Abstract
The endoplasmic reticulum (ER) consists of subcompartments that have distinct protein constituents, morphological appearances, and functions. To understand the mechanisms that regulate the intricate and dynamic organization of the endoplasmic reticulum, it is important to identify and characterize the molecular machinery involved in the assembly and maintenance of the different subcompartments. Here we report that syntaxin 17 is abundantly expressed in steroidogenic cell types and specifically localizes to smooth membranes of the ER. By immunoprecipitation analyses, syntaxin 17 exists in complexes with a syntaxin regulatory protein, rsly1, and/or two intermediate compartment SNARE proteins, rsec22b and rbet1. Furthermore, we found that syntaxin 17 is anchored to the smooth endoplasmic reticulum through an unusual mechanism, requiring two adjacent hydrophobic domains near its carboxyl terminus. Converging lines of evidence indicate that syntaxin 17 functions in a vesicle-trafficking step to the smooth-surfaced tubular ER membranes that are abundant in steroidogenic cells.
INTRODUCTION
The endoplasmic reticulum (ER) is the largest endomembrane system within eukaryotic cells and performs a wide range of functions, including lipid and protein synthesis, protein translocation, folding, modification, and concentration, export to the Golgi complex, and calcium uptake and release (Matlack et al., 1998; Meldolesi and Pozzan, 1998; Ellgaard et al., 1999; Herrmann et al., 1999). It consists of a complex system of cisternae, tubules, and lamellae, which can assume a large variety of morphologies in different cells. Classically, the ER has been recognized to be composed of three morphologically distinct subcompartments, rough ER (RER), smooth ER (SER), and the nuclear envelope. Recently, however, it has become apparent that the complexity of the ER is greater than that expected from the traditional tripartite structure. Rather, the ER can be further subdivided into specialized subdomains that are distinct in terms of protein constituents, morphological appearance, and function (Sitia and Meldolesi, 1992). Furthermore, the abundance of specialized subdomains of the ER is characteristic of certain cell types.
An understanding of the intricate and dynamic organization of the ER is based on the ability to establish relationships among the morphological, biochemical, and functional definitions of the different subcompartments. Several classes of proteins have been identified that mediate and regulate membrane dynamics throughout the eukaryotic cell. One class of membrane-trafficking proteins, referred to as soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs), proteins of the vesicle-associated membrane protein (VAMP) and syntaxin families, have been implicated in mediating membrane fusion through their compartment-specific localizations and protein interactions (Hay and Scheller, 1997; Bock and Scheller, 1999; Pfeffer, 1999). Formation of a very stable four-stranded helical bundle, composed of SNAREs from opposing membranes, is thought to be the biochemical event that drives membrane fusion (Hanson et al., 1997; Lin and Scheller, 1997; Poirier et al., 1998; Sutton et al., 1998).
Many different SNARE proteins of the VAMP and syntaxin families have been identified, and each of these proteins is specifically localized throughout the mammalian secretory pathway, including the ER, the Golgi apparatus, and membranes of the endocytic pathway (Bock and Scheller, 1999). Several mammalian SNAREs, including syntaxin 5, rsec22b, rbet1, and membrin, have been localized to the interface between the ER and the Golgi complex (Hay et al., 1997, 1998). In detergent extracts, syntaxin 5 forms a stable SNARE complex with rsec22b, rbet1, and membrin that is thought to mediate two membrane fusion events: the fusion of ER-derived vesicles with vesicular tubular clusters (VTCs), and the assembly of VTCs to form cis-Golgi elements (Hay et al., 1997, 1998). Although SNAREs are clearly important for membrane fusion, an increasing body of information indicates that additional sets of proteins are required to ensure that accurate targeting and docking of vesicles occurs. For example, members of the Sec1 family of proteins, the low-molecular-weight GTPases, and the Rabs and their effectors are implicated in the regulation of SNARE interactions (Halachmi and Lev, 1996; Novick and Zerial, 1997; Gonzalez and Scheller, 1999).
If each distinct trafficking step requires the interaction of a specific set of VAMP and syntaxin proteins, many SNAREs would be expected to be required for the assembly and maintenance of certain ER subcompartments. Furthermore, one would expect these SNARE proteins to be differentially expressed in certain cell types in which specialized subcompartments of the ER are significantly expanded. Here we show that syntaxin 17 (Steegmaier et al., 1998) is abundantly expressed in steroidogenic cell types and localizes to specialized smooth membranes of the ER. Moreover, syntaxin 17 forms a stable SNARE complex that includes the rsec22b and rbet1 proteins. Converging lines of evidence indicate that syntaxin 17 functions in a vesicle-trafficking step to the smooth-surfaced tubular ER membranes abundant in steroidogenic cells.
MATERIALS AND METHODS
Antibodies
Rabbit antisera for sec22b (Hay et al., 1998), VAMP2 (Pevsner et al., 1994), and VAMP4 (Steegmaier et al., 1999), and mouse mAbs for rbet1 (Hay et al., 1998), syntaxin 1 (Inoue et al., 1992), syntaxin 6 (Bock et al., 1997), and syntaxin 13 (Prekeris et al., 1998), have been described previously. Antibodies for calnexin, cytochrome C, human LAMP1, and myc (clone 9E10) were purchased from Stressgen (Victoria, British Columbia, Canada), PharMingen (San Diego, CA), Transduction Laboratories (Lexington, KY), and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Mouse mAbs against syntaxin 17 were prepared by intraperitoneal injection of purified soluble bacterially expressed full-length cytoplasmic domain of rat syntaxin 17 (amino acids 1–227) produced as a GST–syntaxin 17 fusion protein, that was thrombin-cleaved and separated from GST. Hybridoma production, ELISA and Western blot screening, subcloning, and expansion were carried out as described previously (Hsu et al., 1996). Goat polyclonal antibodies against syntaxin 17 were prepared by immunization with bacterially expressed rat syntaxin 17 produced as described above. For affinity purification, the antiserum was first incubated with Affi-Gel 10/15 beads (Bio-Rad, Hercules, CA) coupled with GST. The flow-through was then incubated with thrombin-cleaved recombinant amino-terminal cytoplasmic domain of syntaxin 17 (amino acids 1–132) coupled to Affi-Gel 10/15 beads (2 mg protein/ml beads). The beads were then washed extensively, and bound antibodies were eluted with the use of 0.1 M glycine, pH 2.5. Affinity-purified rabbit polyclonal anti-KDEL receptor (ERD2) was a generous gift of Dr. I. Majoul (University of Göttingen, Göttingen, Germany). COPII was visualized with a polyclonal antibody against the mammalian homologue of sec13 (hsec13), kindly provided by Dr. W.J. Hong (Institute of Molecular and Cellular Biology, Singapore). Albumin was localized with a polyclonal antiserum that was described previously (Brands et al., 1983). Texas Red-, FITC-, or HRP-labeled secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). Routine Western immunoblotting experiments were carried out with the use of ECL (Amersham Pharmacia Biotech, Arlington Heights, IL) and autoradiography.
