Abstract
OBJECTIVE
To investigate via the vitamin D status whether patients with peripheral arterial disease (PAD) tend to develop vitamin D deficiency that in turn influences their clinical symptoms.
DESIGN
Cross-sectional.
SETTING
University hospital.
PATIENTS AND PARTICIPANTS
Three hundred twenty-seven patients were evaluated; subjects with secondary causes of bone disease or bone active medication were excluded. One hundred sixty-one patients with either PAD stage II (n = 84) or stage IV (n = 77) were enrolled and compared to 45 age- and sex-matched healthy controls.
MEASUREMENTS AND MAIN RESULTS
All patients underwent determinations of serum chemistry, 25-hydroxyvitamin D (vitamin D3) intact parathyroid hormone (iPTH), alkaline phosphatase (ALP), and osteocalcin and were further stratified according to an individual restriction score into 3 groups: mildly, moderately, or severely restricted in daily life due to the underlying disease. Patients with PAD IV showed significantly lower vitamin D3 (P = .0001), and calcium (P = .0001) values and significantly higher iPTH (P = .0001), osteocalcin (P = .0001) and ALP (P = .02) levels as compared to patients with PAD II. Patients considering themselves as severely restricted due to the underlying disease showed lower vitamin D3 and higher iPTH levels than those who described only a moderate (vitamin D3: P < .001; iPTH: P < .01) or mild (vitamin D3: P < .001; iPTH: P < .001) restriction in daily life.
CONCLUSION
Patients with PAD IV, especially those who feel severely restricted due to the disease, are at high risk of developing vitamin D deficiency, secondary hyperparathyroidism, and ultimately osteomalacia due to immobilization and subsequent lack of exposure to sunlight, all of which in turn lead to further deterioration. Monitoring of vitamin D metabolism and vitamin D replacement therapy could be a simple, inexpensive approach to mitigating clinical symptoms and improving quality of life in patients with advanced PAD.
Keywords: vitamin D3, secondary hyperparathyroidism, osteomalacia, immobilization, peripheral arterial disease
Pain is the main clinical complaint of patients suffering from peripheral arterial disease (PAD). Intermittent claudication is typical for the disease, with pain in the calves and/or thighs during exercise that is relieved with rest, and/or pain caused by local ulcers. In addition to pain, patients frequently complain of muscle weakness with difficulty in rising from a sitting position or climbing stairs, along with paresthesia, arthralgia, fatigue, and deep bone pain.
These symptoms are less typical for PAD than for osteomalacia and vitamin D deficiency,1–8 which is commonly defined by decreased serum 25-hydroxyvitamin D (vitamin D3) levels below 9 ng/mL (22 nmol/L).7–10 There is evidence that even individuals with serum 25-hydroxyvitamin D levels less than 20 ng/mL7 or 50 nmol/L8 show increased serum PTH levels,7,8 indicating that the definition of vitamin D deficiency should perhaps be broadened.8 Although osteomalacia is a histomorphometric diagnosis, individuals with serum 25-hydroxyvitamin D levels below 5 ng/mL are at high risk of developing such a deficiency.7,9
Vitamin D deprivation has been frequently described in elderly populations.11–21 The major source of vitamin D is the skin. Because only approximately 15% to 20% of vitamin D is derived from food22–26 and more than 80% of the vitamin D requirement is synthesized in the skin, lack of exposure to sunlight is an important risk factor for developing vitamin D deficiency, secondary hyperparathyroidism, and ultimately, osteomalacia.13,14,16,21,27–32 Other causes of vitamin D deficiency are renal diseases, malnutrition, malabsorption, hepatic failure, and drugs influencing vitamin D and calcium metabolism.10,33,34 With increasing age, the capacity of the skin to synthesize and store vitamin D3 decreases, as do reabsorption in the kidney and absorption in the gut. In PAD patients, immobilization due to the underlying disease could be an important risk factor for the development of vitamin D deficiency even in the absence of any additional secondary causes of bone disease.
The aim of this study was to determine the prevalence of vitamin D deficiency and secondary hyperparathyroidism in patients with confirmed PAD in relation to severity and duration of the disease and a subjective score of restriction due to PAD.
PATIENTS AND METHODS
Approximately 50 patients per day are referred to our angiologic outpatient clinic by their primary health care physicians. On average, 80% of these patients have established PAD and undergo angiography, bypass surgery, or percutaneous transluminal angioplasty; they are included in an aftercare program with regular ultrasound measurements, laboratory analysis, and clinical examination.
