti-Flag M2 beads; Sigma) bound to E2 protein. PERK was visualized by Western blot analysis with anti-c-Myc antibodies. Conversely, we used anti-c-Myc antibodies to immunopurify c-Myc-tagged PERK and visualized 35S-metabolically labeled, unglycosylated E2 (data not shown). These data suggest that PERK and E2 interact in vitro and in cells, similar to our previous results demonstrating that the unglycosylated form of E2 coimmunoprecipitates with PKR (18).
FIG. 1.
E2 binds to PERK in vitro. (A) In vitro-translated, 35S-labeled E2 was incubated with GST, GST-PKR, or GST-PERK fusion protein bound to glutathione beads. Bound proteins were separated by SDS-PAGE and visualized by autoradiography. (B) In vitro-translated, 35S-labeled full-length PERK (P) or hnRNPK (h) was incubated with GST or GST-E2 fusion proteins bound to glutathione beads. Input represents 10% of the input volume of in vitro translation products used for the binding reactions. hnRNPK (small nuclear RNA binding protein K) was used as a negative binding control. (C) HEK 293 cells were transfected with PERK-c-Myc. The cell lysate was incubated with GST- or GST-E2 bound to glutathione beads. Beads were washed, and bound proteins were eluted and separated by SDS-PAGE. Western blot transfers were probed with anti-c-Myc antibodies, and proteins were visualized by chemiluminescence. (D) Coimmunoprecipitation assay. HEK 293 cells were transfected with PERK-c-Myc, E2-flag, or both PERK-c-Myc and E2-flag. Cell lysates were immunoprecipitated with anti-Flag M2 agarose beads (Sigma) for immunoprecipitation of E2 and separated by SDS-PAGE. Western blot transfers were probed with anti-c-Myc antibodies for detection of coimmunoprecipitated PERK, and proteins were visualized by chemiluminescence. Mouse heavy chain of the antibody used for immunoprecipitation is shown as a gel loading control.
E2 inhibits PERK kinase activity.
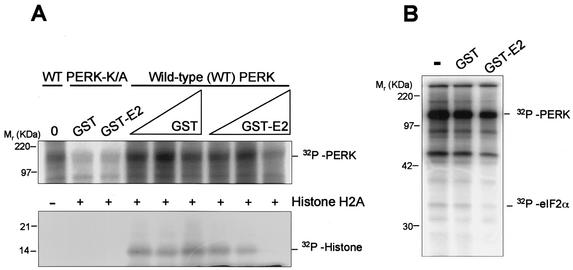
To assess the effect of E2 on PERK kinase activity in vitro, we performed kinase assays with wild-type PERK that was immunopurified from PERK-transfected HEK 293 cells. The kinase assay was performed with the immunoprecipitates in the presence of the purified GST or GST-E2 fusion proteins, using histone H2a as a substrate (Fig. 2A). Incubation with GST-E2, but not GST, inhibited both autophosphorylation of PERK and histone phosphorylation. Catalytically inactive PERK (PERK K/A) was not significantly phosphorylated. While some phospho-PERK K/A can be seen, this may be due to self-association with endogenous wild-type PERK. H2A phosphorylation was not observed in these assays, confirming that PERK K/A is not active. Phosphorylation of eIF2α was also inhibited by GST-E2 (Fig. 2B). The presence of GST-E2 (Mr= 66) did not result in the appearance of any additional phosphorylated protein, indicating that GST-E2 was not phosphorylated by PERK in vitro. These results suggest that E2 serves as an inhibitor of PERK kinase activity in vitro.
FIG. 2.
E2 inhibits PERK kinase activity in vitro. (A) Protein kinase assay of immunoprecipitated PERK. PERK-c-Myc was transfected into HEK 293 cells, and at 48 h posttransfection cells were harvested, lysed, and immunoprecipitated with anti-c-Myc antibody. No added protein (0), GST (2.5 and 5 μg of protein), or GST-E2 (2 and 5 μg of protein) was incubated with the immunoprecipitates before the kinase reaction began. Histone H2a was added as a substrate after an initial incubation with [γ-32P]ATP. PERK K/A was used as a negative control for histone phosphorylation. PERK serves as an autophosphorylation substrate. (B) Immunoprecipitation kinase assay with transfected PERK-c-Myc and eIF2α used as a substrate.