Tissue Sectioning and Immunolabeling
For immunohistochemical studies, 10-wk-old male Sprague-Dawley rats were anesthetized by an intraperitoneal injection of Avertin and perfused with ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Adrenal glands and testicles were removed from the fixed animal, immediately submerged in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 1.5 h, and then transferred to 20% sucrose in PBS for 24 h. Tissue samples were embedded in Tissue-Tek O.C.T. compound (Sakura Finetechnical, Tokyo, Japan) and frozen into plastic molds (Polyscience, Warrington, PA) in a dry ice/methanol bath. Fourteen-micrometer sections were cut with the use of a cryostat, and sections were applied to Superfrost*/Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were first incubated with 0.1 M glycine in PBS for 30 min, and then they were blocked-permeabilized in PBS containing 5% donor goat serum, 0.1% BSA, and 0.3% Triton X-100 for 1 h (permeabilization buffer). Primary antibodies were applied in permeabilization buffer for 3 h in a humidified chamber at 37°C. For anti-syntaxin 17 staining, the mAbs 6A6-3 and 14C10-2 were used at 5 μg/ml purified immunoglobulin G (IgG). Anti-VAMP4 polyclonal rabbit antibodies were used at 3 μg/ml. After washing with permeabilization buffer, secondary antibodies were applied for 1 h in a humidified chamber at 37°C. Sections were then rinsed with PBS, placed under coverslips, and visualized with the use of a Zeiss (Oberkochen, Germany) Axiophot microscope.
For indirect immunofluorescence microscopy, H295R cells were fixed and processed as described previously (Hay et al., 1996). Antibodies and antisera were used at the following dilutions: goat anti-syntaxin 17 antibodies at 5 μg/ml, anti-LAMP1 at 1:1000, anti-cytochrome C at 1:300, and anti-calnexin at 1:2000. Immunofluorescence localization was visualized with the use of a Molecular Dynamics (Sunnyvale, CA) laser confocal imaging system (Beckman Center Imaging Facility, Stanford University).
Electron Microscopy
For the preparation of cryosections, male Wistar rats (∼200 g) were fed a normal diet and fasted overnight. After anesthetization with Nembutal, a whole body perfusion fixation was performed with 2% formaldehyde plus 0.2% glutaraldehyde in 0.1 M phosphate buffer (Posthuma et al., 1988). After fixation, liver and adrenal gland were removed and cut into small blocks that were postfixed in the same fixative overnight at 4°C. Next, the blocks were rinsed with PHEM buffer (60 mM piperazine-N,N′-bis[2-ethanesulfonic acid], 25 mM HEPES, 2 mM MgCl2, 10 mM EGTA, pH 6.9). Tissue was prepared for cryosectioning and immunogold labeling as described (Martinez-Menarguez et al., 1999). Sections were picked up in a 1:1 mixture of methylcellulose and 2.3 M sucrose. In the case of syntaxin 17 labeling, 0.2% uranyl acetate, pH 4, was added. Immunogold labeling for syntaxin 17 was performed with mAb 6A6-3 at a dilution of 1:15 followed by rabbit anti-mouse IgG (DAKO, Glostrup, Denmark) to provide binding sites for protein A–gold. Because multiple secondary antibodies may bind to the primary antibody, this approach can result in a clustered labeling pattern, as is also observed for syntaxin 17. For conventional electron microscopy, a perfusion fixation with 2.5% glutaraldehyde plus 2% formaldehyde in 0.1 M Na-cacodylate buffer, pH 7.4, was performed. The liver was then removed and postfixed in the same fixative for 2 h at room temperature, and additionally in 1% osmium tetroxide (OsO4) plus 1% K-ruthenium-cyanide for 2 h at 4°C. After embedding in Epon, ultrathin sections were prepared and stained with 2% uranyl acetate in Aquadest for 45 min at 63°C.
Membrane Extraction and Glycerol Gradients
Membrane extraction studies of syntaxin 17, syntaxin 6, syntaxin 13, and rsec22b in LC540 Leydig tumor cells were carried out as described previously (Steegmaier et al., 1998). Freshly isolated rat testicle was homogenized and processed as described elsewhere (Steegmaier et al., 1999). Glycerol gradients (11–35%) were prepared and analyzed as described previously (Hay et al., 1997).
Carboxyl-terminal Deletion Mutants of Syntaxin 17 and Transfections
DNA constructs encoding full-length or carboxyl-terminal truncated versions of syntaxin 17 were engineered with an amino-terminal myc epitope tag and subcloned into the mammalian expression vector pcDNA3 (Invitrogen, Carlsbad, CA), with the use of previously described technology (Steegmaier et al., 1998). myc-syntaxin 17Δtail, myc-syntaxin 17ΔTM2nd, and myc-syntaxin 17ΔTM were truncated after amino acids 272, 253, and 227, respectively. NRK and COS-7 cells were maintained and transiently transfected as described previously (Steegmaier et al., 1998).
Immunoprecipitation Experiments and Protein Sequencing
Monoclonal anti-syntaxin 17 (clone 2A9-1) and control mouse antibodies were bound to protein G–Sepharose beads (Amersham Pharmacia Biotech) and cross-linked with dimethylpimidilate (Pierce, Rockford, IL). Membranes from 250 frozen rat testicles (Harlan Bioproducts) were isolated, solubilized with Triton X-100, and used for immunoprecipitation experiments essentially as described (Steegmaier et al., 1999). Precipitates were separated on a 14% SDS-polyacrylamide gel and stained with Coomassie blue. Individual protein bands were cut out and subjected to in-gel proteolysis by trypsin. Aliquots of the digested peptides were subjected to mass spectrometry, and remaining peptide mixtures were fractionated by HPLC and microsequenced as described previously (Hsu et al., 1996). In addition, the identities of the precipitated proteins were verified by Western blot analyses.
RESULTS
Syntaxin 17 Is Abundantly Expressed in Steroidogenic Cells
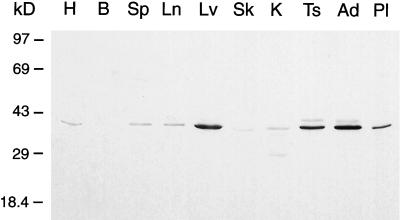
Previously reported Northern blot analysis demonstrated that syntaxin 17 is highly expressed in testis and liver; however, a low level of expression was observed in all other tissues examined (Steegmaier et al., 1998). To extend these observations, we raised a set of mAbs against syntaxin 17. In Western blot analysis on postnuclear supernatants of various tissues, these antibodies recognize a single band of 38 kDa (Figure 1). Preincubation of the mAbs with recombinant syntaxin 17, but not with recombinant syntaxin 1a, eliminated the 38-kDa band on Western blots completely, indicating the specificity of the antibodies (our unpublished results). In addition to the high level of expression of the syntaxin 17 protein in testis and liver, we also found syntaxin 17 abundantly expressed in adrenal gland, placenta, and ovary (Figure 1 and our unpublished results). Although syntaxin 17 is detectable in all other tissues examined, its expression level is drastically reduced compared with that in steroidogenic tissues (Figure 1).
Figure 1.
Syntaxin 17 is abundantly expressed in steroidogenic tissues. Postnuclear supernatants from rat heart (H), brain (B), spleen (Sp), lung (Ln), liver (Lv), skeletal muscle (Sk), kidney (K), testis (Ts), adrenal gland (Ad), and placenta (Pl) (30 μg/lane) were analyzed by Western blotting with the use of the mouse monoclonal anti-syntaxin 17 antibody clone 14C10-2. A major band is detected at 38 kDa, with high expression in liver, testis, adrenal gland, and placenta.