Between December 1999 and March 2000, a total of 327 patients with either PAD stage II (pain in calves and/or thighs only during exercise, relieved by rest) or PAD stage IV (history of, or presence of local ulcers) were evaluated. Clinical diagnosis was always confirmed by angiography. Sixty-six patients were excluded because of impaired renal function, gastrointestinal diseases, or special diets. A further 99 patients were excluded because of drugs with known influence on calcium and vitamin D metabolism (heparin, cumarine, loop diuretics, statins, prostaglandins, magnesium, calcium, vitamin D, hormone replacement therapy, or osteoprotective therapy). One woman was excluded due to hypercalcemia.
The remaining 161 patients (64 women, 97 men) were assigned, according to the clinical stage of disease, to either group A: PAD stage II (n = 84; 29 females and 55 males) with history of intermittent claudication, or to group B: PAD stage IV (n = 77; 35 females and 42 males) with local ulcers current or past. Patients with PAD stage I or stage III were not included in this study because PAD I is an angiographical diagnosis without clinical symptoms and stage III usually requires prompt intervention either conservatively with prostaglandins or with percutaneous transluminal angioplasty. Stage III is thus transient and patients either progress to stage IV or improve enough to be reclassified as PAD II. Vitamin D storage lasts for a few months. Patients with a history of shorter duration may therefore still have “full vitamins D stores” and may misrepresent the analysis (See also DISCUSSION.)
All patients were ambulatory, white, and willing to answer a questionnaire concerning duration of disease, comorbidities, comedication, clinical symptoms, and subjective restriction (feeling mildly, moderately, or severely handicapped in daily life due to the disease). Retrospectively, patients were stratified into 3 categories according to the duration of the disease—up to 1 year, between 1 and 2 years, or longer than 2 years.
As controls, 45 age- and sex-matched healthy subjects from the same geographical area were invited to undergo laboratory analysis of serum chemistry, vitamin D3 and intact parathyroid hormone (iPTH). These individuals were recruited for a nationwide study by articles in local newspapers which invited the general public to undergo evaluation of vitamin D status and bone metabolism at 1 of 5 centers in Austria. A total of 1,350 healthy people were investigated, and these data are currently being analyzed.
All patients and healthy volunteers gave written informed consent to participate in this study.
Laboratory Analysis
In addition to the routine laboratory blood parameters, including serum chemistry and alkaline phosphatase (ALP), blood count, lipid profile, and immunologic parameters, an additional vial for later analysis of iPTH, osteocalcin and vitamin D3 was collected. All blood samples were drawn between 8 and 10 am after an overnight fast. The vial for special analysis was immediately cooled, centrifuged at +4°C, aliquoted, and frozen at −80°C until further analysis.
Routine serum parameters were measured by autoanalyzer (Hitachi 747; Roche Diagnostics, Vienna, Austria). Osteocalcin and iPTH were analyzed using a chemoluminescence immunometric assay (Nichols Diagnostics, San Juan Capistrano, Calif), and vitamin D3 was measured by radioimmunoassay following extraction. The intra-assay precision was between 5% and 6.3% and the interassay precision between 7.3% and 8.2% (Immunodiagnostic Systems, Boldon, U.K.). The measurements were performed in undiluted samples according to the manufacturers' instructions. All samples were measured in duplicate and averaged. If the coefficient of variation was greater than 10%, the sample was re-assayed.
The definition of vitamin D deficiency was based on serum 25-hydroxyvitamin D levels below 9 ng/mL (22 nmol/L) according to Thomas et al.10
All blood samples were collected in wintertime, when vitamin D3 concentrations are lowest.35–37
Statistical Analysis
All data are presented as mean ± SE unless otherwise indicated. All data were checked for normality using the Kolmogorov-Smirnov test. For between-group comparisons either the unpaired Student's t test or χ2 test was used. Analysis of variance was followed by Fisher's multiple comparison test to distinguish differences in variables between the groups. Simple linear regression calculations were used to analyze the correlation between the various parameters. All statistical analyses were performed using the statistical software package STATVIEW 4.0 (Abacus Concepts Inc., Berkeley, Calif).
RESULTS
Age and gender distribution were statistically comparable in all groups. Median ages 69 ± 2 year for Group A, 66 ± 1 year for Group B, and 66 ± 1 year for the Control Group. Females comprised 35% (n = 29) of the patients in Group A, 45% (n = 35) in Group B, and 48% (n = 21) in the Control Group.