E2 reverses PERK-mediated translational repression.
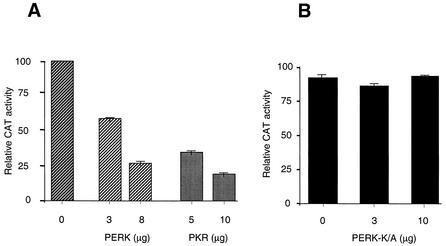
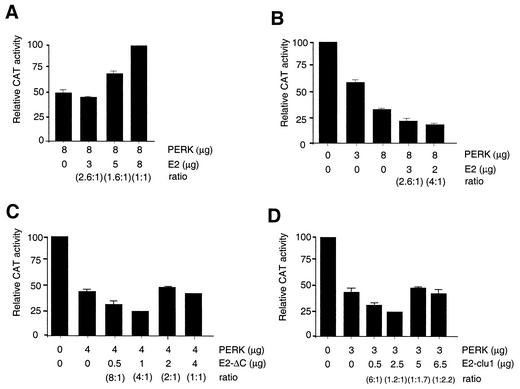
Since E2 repressed PERK kinase activity in vitro, we predicted that E2 would enhance translation in mammalian cells. CAT-expressing plasmids were cotransfected with PKR- or PERK-expressing plasmids, which resulted in reduced CAT activity (Fig. 3), suggesting that translation was inhibited by overexpression of these kinases, which induces their autoactivation (24). Furthermore, the translational inhibition by PERK was seen only when the catalytically active kinase was used and not when PERK K/A was used (Fig. 3B). In reporter assays, exogenously expressed PERK was used to separate the effects of E2 from those of endogenous PKR and PERK. Increasing amounts of E2, transfected into these cells, rescued the negative effects of PERK (Fig. 4A). The extent of translational enhancement by E2 correlated with the amount of transfected E2 plasmid (Fig. 4A). We previously showed that enhanced CAT activity in the presence of E2 was due to increased translation and not transcription (23). Low levels of transfected E2 caused an enhancement of PERK's inhibitory effect (Fig. 4B), suggesting that inhibition of translation by PERK was exacerbated when E2 was present at low protein concentrations. At higher concentrations (e.g., 1:1 ratio of PERK to E2), translation was rescued (Fig. 4A). An E2 truncation mutant, which lacks most of the C terminus including the PePHD (E2-ΔC), was not capable of enhancing translation even at high concentrations (Fig. 4C). Furthermore, the E2 variant containing the PePHD sequence derived from the generally IFN-sensitive HCV genotypes 2 and 3 (E2-clu1) (Fig. 4D) also showed a reduced ability to enhance translation, and at low concentrations both variants exacerbated the inhibitory effects of PERK. Amounts were chosen to maximize the ratios, and the ratios can be used to compare experiments. Absolute protein ratios were not analyzed in these experiments however. Taken together, these data suggest that E2 induces an ER stress response at low expression levels and exacerbates the negative effect on translation caused by overexpression of PERK. E2 can also enhance translation of cellular genes at high expression levels by inhibition of PERK through interaction with the PePHD region.
FIG. 3.
Overexpression of PERK inhibits protein synthesis in mammalian cells. (A) HEK 293 cells were transfected with equal amounts of total plasmid DNA using pcDNA3 as the carrier. Samples contained CAT-pcDNA3 (0.3 μg) and pcDNA3, PERK-pcDNA1 (0, 3, or 8 μg), or PKR-pcDNA3 (0, 5, or 10 μg). Cell lysates were assayed for CAT enzyme activity 48 h after transfection. Transfections were performed in triplicate, and the values were obtained from means of ≥3 separate experiments each (± standard deviation). CAT activity is expressed in relative units, where the pcDNA3 control (0) was 100%. (B) HEK 293 cells were cotransfected with 0.3 μg of CAT-pcDNA3 and 0, 3, or 10 μg of PERK K/A.
FIG. 4.