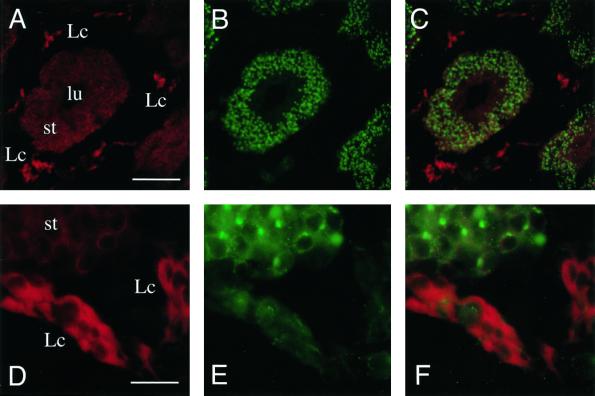
The differential expression of syntaxin 17 in steroidogenic tissues, such as testis and adrenal gland, prompted us to further localize the expression of this membrane-trafficking molecule to determine if it is indeed associated with cell types involved in steroid hormone synthesis. We sectioned rat testicles and immunolabeled the sections with mAbs to syntaxin 17 and affinity-purified antibodies to the ubiquitously expressed vesicle-trafficking protein VAMP4 (Steegmaier et al., 1999). In these sections, high levels of syntaxin 17 immunoreactivity were restricted to the testosterone-secreting Leydig cells, whereas cells of the seminiferous tubules expressed no or much reduced levels of syntaxin 17 (Figure 2, A and C). In contrast, the trans-Golgi network SNARE, VAMP4, is highly expressed in the cells of the seminiferous tubules and shows only moderate expression in Leydig cells (Figure 2, B and C). At higher magnification, it becomes apparent that syntaxin 17 immunoreactivity is distributed throughout the cytoplasmic part of the Leydig cells, whereas anti-VAMP4 immunoreactivity is localized in a small region next to the nucleus in the cells of the seminiferous tubules (Figure 2, D, E, and F).
Figure 2.
Syntaxin 17 is highly expressed in testosterone-synthesizing Leydig cells. Sections of rat testis were labeled with antibodies directed against syntaxin 17 (A and D) and VAMP4 (B and E). (C and F) Overlays of images in A and B and in D and E, respectively. Note that anti-syntaxin 17 antibodies strongly labeled the steroid hormone–secreting Leydig cells (Lc), whereas syntaxin 17 label was drastically reduced in cells of the seminiferous tubules (st) and in the lumen (lu) of the tubules. Bars, 400 μm for A, B, and C (in A) and 100 μm for D, E, and F (in D).
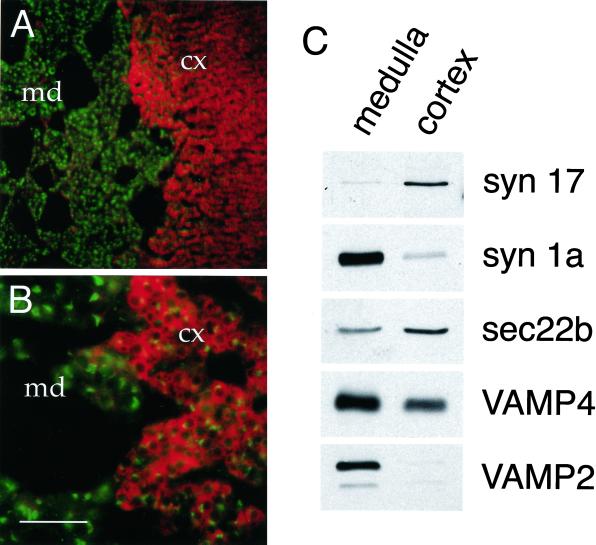
In addition to the gonadal tissues, the adrenal gland is a major site of steroid hormone synthesis in mammalian organisms. Cryosections of rat adrenal gland stained with anti-syntaxin 17 antibodies revealed syntaxin 17 immunoreactivity in the cells of the adrenal cortex, the source of mineralocorticoids and glucocorticoids. Only low or nondetectable syntaxin 17 expression was found in the adrenal medulla, the site of catecholamine synthesis (Figure 3, A and B). VAMP4 expression was detected in both areas of the adrenal gland, with slightly higher expression in the medulla. As observed in Leydig cells, syntaxin 17 immunoreactivity was distributed throughout the cytoplasmic space of adrenal cortical cells (Figure 3, A and B). Bovine adrenal gland was dissected into cortex and medulla, postnuclear supernatant was prepared from both materials, and the expression of different SNARE proteins was analyzed. In agreement with our immunohistochemical observation, we found syntaxin 17 predominantly expressed in the adrenal cortex (Figure 3C). Syntaxin 1a and VAMP2, which are known to function in neurotransmitter release at the synapse as well as in catecholamine release from chromaffin cells, were predominantly expressed in the adrenal medulla (Figure 3C). As observed by our immunohistochemical analysis, VAMP4 was present in postnuclear supernatants of the medulla and the adrenal cortex, with significantly higher expression in the medulla (Figure 3C). Sec22b, a ubiquitously expressed SNARE protein that functions in membrane-trafficking events between the ER and the Golgi apparatus (Hay et al., 1997), was about twofold enriched in cortical postnuclear supernatant (Figure 3C). Interestingly, by immunohistochemical analysis, sec22b was also found enriched in adrenal cortical cells as well as in Leydig cells (our unpublished results).
Figure 3.
Syntaxin 17 is abundantly expressed in steroid hormone–synthesizing cells of the adrenal cortex. (A and B) Sections of rat adrenal gland were double labeled with antibodies directed against syntaxin 17 (Texas Red–conjugated) and VAMP4 (FITC-conjugated). Syntaxin 17 is highly expressed in steroid hormone–synthesizing cells of the adrenal cortex (cx), whereas syntaxin 17 label is drastically reduced in the catecholamine-secreting cells of the adrenal medulla (md). Bars, 400 μm in A and 100 μm in B. (C) Bovine adrenal gland was dissected into cortex and medulla, postnuclear supernatant was prepared, and the expression of different SNARE proteins was analyzed by Western blotting. Labels on the right indicate the antibodies used for immunoblotting.
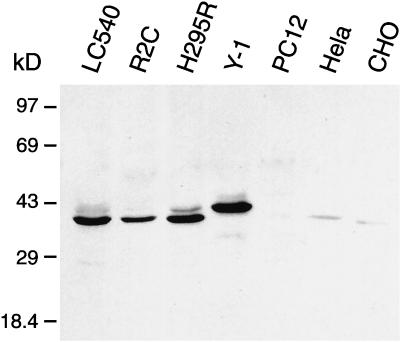
Cell lines derived from Leydig cell tumors such as LC540 and R2C, or cell lines derived from adrenal cortical tumors such as H295R and Y-1, expressed high levels of syntaxin 17. In contrast, nonsteroidogenic cell lines such as PC12, HeLa, and CHO (Chinese hamster ovary) expressed syntaxin 17 at low levels (Figure 4). Because our mAbs do not cross-react with human or mouse syntaxin 17, we used polyclonal goat anti-syntaxin 17 antibodies for these studies. These antibodies recognize a major band of 38 kDa in postnuclear supernatants from LC540, R2C, and H295R cell lines (Figure 4). Interestingly, in the Y-1 adrenocortical cell line, syntaxin 17 has a slightly higher apparent molecular mass. This size difference is attributable to species differences, because Y-1 cells are derived from mouse tissue, whereas LC540 and R2C are derived from rat tissue and H295R is derived from human tissue. Together, our immunohistochemical data and our Western analyses demonstrate that syntaxin 17 is abundantly expressed in steroid hormone secreting cell types.