Patients with PAD IV (Group B) showed significantly lower vitamin D3 levels as compared to Group A (P = .0001), which was paralleled by a decreased serum calcium level (P = .0001). Intact parathyroid hormone (P = .0001), OC (P = .0001) and ALP levels (P = .02) were significantly higher than in Group A patients. There was no significant difference in kidney or liver function, serum magnesium, or albumin levels between patients in Groups A and B (see Table 1).
Table 1.
Biochemical Characteristics of the PAD Groups and Controls
| Group A (PAD II) ±SE | Group B (PAD IV) ±SE | Control Group ±SE | |
|---|---|---|---|
| Serum creatinine (0.6–1.3 mg/dL) | 0.95 ± 0.02 | 0.93 ± 0.02 | 0.96 ± 0.02 |
| Gamma glutamyl transferase (<29 U/L) | 35 ± 1 | 33 ± 1 | 34 ± 3 |
| Serum magnesium (0.7–1.1 mmol/L) | 0.92 ± 0.01 | 0.89 ± 0.02 | 0.91 ± 0.04 |
| Albumin (3.5–5.3 g/dL) | 4.5 ± 0.05 | 4.4 ± 0.05 | 4.3 ± 0.07 |
| Serum phosphate (2.6–4.5 mg/dL) | 3.38 ± 0.06 | 3.31 ± 0.06 | 3.34 ± 0.09 |
| Total serum calcium (2.2–2.65 mmol/L) | 2.46 ± 0.01 | 2.37 ± 0.02*,‡ | 2.45 ± 0.04 |
| Alkaline phophatase (55–170 U/L) | 102 ± 2 | 111 ± 3†,‡ | 97 ± 9 |
| Osteocalcin (1–35 ng/mL) | 15 ± 0.6 | 24.7 ± 1.6* | NA |
| Vitamin D3 (9–45 ng/mL) | 23.4 ± 1.4 | 9.4 ± 1*,‡ | 20.5 ± 1.2 |
| Intact PTH (10–65 pg/mL) | 30.7 ± 1.3 | 55.8 ± 3*,‡ | 34.2 ± 2 |
To convert values of intact parathyroid hormone (iPTH) into picomoles per liter multiply by 0.11; to convert 25-hydroxyvitamin D (vitamin D3) levels into nanomoles per liter, multiply by 2.5; to convert values for alkaline phosphatase (ALP) and gamma glutamyl transferase into microkatals per liter, multiply by 0.0167; to convert creatinine into micromoles per liter, multiply by 88.4; to convert serum phosphate into mmol/L, multiply by 0.3229.
P = .0001 vs group A.
P = .05 vs. group A.
P = .0001 vs control group.
NA, not applicable; PAD, peripheral arterial disease.
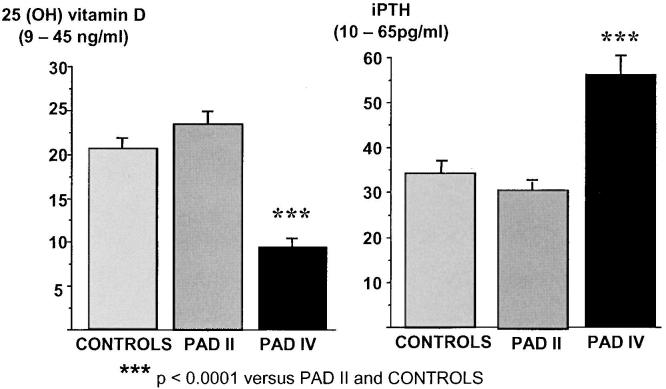
Comparing the patient groups to an age- and sex-matched control group, no differences in vitamin D status and iPTH levels could be found in relation to patients with PAD II (Group A). PAD IV (Group B) patients, however, had significantly lower vitamin D3 (P = .0001) and higher iPTH levels (P = .0001) than the Control Group (See Fig. 1, Table 1).
FIGURE 1.
Comparisons of the mean vitamin D3 (25-hydroxyvitamin D) and intact parathyroid hormone (iPTH) levels between Group A, Group B, and the Control Group. Patients with peripheral arterial disease (PAD) IV had highly significant lower vitamin D3 and significantly higher iPTH levels as compared to the Control Group and to patients with PAD II.