(A) E2 enhances protein synthesis in mammalian cells. CAT enzyme activity from cells transfected with CAT-pcDNA3 (0.3 μg), PERK-c-Myc (8 μg), and increasing amounts of pcDNA3-E2 (0 to 8 μg) is shown. (B) Low levels of E2 exacerbate the negative effects of overexpressed PERK on translation. CAT activity in cells transfected with CAT-pcDNA3 (0.3 μg), PERK-c-Myc (0 to 8 μg), and E2 (0 to 3 μg) is shown. (C and D) Mutant E2 inhibits translation at low levels but does not stimulate translation at high levels. CAT activity from cells transfected with CAT-pcDNA3 (0.3 μg), PERK (0 to 4 μg), and E2-ΔC (C) or E2-clu1 (D) (0 to 6.5 μg) is shown.
E2 confers resistance to ER stress.
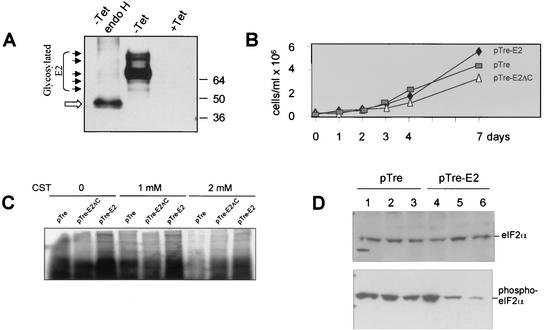
To study the effects of ER stress in the presence of E2, we established a stable HeLa cell line with inducible E2 expression targeted to the ER. E2 is glycosylated at the ER membrane (Fig. 5A), and its increased gel mobility after endoglycosidase H treatment (Fig. 5A), which removes carbohydrate residues, demonstrates that E2 is being expressed. Little difference in cell growth was observed between the different cell lines, although E2-expressing cells grew to larger numbers, suggesting a slight enhancement of cell growth (Fig. 5B). To examine the effect of E2 during ER stress, these cell lines were treated with CST, an inhibitor of α-glucosidase (Fig. 5C). CST interferes with the ability of calnexin to associate with glycoproteins in the ER and results in the induction of ER stress and inhibition of protein synthesis. Calnexin binds specifically to E2 and plays a role in folding of HCV glycoproteins (2). Cell lines expressing either E2 or the E2-ΔC mutant were refractory to CST treatment, demonstrated by increased incorporation of [35S]methionine into proteins (Fig. 5C). This shows that E2 can evade the ER stress response induced by CST. The effect may be independent of the C-terminal portion of E2. To determine if E2 was evading the effects of ER stress specifically through inhibition of PERK, we analyzed eIF2α phosphorylation under ER stress conditions in the E2-expressing cell lines. Tg, which promotes the depletion of lumenal calcium stores, and Tu, which blocks N glycosylation, were used to induce ER stress. In the presence of E2, decreased eIF2α phosphorylation was observed in cells that were treated with Tg or Tu (Fig. 5D, lanes 5 and 6). Interestingly, in the presence of TET, for control cells and those that express E2 (lanes 1 and 4), eIF2α was phosphorylated in the absence of Tg and Tu, perhaps due to the presence of the TET-off inducible plasmid or because the cells were stressed due to a high population density, which may result in PERK activation as well (P. R. Romano, unpublished observations). Taken together, these results demonstrate that E2 can reverse ER stress-mediated eIF2α phosphorylation and the resulting inhibition of translation by inhibiting PERK.
FIG. 5.
E2-expressing cells are resistant to ER stress transducers. (A) E2-expressing cells were grown in the absence of TET (−) or in the absence of TET, followed by endoglycosidase H treatment (endo H), and in the presence of TET (+). Arrowheads denote full and partially glycosylated forms of E2; the open arrow denotes unglycosylated E2. Cell lysates were separated by SDS-PAGE and analyzed by Western blotting, probed with the monoclonal anti-E2 antibody H52 (3). (B) Cell growth of stable cell lines in the absence of TET. Cell lines are designated by ♦ for pTre-E2, ▵ for pTre-E2ΔC, and □ for pTre vector alone. (C) Cell lines grown in the absence of TET and treated with 0, 1, and 2 mM CST and metabolically labeled with [35S]methionine. Cytoplasmic extracts were separated by SDS-PAGE. (D) Cells were grown in the presence of (lanes 1 and 4) or absence of TET and treated with Tg (lanes 2 and 5) or Tu (lanes 3 and 6). Lysates were separated by SDS-PAGE, analyzed by Western blotting, and probed with anti-eIF2α antibody or anti-phospho-eIF2α antibody.