Figure 4.
Cell lines derived from Leydig cell and adrenal cortical cell tumors express high levels of syntaxin 17. Postnuclear supernatants (30 μg/lane) derived from the cell lines indicated on the top of the panel were separated by SDS-PAGE and immunoblotted with affinity-purified goat anti-syntaxin 17 antibodies. LC540 and R2C are cell lines derived from rat Leydig cell tumor, and H295R and Y-1 are cell lines derived from human and mouse adrenal tumors, respectively. The antibodies recognize a major band of 38 kDa in cell lines of rat or human origin and an ∼40-kDa band in the cell line of mouse origin. Note that only cell lines derived from steroidogenic tissues express high levels of syntaxin 17.
Syntaxin 17 Is Localized to the SER
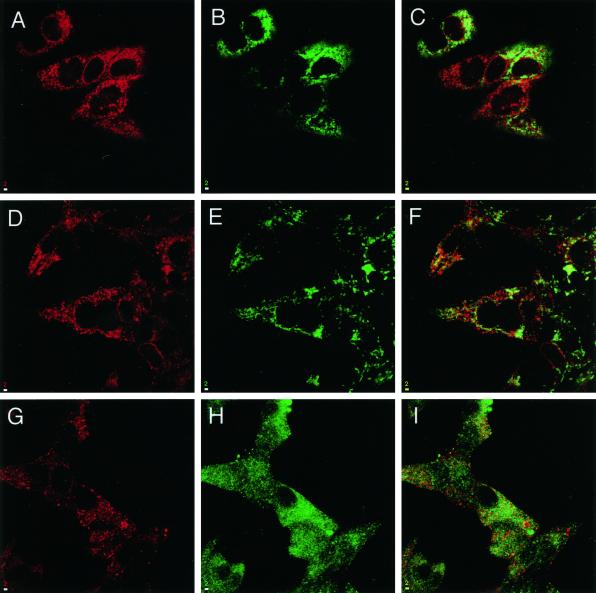
Many enzymes involved in steroid hormone synthesis have been mapped to mitochondrial and microsomal cell fractions. This prompted us to test if syntaxin 17 colocalizes with markers of these compartments. We previously reported that epitope-tagged transiently transfected syntaxin 17 was associated with a tubular membrane compartment, perhaps a subcompartment of the ER (Steegmaier et al., 1998). To extend these studies, we costained syntaxin 17 with markers for mitochondria, lysosomes, RER (Rajagopalan et al., 1994), and intermediate compartment markers in LC540, R2C, and H295R steroidogenic cells (Figure 5 and our unpublished results). However, the staining pattern with anti-syntaxin 17 antibodies did not reveal a pattern similar to that of any of the other markers we tested. Treatment of H295R cells with the fungal metabolite brefeldin A or with the microtubule-depolymerizing drug nocodazole did not result in an alteration of the syntaxin 17 staining pattern (our unpublished results).
Figure 5.
Syntaxin 17 does not colocalize with markers of lysosomes, mitochondria, or RER. H295R adrenal cortical cells were fixed with 4% paraformaldehyde, permeabilized with saponin, and stained with affinity-purified goat anti-syntaxin 17 antibodies (A, D, and G). The cells were costained with mouse mAbs against LAMP1 (B) and cytochrome C (E) and rabbit antibodies against calnexin (H). Texas Red–labeled anti-goat IgG and FITC-labeled anti-mouse and anti-rabbit IgG were used as secondary antibodies. Cells were visualized with confocal microscopy. C, F, and I show merged images. Bars, 2 μm.
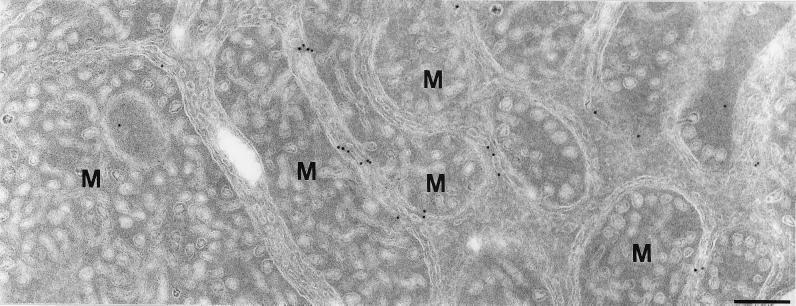
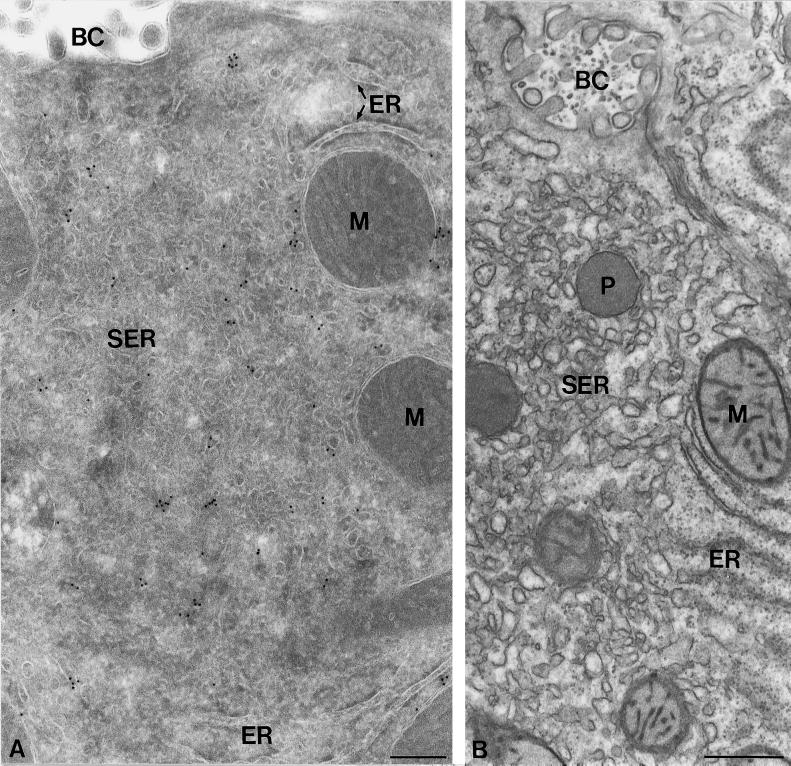
One hallmark of steroidogenic cells is a high abundance of membranes of the SER. To gain a more precise understanding of the subcellular distribution of syntaxin 17 in steroidogenic tissue, we performed cryoimmunogold electron microscopy on two tissues with high levels of expression, the adrenal cortex (Figure 6) and the liver (Figures 7–9). Consistent with the immunofluorescence data, in adrenal cortex syntaxin 17 was found throughout the cytoplasm, where it was present on smooth-surfaced vesicles and tubules of the SER. Mitochondria, with the characteristic tubular cristae, were unstained. Similarly, syntaxin 17 prominently stained large areas of smooth-surfaced SER membranes in rat liver hepatocytes (Figure 7A). Because of the relatively poor preservation of the SER membranes in cryosections, we also visualized SER in conventionally prepared liver sections with the use of OsO4 fixation and Epon embedding (Figure 7B). OsO4 reacts rapidly with unsaturated acyl chains of membrane lipids, resulting in the characteristic dark staining of membranes. However, because OsO4 reduces antigenicity, it is less suitable for immunolabeling protocols. Figure 7, A and B, shows the cellular region adjacent to a bile capillary, which is especially rich in SER.