More than 71% (n = 55) of Group B patients had serum vitamin D3 levels below 9 ng/mL, 13% (n = 10) had serum calcium levels below the normal range, and 40% (n = 31) had iPTH levels above the upper limit of normal, indicating secondary hyperparathyroidism. About one third of vitamin D–deficient individuals in this group (31%, n = 17) showed serum vitamin D3 levels below 5 ng/mL, indicating that these patients could be at high risk for developing osteomalacia.7,9
Serum vitamin D3 showed a significant negative correlation with iPTH(r = −.42; P =.0001) and osteocalcin (r = −.24; P = .002). There was no correlation to age, creatinine, calcium, magnesium, phosphate or ALP.
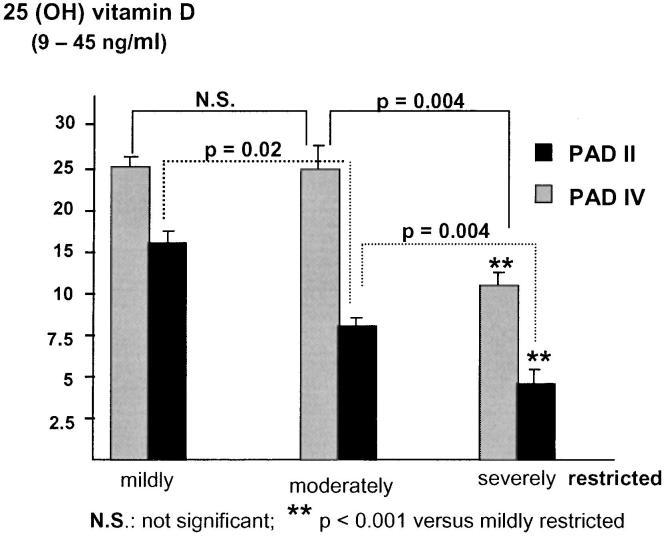
According to an individual restriction score, patients were further categorized into 3 groups: mildly, moderately, or severely handicapped in daily life due to claudication or pain caused by the disease. We found a significant difference in vitamin D status, iPTH, and serum calcium levels between the 3 groups (Table 2). Patients describing themselves as severely handicapped had lower mean vitamin D3 and calcium levels and higher iPTH levels than those who hardly felt disabled (Table 2). These differences in vitamin D3 levels were consistent not only for the whole study population but also when patients were stratified according to their stage of disease (Fig. 2); more patients with PAD IV described themselves as severely handicapped than did patients with PAD II (Table 3). The differences in vitamin D3 levels remained significant between grade II and grade IV PAD patients throughout all 3 individual restriction score categories.
Table 2.
Comparison Between PAD Patients Stratified into 3 Groups According to Their Subjective Feeling of Restriction
| Individual Restriction Score | Mildly Restricted (A) ±SE | Moderately Restricted (B) ±SE | Severely Restricted (C) ±SE | P Values |
|---|---|---|---|---|
| Age, y | 68 ± 1.3 | 68 ± 1.2 | 66 ± 1.2 | |
| Group C versus B | NS | |||
| Group C versus A | NS | |||
| Group B versus A | NS | |||
| Vitamin D3 (9–45 ng/mL) | 23 ± 1.9 | 17.7 ± 1.7 | 8.7 ± 0.8 | |
| Group C versus B | .0001 | |||
| Group C versus A | .0001 | |||
| Group B versus A | .05 | |||
| Serum calcium (2.2–2.65 mmol/L) | 2.40 ± 0.02 | 2.41 ± 0.02 | 2.40 ± 0.02 | |
| Group C versus B | NS | |||
| Group C versus A | NS | |||
| Group B versus A | NS | |||
| Intact PTH (10–65 pg/mL) | 35.8 ± 3 | 39.7 ± 2.3 | 52.3 ± 3.8 | |
| Group C versus B | .01 | |||
| Group C versus A | .001 | |||
| Group B versus A | NS | |||
| Osteocalcin (1–35 ng/mL) | 17.1 ± 1.4 | 18.2 ± 1.1 | 24 ± 2.2 | |
| Group C versus B | .01 | |||
| Group C versus A | .01 | |||
| Group B versus A | NS |
To convert values of intact parathyroid hormone (iPTH) into picomoles per liter, multiply by 0.11; to convert 25-hydroxyvitamin D (vitamin D3) levels into nanomoles per liter, multiply by 2.5.
NS, not significant.
FIGURE 2.
Vitamin D3 (25-hydroxyvitamin D) serum levels in relation to the patient's individual restriction score.Patients with a subjective feeling of being severely immobilized and restricted in personal life had significantly lower vitamin D3 levels as compared to those who described themselves as only moderately or mildly handicapped. Throughout the 3 groups, it was evident that patients with PAD IV (black columns) had significantly lower vitamin D3 levels compared to PAD II patients (gray columns).