DISCUSSION
These results combined suggest that the E2 protein of HCV genotype 1 binds to and inhibits PERK in vitro and in mammalian cells. These effects require a region within the E2 protein which bears sequence homology to the eIF2α phosphorylation site, although we cannot exclude the possibility that other sequences in E2 may be important for its interaction with PERK. Taken together, these results suggest that in addition to providing the relative resistance of HCV genotype 1 to IFN, E2 could provide the virus with an ability to escape the negative translational effects of ER stress.
E2 is localized to the perinuclear region of the cytoplasm and contains a transmembrane domain in the last 29 amino acids of its C terminus that retains E2 at the ER membrane (3). The signal peptide leader sequence is also required for the proper folding, localization, and processing of E2 proteins. Interestingly, PKR also localizes to the perinuclear region of the cytoplasm and the nucleolar region (11), and both of these proteins colocalize in cells (23). This is consistent with the finding that PKR is closely associated with ribosomes through binding to the L18 protein (13), including most likely ER-bound ribosomes. PKR interacts with the abundant unglycosylated form of E2 on the cytoplasmic side of the ER, and overexpression of PKR leads to an accumulation of this unprocessed form of E2 (18). Therefore, E2 and PERK also likely interact with each other on the cytoplasmic side of the ER as the kinase domain of PERK is cytoplasmic. Because BiP is activated during E2 expression, the ER overload response or unfolded-protein response are probably activated. Misfolding of E2 probably occurs (2), leading to an accumulation of unglycosylated E2 that gets retained in the cytoplasm. These signals may be detected by BiP and mediated by the recognition of hydrophobic residues in E2, which would be exposed in its misfolded state but concealed in its properly folded state.
Cells have the ability to detect foreign, nonhost molecules, such as viruses. PKR acts as an antiviral protein by detecting double-stranded RNA, produced during viral replication. PERK, however, senses misfolded or overexpressed proteins and possibly additional signals that induce ER stress (e.g., Ca2+ flux). Both kinases regulate translation through phosphorylation of eIF2α. The ER overload response occurs during viral infection where an abundance of viral glycoproteins and excessive membrane traffic result in ER stress (17). A cytopathic strain of bovine viral diarrhea virus, another member of the flaviviridae family, induces ER stress-mediated apoptosis (12). Consequently, persistent infection with noncytopathic strains of bovine viral diarrhea virus may result from a viral strategy that allows it to evade the ER stress response.
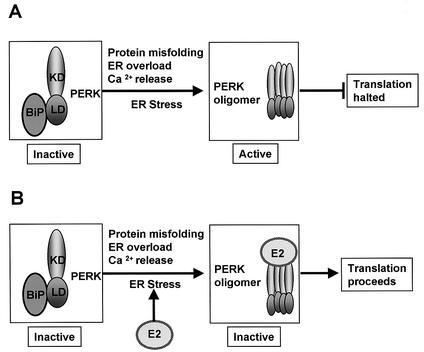
HCV E2 can induce ER stress, leading to the transactivation of BiP (14), which is activated during the unfolded-protein response and mediates the folding of newly synthesized proteins in the ER lumen (for a review, see reference 13). PERK is held at the ER in an inactive, monomeric form by BiP and possibly by GRP94 (1, 15) (Fig. 6A). During ER stress, including stress induced by viral infection (12), the latent PERK-BiP heterodimer dissociates, whereby PERK oligomerizes and becomes active, resulting in phosphorylation of eIF2α and the inhibition of both cap-dependent and internal ribosome entry site (IRES)-dependent translation initiation (Fig. 6A). Overexpression of PERK in mammalian cells can also lead to autophosphorylation and activation of the kinase (8). In our model (Fig. 6B), HCV E2 is multiply glycosylated and probably induces ER stress by protein overexpression, ER overload, or underglycosylation and, thus, misfolding. At first this leads to ER stress, but as E2 is expressed, PERK binds to and possibly recognizes E2 as a pseudosubstrate, and PERK then may be sequestered by E2 from its substrate eIF2, allowing translation to proceed (Fig. 6B). Similarly, PERK is inhibited through a pseudosubstrate mechanism by the vaccinia virus protein K3L, which is also a PKR inhibitor (20). The finding that PERK activity increases upon dissociation from BiP, and BiP activity increases in the presence of E2, provides a basis for how E2 can induce the ER stress response, thus allowing for proper folding of viral proteins while maintaining translation through inhibition of at least two eIF2α kinases.