Figure 6.
Ultrathin cryosection of an adrenal cortical cell. Immunogold labeling of syntaxin 17 (10-nm gold). Syntaxin 17 is present on smooth-surfaced membranes typical of the SER. The appearance of holes in the mitochondria (M) is due to tubular cristae in cross-section. The clustering of gold particles that is seen at some points is due to the labeling method (see MATERIALS AND METHODS) and does not represent local concentrations of syntaxin 17. Bar, 200 nm.
Figure 7.
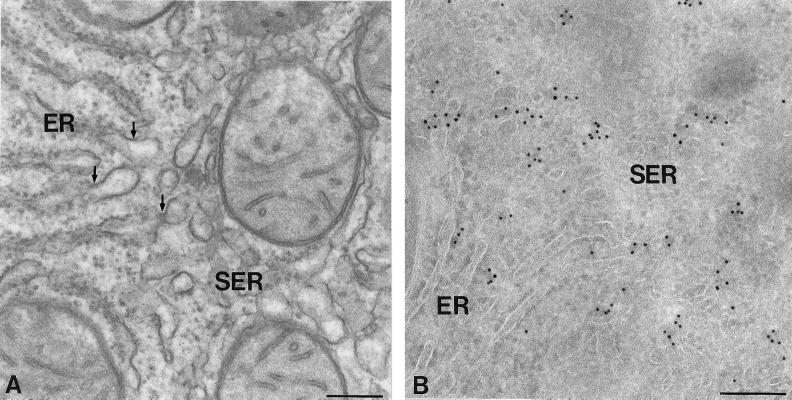
Syntaxin 17 localization in hepatocytes. Ultrathin cryosection (A) and Epon section (B) of rat hepatocytes, both showing the SER-rich region adjacent to a bile capillary (BC). (A) Immunogold label for syntaxin 17 (10-nm gold) is predominantly associated with the SER, whereas RER cisternae (ER) show little or no labeling. (B) The highly convoluted SER membranes are more clearly delineated in Epon sections. M, mitochondrion; P, peroxisome. Bars, 200 nm (A) and 500 nm (B).
Figure 9.
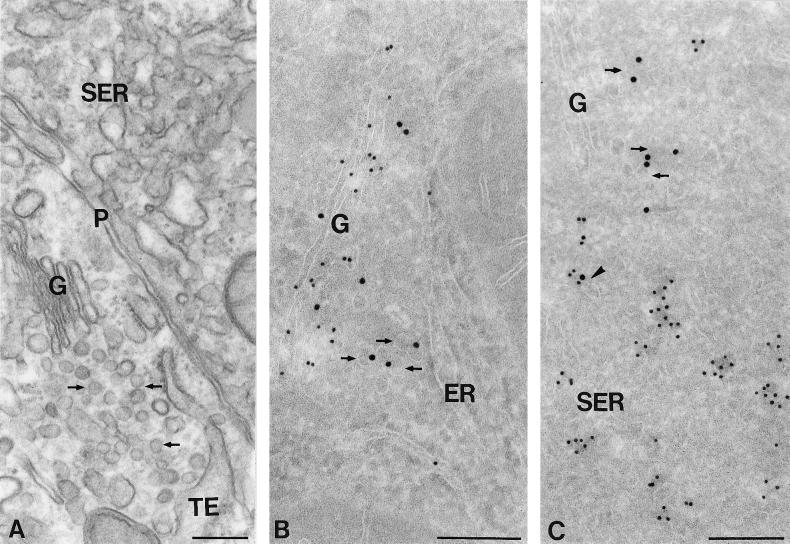
Comparison of SER and VTC membranes in rat hepatocytes. (A) Epon section showing a transitional element (TE) of the ER facing a cluster of VTCs (arrows) that extends up to the Golgi (G). The VTC membranes are morphologically clearly different from the SER membranes shown in the same panel. (B) Cryosection double immunogold labeled for albumin (10-nm gold) and KDELr (15-nm gold) showing the presence of KDELr on VTC membranes between the ER and Golgi (arrows). The Golgi is outlined by the albumin labeling. (C) Cryosection double labeled for syntaxin 17 (10-nm gold) and COPII (15-nm gold). COPII labels some of the VTC membranes between the ER and Golgi (arrows). Syntaxin 17 is only occasionally found on VTC membranes (arrowhead) but strongly labels membranes of the SER. Note that syntaxin 17 is absent from the Golgi. P, plasma membrane. Bars, 200 nm.
In higher-magnification images of the transition between RER and SER, continuities between these two subcompartments are often visible (Figure 8A). Although low-level syntaxin 17 labeling is associated with the RER cisternae, labeling is obviously most prominent in the adjacent SER tubules (Figure 8B). In Epon sections, membranes belonging to VTCs mediating ER-to-Golgi traffic can be distinguished from SER by their smaller size, more electron-dense lumen, and less convoluted appearance (Figure 9A). In cryosections, similarly shaped and located vesicles stained prominently for KDELr (Figure 9B) and COPII (Figure 9C) but only occasionally contained detectable levels of syntaxin 17 (Figure 9C). KDELr and COPII staining were restricted to typical VTC membranes and were not found in SER regions, which extended over much larger areas. Collectively, these electron microscopy data show that syntaxin 17 is predominantly associated with SER membranes.
Figure 8.
The ER-to-SER transition. Ultrathin Epon section (A) and cryosection (B) of rat hepatocytes. (A) Arrows point to continuities between the ER and SER subdomains. (B) Syntaxin 17 (10-nm gold) is predominantly associated with the SER membranes. Bars, 200 nm.
Two Carboxyl-terminal Hydrophobic Domains Are Required to Anchor Syntaxin 17 to Membranes
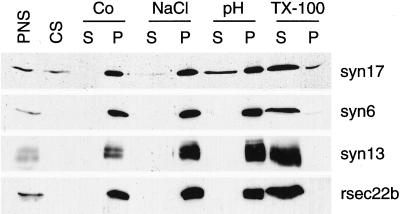
Essentially all syntaxins are anchored in membrane via 20–25 hydrophobic amino acids near the carboxyl terminus. In contrast, syntaxin 17 has a ∼25-amino acid predicted transmembrane domain, followed by a second stretch of 15 hydrophobic amino acids and an additional domain composed of 33 charged and uncharged amino acid residues (Steegmaier et al., 1998). The two hydrophobic domains are separated by a single lysine residue at position 253 (see Figure 11A). Membranes from LC540 Leydig tumor cells were extracted under various conditions and separated into supernatant and pellet fractions. Aliquots of the fractions were separated by SDS-PAGE and immunoblotted with antibodies to syntaxin 17, syntaxin 6, syntaxin 13, and rsec22b (Figure 10). Surprisingly, low levels of syntaxin 17 were present in the cytosolic fraction. A significant fraction of syntaxin 17 was also detected in the cytosol when primary tissue such as liver, testis, or adrenal gland was homogenized and fractionated (our unpublished results). Furthermore, a significant portion of syntaxin 17 was extracted by high pH, and trace amounts of syntaxin 17 were extracted when membranes were treated with 1.5 M NaCl. In contrast, other SNARE proteins, including syntaxins 6 and 13 as well as rsec22b, were not extracted with high pH or with 1.5 M NaCl. When membranes were solubilized with 2% Triton X-100, all of the tested SNARE proteins redistributed to the supernatant. However, a minor fraction of syntaxin 17 was not extracted under this condition.