Table 3.
Distribution of Study Patients According to the Stage of Disease (PAD II/PAD IV) as Well as to Their Individual Restriction Score
| Restriction | Mild, n (%) | Moderate, n (%) | Severe, n (%) |
|---|---|---|---|
| Total group | 50 (31) | 63 (39) | 48 (30) |
| PAD II | 36 (43) | 33 (39) | 15 (18) |
| PAD IV | 14 (18) | 30 (39) | 33 (43) |
PAD, peripheral arterial disease.
When patients were stratified according to the duration of the disease in categories of up to 1 year, between 1 and 2 years, and longer than 2 years, there were no significant between-category differences in any of the parameters investigated.
DISCUSSION
We found a strikingly high prevalence of vitamin D deficiency paralleled by secondary hyperparathyroidism in patients with peripheral arterial disease. It appears to us that vitamin D deficiency is an underestimated complication in patients with PAD.
Impaired vitamin D metabolism is accompanied by insufficient intestinal calcium absorption and renal calcium loss, resulting in secondary hyperparathyroidism and, ultimately, osteomalacia.7,9 Symptoms typical for osteomalacia, such as arthralgia, paresthesia, fatigue, muscle pain and weakness, deep bone pain, and difficulties in walking, are frequently described in these patients and contribute significantly to the clinical picture of PAD. Common causes of vitamin D deficiency, including impaired renal function, malnutrition, malabsorption, hepatic failure, or bone-active drugs9,10,26,34 were excluded from our study. Nevertheless, our population was an elderly one, and age is associated with decreasing vitamin D3 synthesis and storage in the skin as well as decreased absorption in the gut and kidneys. It is, however, well known that restricted mobility with subsequent lack of sunlight exposure leads to vitamin D deficiency, even in patients with normal renal function.13,14,16,22,33,34,38–40
Patients with PAD, especially those with local ulcers, are frequently immobilized by pain and may become socially isolated. The lack of sunlight exposure caused by partial or total immobility would be a reasonable explanation for the deficiency or inadequacy of vitamin D even in the absence of renal or liver disease or bone-active medication. On the basis of this hypothesis, we were able to show significantly lower vitamin D3 and serum calcium levels in patients with PAD grade IV than in patients with PAD grade II or control subjects. It must be pointed out that there were no differences when the latter 2 groups were compared to each other. Vitamin D deficiency was found in more than 70% of PAD grade IV patients, of which osteomalacia could be assumed in 31%, with serum vitamin D3 levels even below 5 ng/mL. Even though vitamin D deficiency is common in the elderly, the magnitude of the problem in PAD patients was surprising. That we found no correlation of vitamin D3 to the patients' age in the study population may be due to the narrow age range of our patient group.
We found elevated iPTH values in patients with decreased serum vitamin D3 levels, as previously described.10,41 The prevalence of secondary hyperparathyroidism in our study was only 40% in Group B patients, a relatively low value despite a high prevalence of vitamin D deficiency (71%). Chapuy et al. described an increased iPTH level in virtually all elderly subjects investigated despite a prevalence of hypovitaminosis D of only 39%, but they studied only older women and did not exclude those with impaired renal function.41 In contrast, Thomas et al. described a relatively low percentage of severe vitamin D deficiency (<9 ng/mL) of 22% in younger medical inpatients, although many of the patients had impaired renal or liver function or co-medications with known influence on vitamin D metabolism.10 The discrepancy between this study and our findings may be due to the fact that those patients were younger and hospitalization only lasted for a few days—not long enough to deplete vitamin D stores that are likely to be higher in these patients than in ours, because their patients were younger and mobile prior to hospitalization. Another possible explanation may be the fact that fortified milk or dairy products are not available in Austria.
Surprisingly, the duration of the disease had no influence on vitamin D status in our patients. There is some evidence that vitamin D depletion occurs within a relatively short time.16,20 We included only patients with a PAD history of at least 6 months. After this time, vitamin D depletion already seems to be established.