FIG. 6.
Model of ER stress and PERK activation and inhibition. (A) Classical ER stress induces the dissociation of the latent BiP-PERK heterodimer, leading to PERK oligomerization. PERK oligomerization results in activation of the kinase, which phosphorylates eIF2α, leading to the inhibition of translation. (B) E2 stimulates ER stress, leading to PERK oligomerization, but blocks the kinase through binding and prevents productive activation, preventing eIF2α phosphorylation, thus allowing translation to proceed. KD, kinase domain; LD, lumenal domain.
ER stress mediated by glucose deprivation stimulated translation from the cellular IRES of cat-1 mRNA (5). Although cat-1 translation increased in a PERK/phospho-eIF2α-independent manner, BiP IRES-dependent translation was not stimulated by ER stress (6). Furthermore, expression of HCV subgenomic replicons that contain only nonstructural genes and lack the structural genes (e.g., E2) induces ER stress and stimulates HCV IRES activity in a PERK/BiP-independent pathway (21). In fact, lower levels of eIF2α phosphorylation correlates with increased HCV IRES activity (21; D. R. Taylor, unpublished results) and BiP IRES activity (21). Thus, HCV may benefit from ER stress inducers that are unrelated to PERK.
It is further noted that loss of PERK in mice (9) and humans (4) resulted in the destruction of pancreatic beta cells, characterized by insulin-dependent diabetes mellitus. HCV infection has been associated with diabetes mellitus (reference 16 and references therein), and it will be interesting to find out if the inhibition of PERK by E2 plays a role in its etiology.
Our model (Fig. 6) is consistent with previous findings for HCV E2, PERK, and BiP and suggests that HCV E2 may help to circumvent the coordinated antiviral response that includes the ER stress response pathways in addition to the IFN-induced antiviral pathways. These findings suggest that E2 may play a role in HCV persistent infection by overcoming the cellular ER stress response and may point to new directions for the development of therapeutics for the treatment of HCV.
Acknowledgments
We thank Heather Harding and David Ron for the PERK constructs and Glen Barber for purified eIF2α protein. The H52 antibody was a gift from J. Dubuisson. We also thank Michael Lai for helpful discussions in the early part of this work.
This work was supported by a fellowship to D.R.T. from the Oak Ridge Institute. N.P. is a fellow of the Howard Hughes Medical Institute.