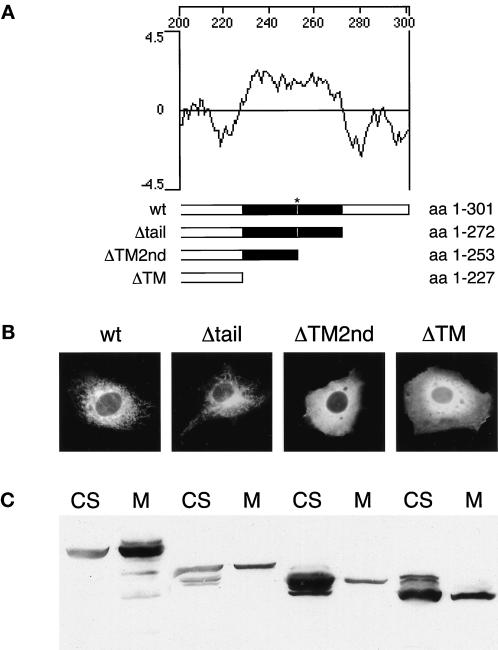
Figure 11.
Two carboxyl-terminal hydrophobic domains are required to anchor syntaxin 17 to membranes. (A) Kyte-Doolittle hydrophobicity plot of the carboxyl-terminal part of syntaxin 17 generated with the DNA-Star program. Shown below are the individual carboxyl-terminal deletion constructs. The putative transmembrane domains (in black) are separated by a single positively charged amino acid (K253, *). (B) NRK cells were transfected with amino-terminally myc-tagged syntaxin 17 full-length protein (amino acids [aa] 1–301; wt), myc–syntaxin 17 deleted of its carboxyl-terminal hydrophilic tail (aa 1–272; Δtail), myc–syntaxin 17 deleted of its carboxyl-terminal hydrophilic tail and its second hydrophobic domain (aa 1–253; ΔTM2nd), or myc–syntaxin 17 deleted of its carboxyl-terminal hydrophilic tail and both hydrophobic domains (aa 1–227; ΔTM). Cells were then fixed, permeabilized, and stained with anti-myc mAb. (C) COS-7 cells were transfected with the same syntaxin 17 deletion constructs as in B. Transfected cells were then fractionated into cytosolic (CS) and postnuclear membrane (M) fractions. Ten micrograms of protein was loaded onto each lane, separated by SDS-PAGE, and immunoblotted with anti-myc antibody. Note that the ratio of cytosolic to membrane-bound syntaxin17ΔTM2nd is dramatically increased compared with the full-length construct.
Figure 10.
Syntaxin 17 is extractable from membranes with high pH. LC540 Leydig tumor cells were homogenized and divided into postnuclear (PNS), cytosolic (CS), and postnuclear membrane fractions. Membrane fractions were extracted with either control buffer (Co), 1.5 M NaCl (NaCl), 0.2 M sodium bicarbonate at pH 11 (pH), or 2% Triton X-100 (TX-100) and centrifuged at 100,000 × g. The resulting supernatants (S) and pellets (P) were analyzed by SDS-PAGE and immunoblotted with the antibodies indicated on the right. Note that only syntaxin 17 and not the other SNARE proteins were extracted from membranes with high pH.
To further investigate the molecular basis underlying the membrane anchoring of syntaxin 17, we generated a set of myc-tagged carboxyl-terminal deletion mutants (Figure 11A). We deleted syntaxin 17's hydrophilic tail (syntaxin17Δtail) or one or both of its hydrophobic domains (syntaxin17ΔTM2nd and syntaxin17ΔTM). We then transiently expressed the deletion constructs in either NRK cells for immunofluorescent analysis or in COS-7 cells for cell fractionation studies. As shown in Figure 11B, full-length epitope-tagged syntaxin 17 localized to tubular structures extending from the perinuclear region to the cytoplasm, as observed previously (Steegmaier et al., 1998). A minor fraction of the epitope-tagged protein, like endogenously expressed syntaxin 17, was found in the cytosol (Figure 11C). When the hydrophilic lumenal tail was deleted, syntaxin 17 still localized to the same tubular membranes, and cell fractionation revealed a cytosolic as well as a membrane pool of syntaxin17Δtail (Figure 11, B and C). Strikingly, when the second carboxyl-terminal stretch of hydrophobic amino acids was deleted in addition to the hydrophilic tail, leaving one intact hydrophobic transmembrane domain on the protein, ∼90% of the syntaxin17ΔTM2nd construct redistributed to the cytosol (Figure 11, A–C). This finding is unexpected, because syntaxin17ΔTM2nd is left with a membrane anchor typical of other syntaxins and these proteins behave as integral transmembrane proteins. As expected, the deletion of both hydrophobic domains in addition to the hydrophilic tail resulted in a predominant cytosolic pool of syntaxin17ΔTM. These data demonstrate that syntaxin 17 does not behave as the other SNARE proteins in that it requires two hydrophobic domains for membrane anchoring. Its extractability by high pH, not observed for the other SNAREs, might be attributed to a unique lipid composition of the tubular membranes of the SER.
Characterization of Syntaxin 17 Complex(es)
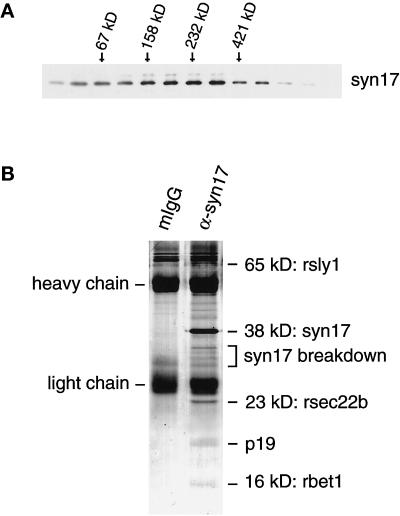
A key step in the understanding of the function of a SNARE protein is the identification of its interacting membrane-trafficking proteins. To determine whether syntaxin 17 exists in high-molecular-mass protein complex(es), soluble Triton X-100 extracts of rat testis were fractionated by velocity sedimentation, and the fractions were immunoblotted for syntaxin 17. As shown in Figure 12A, syntaxin 17 immunoreactivity peaked in fractions corresponding to a molecular mass of ∼160–200 kDa. The entire pool of syntaxin 17 appears to be present in an oligomeric complex(es), because syntaxin 17 is virtually absent from the monomer fraction corresponding to ∼40 kDa.
Figure 12.