The individual disability score was a major determinant of vitamin D status in our study. Bischoff et al. described a decrease of vitamin D3 combined with elevated bone resorption markers with increasing immobility in a population of residents of a geriatric ward stratified by a grade 4 mobility score.42 In another study, patients with a high personal outdoor score had higher vitamin D3 and lower iPTH levels than more-or-less homebound individuals.41 Our patients were categorized in a similar fashion and were asked to quantify their personal assessment of being restricted and housebound by their disease. Subjects who felt severely disabled had significantly lower vitamin D3 and higher serum iPTH levels than those who described themselves as only mildly restricted, regardless of the stage of PAD. This finding again supports our hypothesis that lack of sunlight exposure is the key factor for the development of vitamin D3 deficiency. As mobility decreases, the problem increases, since vitamin D deficiency aggravates fatigue, muscle weakness, and bone pain. In parallel, PAD progresses with decreasing mobility and lack of exercise, creating a vicious cycle from which patients cannot escape. Another interesting aspect could be that vitamin D–deficient individuals may be at high risk of developing PAD due to fatigue, pain, and the consequent lack of exercise.
A low serum vitamin D3 concentration is not a simple biochemical abnormality. Pfeifer et al. have described how hypovitaminosis D is associated with increased body sway and an elevated risk for falls and fall-related fractures.43 Because vitamin D3 is required for calcium homeostasis, secondary hyperparathyroidism may develop in patients with vitamin D deficiency who are on a normal- or low-calcium diet. With increasing severity of vitamin D deficiency and secondary hyperparathyroidism, patients may progress from initially increased bone turnover to greatly impaired mineralization with generalized osteomalacia.
We found significantly higher osteocalcin levels in patients with PAD grade IV as compared to Group A, indicating increased bone turnover. The present study is of course limited by the absence of measurements of resorption markers and/or bone density. Nonetheless, surrogates of bone metabolism, such as ALP, the most commonly used marker for hypovitaminosis D osteopathy,12,44,45 as well as OC as a further parameter for this condition,46 were significantly higher in patients in Group B than in those in the other groups. A higher prevalence of osteopathy is therefore likely in at least some of these patients. Falls and bone fractures may also aggravate the clinical condition of PAD patients. Further studies are needed to elucidate whether the biochemical findings in our study would also document low bone mass, osteoporosis, and increased fracture incidence in PAD.
There is evidence that vitamin D–deficient patients profit from simple vitamin D3 substitution47–49 and, after a few months of substitution, muscle strength improves remarkably.44,45,48,49 There is evidence that calcium and vitamin D3 substitution significantly reduce the risk of falls and therefore the incidence of bone fractures.49–53
The magnitude of vitamin D depletion in our patients justifies regular and routine monitoring of vitamin D status in the management of patients with peripheral arterial occlusive disease. In the elderly populations of industrialized countries, PAD has a known prevalence of about 10%, and there is evidence for even more unrecognized cases.54 Management of PAD, therefore, raises public health expenditures for specific therapy and additional medication and physiotherapy for such symptoms and consequences of vitamin D deficiency and osteomalacia as fatigue, myalgia, muscle weakness, bone pain, and fractures.
Replacement therapy with vitamin D3 could be an inexpensive, well-tolerated, and highly effective supportive treatment to mitigate pain and the risk of falls with subsequent bone fractures, and ultimately to improve the quality of life and outcome in PAD patients. Further studies should investigate the influence of vitamin D3 replacement therapy on quality of life and outcome in this special group of patients.
Acknowledgments
We are grateful to Mrs. Eugenia Lamont for reading the manuscript.
REFERENCES
- 1.Chalmers J, Conacher WD, Gardener DL, Scott PJ. Osteomalacia—a common disease in elderly women. J Bone Joint Surg Br. 1967;49:403–23. [PubMed] [Google Scholar]
- 2.Smith R, Stern G. Myopathy, osteomalacia and hyperparathyroidism. Brain. 1967;90:593–602. doi: 10.1093/brain/90.3.593. [DOI] [PubMed] [Google Scholar]