REFERENCES
- 1.Bertolotti, A., Y. Zhang, L. M. Hendershot, H. P. Harding, and D. Ron. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2:326-332. [DOI] [PubMed] [Google Scholar]
- 2.Choukhi, A., S. Ung, C. Wychowski, and J. Dubuisson. 1998. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J. Virol. 72:3851-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cocquerel, L., J.-C. Meunier, A. Pillez, C. Wychowski, and J. Dubuisson. 1998. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J. Virol. 72:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delepine, M., M. Nicolino, T. Barrett, M. Golamaully, G. M. Lathrop, and C. Julier. 2000. EIF3AK3, encoding translation initiation factor 2-α kinase3, is mutated in patients with Wolcott-Rallison syndrome. Nat. Genet. 25:406-409. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez, J. M., B. Bode, A. Koromilas, J. A. Diehl, I. Krukovets, M. D. Snider, and M. Hatzoglou. 2002. Translation mediated by the internal ribosome entry site of the cat-1 mRNA is regulated by glucose availability in a PERK-kinase-dependent manner. J. Biol. Chem. 277:11780-11787. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez, J. M., I. Yaman, P. Sarnow, M. D. Snider, and M. Hatzoglou. 2002. Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2alpha. J. Biol. Chem. 277:19198-19205. [DOI] [PubMed] [Google Scholar]
- 7.Gale, M. J., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 8.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271-274. [DOI] [PubMed] [Google Scholar]
- 9.Harding, H. P., H. Zeng, Y. Zhang, R. Jungries, P. Chung, H. Plesken, D. Sabatini, and D. Ron. 2001. Diabetes mellitus and exocrine pancreatic dysfunction in PERK−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7:1153-1163. [DOI] [PubMed] [Google Scholar]
- 10.Hershey, J. W. B., and W. C. Merrick. 2000. The pathway and mechanism of initiation of protein synthesis, p. 33-88. In J. W. B. Hershey, M. B. Mathews, and N. Sonenberg, Translational control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Jimenez-Garcia, L. F., S. R. Green, M. B. Mathews, and D. L. Spector. 1993. Organization of the double-stranded RNA-activated protein kinase DAI and virus-associated VA RNA1 in adenovirus-2 infected cells. J. Cell Sci. 106:11-22. [DOI] [PubMed] [Google Scholar]
- 12.Jordan, R., L. Wang, T. M. Graczyk, T. M. Block, and P. R. Romano. 2002. Replication of a cytopathic strain of bovine diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 76:9588-9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kauffman, R. J. 1999. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13:1211-1233. [DOI] [PubMed] [Google Scholar]
- 14.Liberman, E., Y.-L. Fong, M. J. Selby, Q.-L. Choo, L. Cousens, M. Houghton, and T. S. B. Yen. 1999. Activation of the grp78 and grp94 promoters by hepatitis C virus E2 envelope protein. J. Virol. 73:3718-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma, K., K. M. Vattem, and R. C. Wek. 2002. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 (eIF2) kinase in response to endoplasmic reticulum stress. J. Biol. Chem. 277:18728-18735. [DOI] [PubMed] [Google Scholar]
- 16.Mason, A. L., J. Y. N. Lau, N. Hoang, K. Qian, G. J. M. Alexander, L. Xu, L. Guo, S. Jacob, F. G. Refenstein, R. Zimmerman, J. E. Everhart, C. Wasserfall, N. K. Maclaren, and R. P. Perrillo. 1999. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology 29:328-333. [DOI] [PubMed] [Google Scholar]
- 17.Pahl, H. L., H. G. Burgert, and P. A. Baeuerle. 1996. Activation of transcription factor NF-kappaB by the adenovirus E3/19K protein requires its ER retention. J. Cell Biol. 132:511-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavio, N., D. R. Taylor, and M. M. C. Lai. 2002. Detection of a novel unglycosylated form of hepatitis C virus E2 envelope protein that is located in the cytosol and interacts with PKR. J. Virol. 76:1265-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi, Y., K. M. Vattem, R. Sood, J. An, J. Liang, L. Stramm, and R. C. Wek. 1998. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18:7499-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sood, R., A. C. Porter, K. Ma, L. A. Quilliam, and R. C. Wek. 2000. Pancreatic eukaryotic initiation factor-2a kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem. J. 346:281-293. [PMC free article] [PubMed] [Google Scholar]
- 21.Tardif, K. D., K. Mori, and A. Siddiqui. 2002. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J. Virol. 76:7453-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor, D. R., S. B. Lee, P. R. Romano, D. R. Marshak, A. G. Hinnebusch, M. Esteban, and M. B. Mathews. 1996. Autophosphorylation sites participate in the activation of the double-stranded RNA-activated protein kinase PKR. Mol. Cell. Biol. 16:6295-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor, D. R., S. T. Shi, P. R. Romano, G. N. Barber, and M. M. C. Lai. 1999. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 285:107-110. [DOI] [PubMed] [Google Scholar]
- 24.Taylor, D. R., B. Tian, P. R. Romano, A. G. Hinnebusch, M. M. C. Lai, and M. B. Mathews. 2001. Hepatitis C virus envelope protein E2 does not inhibit PKR by simple competition with autophosphorylation sites in the RNA-binding domain. J. Virol. 75:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]