The syntaxin 17 complex(es) contains five vesicle-trafficking proteins. (A) Rat testis membrane extract was fractionated by centrifugation through an 11–35% glycerol velocity gradient, and sequential fractions were analyzed by electrophoresis and immunoblotting with antibodies against syntaxin 17. Note that the entire pool of syntaxin 17 is present in an oligomeric complex(es). (B) Rat testis membranes were isolated and solubilized with 1% Triton X-100. The solubilized membranes were incubated with protein G–Sepharose beads loaded with either mouse monoclonal anti-syntaxin 17 antibody or with nonspecific mouse IgG. After the binding step, the beads were washed, eluted with SDS protein sample dye, resolved by SDS-PAGE, and visualized with Coomassie blue. The indicated bands were excised from the gel, digested with trypsin, and analyzed by microsequencing and mass spectrometry. The labels to the right indicate the identities of the precipitated proteins.
Large-scale immunoprecipitation studies from rat testis membrane Triton X-100 extracts that used monoclonal anti-syntaxin 17 antibody were used to obtain quantities of protein suitable for Coomassie blue staining of gels (Figure 12B). In addition to syntaxin 17, we found several other protein bands specific to the immune precipitates. These bands were not coprecipitated with mouse control IgG. Five abundant and specific protein bands were excised, digested with trypsin, and subjected to Edman sequence analysis and mass spectroscopy. From the 65-kDa protein, a peptide sequence was obtained corresponding to a peptide stretch present in rsly1 (amino acids 32–41: MLNFNVPHVK), a member of the Sec1 family of vesicle-trafficking regulatory proteins previously shown to bind to syntaxin 5 (Dascher and Balch, 1996; Hay et al., 1997). Additional proof for the identity of this protein was also obtained by mass spectroscopy, in which 18 peptides (37% protein coverage) had masses predicted from the rsly1 sequence. The 38-kDa protein, predicted to be syntaxin 17, was identified by Western blot analysis and mass spectrometry (our unpublished results). The 23-kDa protein band yielded several peptides that were analyzed by mass spectrometry and identified as rsec22b, another protein that has been found previously in a SNARE complex with syntaxin 5 (Hay et al., 1997). One peptide of the 23-kDa protein was subjected to Edman sequencing, and the sequence obtained was identical to a polypeptide in rsec22b (amino acids 10–21: VADGLPLAASMQ).
The tryptic digests of the two low-molecular-mass bands of 19 and 16 kDa did not yield sufficient material to obtain sequence information by either Edman sequencing or mass spectrometric analysis. However, by Western blot analysis, the 16-kDa band could be identified as rbet1 (our unpublished results). This SNARE protein was previously shown to be a member of the syntaxin 5- and rsec22b-containing SNARE complex that mediates vesicular trafficking at the ER and Golgi apparatus interface (Hay et al., 1997). Although we were unable to identify the 19-kDa protein, it is likely that this protein is a SNARE molecule that contributes the fourth coil to a SNARE complex consisting of syntaxin 17, rsec22b, rbet1, and this 19-kDa SNARE protein.
DISCUSSION
Many species share membrane-trafficking pathways, in part as a result of the highly conserved sets of SNAREs that are found to localize similarly from yeast to mammals. For example, mammalian syntaxin 5 and the yeast Sed5p protein are believed to be functional homologues; both have been shown to act as SNAREs for ER-derived vesicles or tubule fusion events (Hardwick and Pelham, 1992; Bennett et al., 1993; Hay et al., 1997). However, the more complex intracellular architecture and multiple differentiated and specialized tissues of mammals relative to yeast predict the existence of additional mammalian SNAREs. Syntaxin 17 is a very divergent member of the syntaxin family. Sequence comparisons did not reveal a close homology to any of the known yeast SNAREs (Steegmaier et al., 1998). Therefore, it is expected that syntaxin 17 functions in a membrane-trafficking step that is of particular importance in higher organisms and may have no direct counterpart in yeast. Our data demonstrate that syntaxin 17 is abundantly expressed in steroidogenic cell types, such as testosterone-producing Leydig cells or mineralocorticoid- and glucocorticoid-synthesizing cells of the adrenal cortex. Hepatocytes, with their well-established steroidogenic functions, such as cholesterol synthesis and bile acid formation, also express syntaxin 17 abundantly. The most conspicuous feature of a steroidogenic cell is the abundance of smooth-surfaced ER, which occurs as a network of branching tubules (Lenz, 1971). Syntaxin 17 is the first membrane-trafficking protein that we know of that specifically localizes to SER membranes. In fact, to our knowledge, only one other endogenous protein, epoxide hydrolase in liver, has been localized to the SER by immunoelectron microscopy (Galteau et al., 1985). Because syntaxin 17 is present at low levels in nonsteroidogenic cells, perhaps even all cells, we propose that this SNARE plays a critical role in SER membrane-trafficking dynamics and that this trafficking is used abundantly in the synthesis of steroids.
Syntaxin and VAMP proteins are unusual in that, unlike most transmembrane proteins, they lack a signal sequence required for cotranslational insertion into the ER and instead associate with membranes posttranslationally (Kutay et al., 1995). Unlike any other known syntaxin or VAMP protein, syntaxin 17 requires two adjacent hydrophobic domains near its carboxyl terminus for proper membrane anchorage. In spite of these two hydrophobic domains, syntaxin 17, but not other SNAREs, is extractable by high-pH treatment, suggesting that the smooth-surfaced ER tubules have a different lipid composition from other membranes in the cell. Consistent with this speculation, we found that membranes labeled by anti-syntaxin 17 antibodies were less well preserved than other intracellular membranes in the cryosections we used for immunogold electron microscopy. Because the SER harbors many enzymes involved in lipid and steroid synthesis or modification, it is likely that these membranes are enriched in distinct classes of lipids and steroids. We postulate that the unique transmembrane domain topology of syntaxin 17 is an adaptation to target and anchor the protein into the SER.
Our biochemical data demonstrate that syntaxin 17 is in a complex(es) with SNAREs and a sec1p homologue important for ER-to-Golgi transport. We found syntaxin 17 associated with rsly1, a sec1 homologue also found to complex with syntaxin 5 (Dascher and Balch, 1996; Hay et al., 1997). We suggest that, by analogy to syntaxin1A and nsec1, the syntaxin 17/rsly1 complex contains the closed conformation of syntaxin 17 and is not able to interact with other proteins to form a SNARE fusion complex. If this is the case, then syntaxin 17 must also be present in a second distinct complex, a SNARE complex, containing rsec22b, rbet1, and p19.