- 3.Smith R, Stern G. Muscular weakness in osteomalacia and hyperparathyroidism. J Neurol Sci. 1969;8:511–20. doi: 10.1016/0022-510x(69)90010-0. [DOI] [PubMed] [Google Scholar]
- 4.Mallette LE, Patten BM, Engel WK. Neuromuscular disease in secondary hyperparathyroidism. Ann Intern Med. 1975;82:474–83. doi: 10.7326/0003-4819-82-4-474. [DOI] [PubMed] [Google Scholar]
- 5.Skaria J, Katiyar BC, Srivastava TP, Dube B. Myopathy and neuropathy associated with osteomalacia. Acta Neurol Scand. 1975;51:37–58. doi: 10.1111/j.1600-0404.1975.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 6.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;1:626–9. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- 7.Kruse HP. Osteomalazie. In: Allolio B, Schulte HM, editors. Praktische Endokrinologie. München, Wien, Baltimore: Urban u. Schwarzenberg; 1996. pp. 302–9. [Google Scholar]
- 8.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–06. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 9.Parfitt AM. Osteomalacia and related disorders. In: Avioli LV, Krane SM, editors. Metabolic Bone Disease. 3rd ed. San Diego, Calif: Academic Press; 1998. pp. 327–86. [Google Scholar]
- 10.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 11.Dattani JT, Exton-Smith AN, Stephen JM. Vitamin D status of the elderly in relation to age and exposure to sunlight. Hum Nutr Clin Nutr. 1984;38:131–7. [PubMed] [Google Scholar]
- 12.McKenna MJ, Freaney R, Meade A, Muldowney FP. Hypovitaminosis D and elevated serum alkaline phosphatase in elderly Irish people. Am J Clin Nutr. 1985;41:101–9. doi: 10.1093/ajcn/41.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Reid IR, Gallagher DJ, Bosworth J. Prophylaxis against vitamin D deficiency in the elderly by regular sunlight exposure. Age Ageing. 1986;15:35–40. doi: 10.1093/ageing/15.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Egsmose C, Lund B, McNair P, Storm T, Sorensen OH. Low serum levels of 25-hydroxyvitamin D and 1.25-dihydroxyvitamin D in institutionalised old people: influence of solar exposure and vitamin D supplementation. Age Ageing. 1987;16:35–40. doi: 10.1093/ageing/16.1.35. [DOI] [PubMed] [Google Scholar]
- 15.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93:69–77. doi: 10.1016/0002-9343(92)90682-2. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Med. 1995;61(3Suppl):638–45. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D and bone health. J Nutr. 1996;126(4Suppl):1159–64. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- 18.Gloth FM, III, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–6. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 19.Gloth FM, III, Tobin JD. Vitamin D deficiency in older people. J Am Geriatr Soc. 1995;43:822–8. doi: 10.1111/j.1532-5415.1995.tb07059.x. [DOI] [PubMed] [Google Scholar]
- 20.van der Wielen RP, Lowik MRH, van den Berg H, et al. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207–10. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 21.Utiger RD. The need for more vitamin D. N Engl J Med. 1998;338:828–9. doi: 10.1056/NEJM199803193381209. [DOI] [PubMed] [Google Scholar]
- 22.Fraser DR. Vitamin D. Lancet. 1995;345:104–7. doi: 10.1016/s0140-6736(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 23.Omdahl JL, Garry PJ, Hunsaker LA, Hunt WC, Goodwin JS. Nutritional status in a healthy elderly population: vitamin D. Am J Clin Nutr. 1982;36:1225–33. doi: 10.1093/ajcn/36.6.1225. [DOI] [PubMed] [Google Scholar]
- 24.Webb AR, Pilbeam C, Hanafin N, Holick MF. An evolution of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr. 1990;51:1075–81. doi: 10.1093/ajcn/51.6.1075. [DOI] [PubMed] [Google Scholar]
- 25.Salamone LM, Dallal GE, Zantos D, Makrauer F, Dawson-Hughes B. Contributions of vitamin D intake and seasonal sunlight exposure to plasma 25-hydroxyvitamin D concentration in elderly women. Am J Clin Nutr. 1994;58:80–6. doi: 10.1093/ajcn/59.1.80. [DOI] [PubMed] [Google Scholar]
- 26.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations and safety. Am J Clin Nutr. 1999;69:825–6. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 27.Preece MA, Tomlinson S, Ribot CA. Studies of vitamin D deficiency in man. Q J Med. 1975;44:575–89. [PubMed] [Google Scholar]
- 28.Lips P, Hackeng WH, Jongen MJ, van Ginkel FC, Netelenbos JC. Seasonal variation in serum concentrations of parathyroid hormone in elderly people. J Clin Endocrinol Metab. 1983;57:204–6. doi: 10.1210/jcem-57-1-204. [DOI] [PubMed] [Google Scholar]
- 29.Lips P, Wiersinga A, van Ginkel FC. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67:644–50. doi: 10.1210/jcem-67-4-644. [DOI] [PubMed] [Google Scholar]
- 30.Dlugos DJ, Perrotta PL, Horn WG. Effects of the submarine environment on renal-stone risk factors and vitamin D metabolism. Undersea Hyperb Med. 1995;22:145–52. [PubMed] [Google Scholar]
- 31.Dawson-Hughes B, Harris SS, Dallal GE. Plasma calcidiol, season and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr. 1997;65:67–71. doi: 10.1093/ajcn/65.1.67. [DOI] [PubMed] [Google Scholar]
- 32.Feleke Y, Abdulkadir J, Mshana R, et al. Low levels of serum calcidiol in an African population compared to a North European population. Eur J Endocrinol. 1999;141:358–60. doi: 10.1530/eje.0.1410358. [DOI] [PubMed] [Google Scholar]
- 33.Sherman SS, Hollis BW, Tobin JD. Vitamin D status and related parameters in a healthy population: the effects of age, sex and season. J Clin Endocrinol Metab. 1990;71:405–13. doi: 10.1210/jcem-71-2-405. [DOI] [PubMed] [Google Scholar]
- 34.Boonen S, Aerssens J, Dequeker J. Age-related endocrine deficiencies and fractures of the proximal femur: II implications of vitamin D deficiency in the elderly. J Endocrinol. 1996;149:13–7. doi: 10.1677/joe.0.1490013. [DOI] [PubMed] [Google Scholar]
- 35.Stamp TCB, Round JM. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature. 1974;247:563–5. doi: 10.1038/247563a0. [DOI] [PubMed] [Google Scholar]
- 36.Lester E, Skinner RK, Wills MR. Seasonal variation in serum-25-hydroxyvitamin D in elderly in Britain. Lancet. 1977;1:979–80. doi: 10.1016/s0140-6736(77)92280-2. [DOI] [PubMed] [Google Scholar]
- 37.Juttmann JR, Visser TJ, Buurman C, de Kam E, Birkenhäger JC. Seasonal fluctuations in serum concentrations of vitamin D metabolites in normal subjects. BMJ. 1981;282:1349–52. doi: 10.1136/bmj.282.6273.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 39.Chel VJ, Ooms ME, Popp-Snijders C, et al. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J Bone Miner Res. 1998;13:1238–42. doi: 10.1359/jbmr.1998.13.8.1238. [DOI] [PubMed] [Google Scholar]
- 40.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85:4125–30. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- 41.Chapuy MC, Schott AM, Garnero P, Hans D, Delmas PD, Meunier PJ EPIDOS STUDY GROUP. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. J Clin Endocrinol Metab. 1996;81:1129–33. doi: 10.1210/jcem.81.3.8772587. [DOI] [PubMed] [Google Scholar]
- 42.Bischoff H, Stahelin HB, Vogt P, et al. Immobility as a major cause of bone remodeling in residents of a long-stay geriatric ward. Calcif Tissue Int. 1999;64:485–9. doi: 10.1007/s002239900638. [DOI] [PubMed] [Google Scholar]
- 43.Pfeifer M, Begerow B, Minne HW, et al. Vitamin D status, trunk muscle strength, body sway, falls and fractures among 237 postmenopausal women with osteoporosis. Exp Clin Endocrinol Diabetes. 2001;109:87–92. doi: 10.1055/s-2001-14831. [DOI] [PubMed] [Google Scholar]
- 44.Lips P, Duong T, Oleksik A. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–21. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 45.Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–24. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 46.Demiaux B, Arlot ME, Chapuy MC, Meunier PJ, Delmas PD. Serum osteocalcin is increased in patients with osteomalacia: correlations with biochemical and histomorphometric findings. J Clin Endocrinol Metab. 1992;74:1146–51. doi: 10.1210/jcem.74.5.1569162. [DOI] [PubMed] [Google Scholar]
- 47.Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab. 1995;80:1052–8. doi: 10.1210/jcem.80.4.7714065. [DOI] [PubMed] [Google Scholar]
- 48.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–8. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 49.Chapuy MC, Arlot ME, Duboeuf F. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327:1637–42. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 50.Chapuy MC, Arlot ME, Delmas PD, Meunier PJ. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. BMJ. 1994;308:1081–2. doi: 10.1136/bmj.308.6936.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meunier PJ, Chapuy MC, Arlot ME, Delmas PD, Duboeuf F. Can we stop bone loss and prevent hip fractures in the elderly? Osteoporos Int. 1994;4(Suppl1):71–6. doi: 10.1007/BF01623440. [DOI] [PubMed] [Google Scholar]
- 52.Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons: a randomised, placebo-controlled trial. 1996. pp. 400–6. [DOI] [PubMed]
- 53.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–6. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 54.McDermott MM, Kerwin DR, Liu K, et al. Prevalence and significance of unrecognised lower extremity peripheral arterial disease in general medicine practice. J Gen Intern Med. 2001;16:384–90. doi: 10.1046/j.1525-1497.2001.016006384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]