What is the trafficking step(s) and fusion event mediated by syntaxin 17? rsec22b and rbet1 have been shown to largely reside on VTC elements as they cycle between peripheral and Golgi-adjacent locations mediating vesicular transport between the ER, the intermediate compartment, and the cis-Golgi (Zhang et al., 1997; Hay et al., 1998). The cis-Golgi and intermediate compartment vesicle receptor syntaxin 5 were previously shown to form a SNARE complex with rbet1, rsec22b, and an additional 25-kDa SNARE protein, membrin (Hay et al., 1997, 1998). The SNARE complex presented in this work does not contain syntaxin 5 or membrin, as determined by immunoblotting (our unpublished results). One interpretation of our data is that the syntaxin 17–containing SNARE complex purified from rat testis represents a functional vesicle-trafficking complex mediating transfer reactions between VTC elements and membranes of the SER. Perhaps in steroidogenic cells, syntaxin 17 and p19 act as vesicle receptors at the SER, whereas rbet1 and rsec22b represent trafficking proteins derived from the donor compartment. Syntaxin 17 may mediate the transport of proteins and/or steroids/lipids needed for steroidogenesis between the intermediate compartment and the SER. An example of this cargo could be newly synthesized steroidogenic enzymes synthesized at the RER and then transported to VTCs and, subsequently, by a syntaxin 17–dependent shuttling event, to the SER. The continuity between the RER and SER we observed by electron microscopy would be another route for these enzymes. We also envision that sterol precursors needed for steroid hormone synthesis are taken up by a receptor-mediated mechanism and travel through VTC elements on their way to the SER, where they become modified. Alternatively, the syntaxin 17 SNARE complex could mediate transport of lipoprotein particles from the SER to the intermediate compartment/VTCs. Finally, syntaxin 17 could be involved in controlling the extent of this ER subcompartment by allowing vesicles budding from membranes of VTCs to fuse with preexisting SER structures. Such a control mechanism would allow an organism to adapt to certain environmental changes or demands, such as increasing the level of steroid hormone secretion or the up-regulation of bile acid formation.
Regulation of the composition of the overlapping syntaxin 5 and syntaxin 17 complexes is likely to involve a series of regulatory events upstream of SNARE complex formation. Although little is known of these regulatory events, it is likely that Rab proteins and their effectors are important components. With syntaxin 17 as a starting point, further studies should better define the function and dynamics of the SER.
ACKNOWLEDGMENTS
We thank R. Prekeris and D. Foletti for many insightful discussions and their technical help. We thank Dr. G. Posthura (Utrecht University, Utrecht, The Netherlands) for excellent help with the perfusions for electron microscopy and Rene Scriwaneck and Tom Van Rijn for preparation of electron micrographs. We also thank R. Winant of the Stanford University PAN facility for amino acid sequencing.
REFERENCES
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bock JB, Klumperman J, Davanger S, Scheller RH. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1997;8:1261–1271. doi: 10.1091/mbc.8.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Scheller RH. SNARE proteins mediate lipid bilayer fusion. Proc Natl Acad Sci USA. 1999;96:12227–12229. doi: 10.1073/pnas.96.22.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands R, Slot JW, Geuze HJ. Albumin localization in rat liver parenchymal cells. Eur J Cell Biol. 1983;32:99–107. [PubMed] [Google Scholar]
- Dascher C, Balch WE. Mammalian Sly1 regulates syntaxin 5 function in endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:15866–15869. doi: 10.1074/jbc.271.27.15866. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- Galteau MM, Antoine B, Reggio H. Epoxide hydrolase is a marker for the smooth endoplasmic reticulum in rat liver. EMBO J. 1985;4:2793–2800. doi: 10.1002/j.1460-2075.1985.tb04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez L, Jr, Scheller RH. Regulation of membrane trafficking: structural insights from a Rab/effector complex. Cell. 1999;96:755–758. doi: 10.1016/s0092-8674(00)80585-1. [DOI] [PubMed] [Google Scholar]
- Halachmi N, Lev Z. The Sec1 family: a novel family of proteins involved in synaptic transmission and general secretion. J Neurochem. 1996;66:889–897. doi: 10.1046/j.1471-4159.1996.66030889.x. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Pelham HR. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JC, Chao DS, Kuo CS, Scheller RH. Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell. 1997;89:149–158. doi: 10.1016/s0092-8674(00)80191-9. [DOI] [PubMed] [Google Scholar]
- Hay JC, Hirling H, Scheller RH. Mammalian vesicle trafficking proteins of the endoplasmic reticulum and Golgi apparatus. J Biol Chem. 1996;271:5671–5679. doi: 10.1074/jbc.271.10.5671. [DOI] [PubMed] [Google Scholar]
- Hay JC, Klumperman J, Oorschot V, Steegmaier M, Kuo CS, Scheller RH. Localization, dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs. J Cell Biol. 1998;141:1489–1502. doi: 10.1083/jcb.141.7.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- Herrmann JM, Malkus P, Schekman R. Out of the ER: outfitters, escorts and guides. Trends Cell Biol. 1999;9:5–7. doi: 10.1016/s0962-8924(98)01414-7. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Ting AE, Hazuka CD, Davanger S, Kenny JW, Kee Y, Scheller RH. The mammalian brain rsec6/8 complex. Neuron. 1996;17:1209–1219. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- Inoue A, Obata K, Akagawa K. Cloning and sequence analysis of cDNA for a neuronal cell membrane antigen, HPC-1. J Biol Chem. 1992;267:10613–10619. [PubMed] [Google Scholar]
- Kutay U, Ahnert-Hilger G, Hartmann E, Wiedenmann B, Rapoport TA. Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane. EMBO J. 1995;14:217–223. doi: 10.1002/j.1460-2075.1995.tb06994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz TL. Cell Fine Structure: An Atlas of Drawings of Whole-Cell Structure. Philadelphia: WB Saunders; 1971. [Google Scholar]
- Lin RC, Scheller RH. Structural organization of the synaptic exocytosis core complex. Neuron. 1997;19:1087–1094. doi: 10.1016/s0896-6273(00)80399-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Menarguez JA, Geuze HJ, Slot JW, Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- Matlack KE, Mothes W, Rapoport TA. Protein translocation: tunnel vision. Cell. 1998;92:381–390. doi: 10.1016/s0092-8674(00)80930-7. [DOI] [PubMed] [Google Scholar]
- Meldolesi J, Pozzan T. The heterogeneity of ER Ca2+ stores has a key role in nonmuscle cell signaling and function. J Cell Biol. 1998;142:1395–1398. doi: 10.1083/jcb.142.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu SC, Braun JE, Calakos N, Ting AE, Bennett MK, Scheller RH. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol. 1999;1:E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- Poirier MA, Xiao W, Macosko JC, Chan C, Shin Y-K, Bennett MK. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- Posthuma G, Slot JW, Veenendaal T, Geuze HJ. Immunogold determination of amylase concentrations in pancreatic subcellular compartments. Eur J Cell Biol. 1988;46:327–335. [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Xu Y, Brenner MB. Retention of unassembled components of integral membrane proteins by calnexin. Science. 1994;262:387–390. doi: 10.1126/science.8278814. [DOI] [PubMed] [Google Scholar]
- Sitia R, Meldolesi J. Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol Biol Cell. 1992;3:1067–1072. doi: 10.1091/mbc.3.10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmaier M, Klumperman J, Foletti DL, Yoo J-S, Scheller RH. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1999;10:1957–1972. doi: 10.1091/mbc.10.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmaier M, Yang B, Yoo J-S, Huang B, Shen M, Yu S, Luo Y, Scheller RH. Three novel proteins of the syntaxin/SNAP-25 family. J Biol Chem. 1998;274:34171–34179. doi: 10.1074/jbc.273.51.34171. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wong SH, Tang BL, Xu Y, Peter F, Subramaniam VN, Hong W. The mammalian protein (rbet1) homologous to yeast Bet1p is primarily associated with the pre-Golgi intermediate compartment and is involved in vesicular transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1997;139:1157–1168. doi: 10.1083/jcb.139.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]