Abstract
We conducted a population-based cohort study using administrative databases to quantify the association between oral and inhaled corticosteroid use and onset of diabetes mellitus in the elderly. Proton pump inhibitor (PPI) users were used as a control group. Relative to PPI users (N = 53,845), oral corticosteroid users (N = 31,864) were more likely to develop diabetes (adjusted rate ratio [aRR], 2.31; 95% confidence interval [95% CI], 2.11 to 2.54); however, inhaled corticosteroid users (N = 38,441) were not (aRR, 1.03; 95% CI, 0.93 to 1.14). The estimated number needed to harm for continuous use of oral corticosteroids relative to PPIs over 1, 2, and 3 years of use were 41, 23, and 16, respectively.
Keywords: corticosteroids, diabetes, drug-induced diabetes, elderly
Hyperglycemia1 is a troubling consequence of chronic corticosteroid therapy. While it is well established that oral corticosteroids have a pronounced hyperglycemic effect, the risk of developing corticosteroid-induced diabetes is poorly characterized quantitatively from a population-based perspective.2–6 A previous case-control study6 in the outpatient setting reported a significant association between oral corticosteroid use and the initiation of hypoglycemic therapy (odds ratio, 2.23); however, the study population, time-to-event, and numbers needed to harm (NNH) were poorly characterized, given the study design. Evidence for the association between inhaled corticosteroids and diabetes mellitus is largely limited to case reports7,8 and warrants further investigation in the general population to ascertain overall risk.
The objective of this study was to quantitatively characterize the association between oral and inhaled corticosteroid use and subsequent development of diabetes.
METHODS
We created a retrospective open cohort by linking administrative databases from July 1, 1991 through March 31, 1999. The Ontario Drug Benefit Program database (ODB) is maintained by the Ontario Ministry of Health and includes patient identifiers and drug information for all residents of Ontario aged 65 years or older. The Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) records discharge information for all patients admitted to Ontario acute care hospitals. The Ontario Health Insurance Plan database contains physician claims data and captures clinic visits. Finally, the Ontario Diabetes Database (ODD) contains unique patient identifiers and demographic information for all persons in Ontario defined as having diabetes through a validated administrative data algorithm.9,10 The ODD examines physician claims data and hospital discharge data for 2 consecutive occurrences of diabetes diagnoses, the first of which is defined as the date of diagnosis, without occurrence in the prior 2 years. Using this approach, validation studies have indicated a sensitivity of 86%, specificity of 97%, and positive predictive value of 80%.9 Using the ODB database, we identified patients with a new prescription (no fills in previous year) of an oral corticosteroid, inhaled corticosteroid, or oral proton pump inhibitor (PPI). PPI users were considered the control group, since they are not known to be associated with diabetes. Hence, the frequency of diabetes over time in this cohort of patients served as a representation of the burden of risk in the general population. By construction, this group controls for insurance status, access to care, temporal placement, and geographic location. By regression techniques, this group also provided a standard for making adjustments for age, gender, and antecedent health status. Patients were excluded if they had short-term therapy (less than 2 prescriptions in 180 days), if they had a prior diagnosis of diabetes (from ODD), transplant (from CIHI-DAD or ODB), or malignancy (from CIHI-DAD) within 5 years prior to study entry. Subject age, gender, and date of death (if applicable) were retrieved from the Registered Persons Database, which is maintained by the Ontario Ministry of Health.
Patients aged 66 years and older were followed from the date of first prescription of the study drug between January 1, 1994 and January 31, 1999 to allow for at least 2 months of follow-up. Observation ceased upon occurrence of diabetes mellitus, exposure to a medication from another study group, 30 days post-discontinuation of study medication (no refill within 180 days of last prescription), death, or reaching 3 years of continuous drug use without an event. For example, observation for an inhaled corticosteroid user with gastroesophageal reflux disease would cease upon initiation of a PPI.
Time-to-event was analyzed using Cox proportional hazards model. Age of subjects was included in the model as an independent continuous variable. Beginning 100 days before study entry to the end of the observation period, use of potentially diabetogenic drugs such as β-blockers, lipid-lowering drugs, diuretics, lithium, atypical antipsychotics, antiepileptics, nasal corticosteroids, intravenous corticosteroids, rectal corticosteroids, or transplant medications was controlled for by attributing independent binary variables for each drug. We adjusted for hospitalization during the observation period and controlled for the number of clinic visits to reduce the risk of detection bias in the diagnosis of diabetes. We also estimated the expected numbers of patients treated with corticosteroids over 1, 2, and 3 years that would be needed to observe a case of corticosteroid-induced diabetes mellitus, or the NNH, using the Cox proportional hazards estimates. All analyses were performed using SAS for UNIX, Version 6.12 (SAS Institute, Cary, NC). Patient characteristics were compared using the Kruskall-Wallis test for continuous variables and the χ2 test for categorical variables.
RESULTS
The average age of the oral corticosteroid users (n = 31,864) was 74.9 (SD 6.7) years, and 42% were men. Similarly, the average age of the inhaled corticosteroid users (N = 38,441) was 74.5 (SD 6.8) years, and 45% were men. For PPI users (N = 53,845), the average age was 74.9 (SD 6.9) years, and 38% were men. Table 1 outlines the demographic and clinical characteristics of the study population.
Table 1.
Patient Demographics and Outcome Data*
| Inhaled Corticosteroid Users (N = 38,441) | Oral Corticosteroid Users (N = 31,864) | Proton Pump Inhibitor Users (N = 53,845) | |
|---|---|---|---|
| Mean age, ±SD | 74.5 ± 6.8 | 74.9 ± 6.7 | 74.9 ± 6.9 |
| Gender, % male | 44.7 | 41.7 | 38.4 |
| Mean number of clinic visits over 1 y prior to study entry ±SD | 28.6 ± 27.9 | 35.9 ± 28.8 | 33.7 ± 30.5 |
| Patients hospitalized during study period, % | 21.8 | 30.5 | 26.1 |
| Patients taking confounding medications, % | |||
| β-blockers | 14.1 | 16.3 | 24.2 |
| Corticosteroids | 16.7 | 11.9 | 12.9 |
| Nasal | 14.6 | 5.7 | 8.9 |
| Parenteral | 2.5 | 6.3 | 4.4 |
| Rectal | 0.1 | 0.4 | 0.3 |
| Lipid-lowering agents | 10.5 | 7.8 | 15.1 |
| Lithium | 0.4 | 0.2 | 0.4 |
| Diuretics | 30.6 | 28.0 | 27.7 |
| Atypical antipsychotics | 0.3 | 0.3 | 0.6 |
| Antiepileptics | 2.8 | 5.5 | 3.0 |
| Transplant medications | 0.1 | 1.8 | 0.1 |
| Events | |||
| Number of patients developing diabetes | 626 | 906 | 1,065 |
| Mean follow-up time, d ±SD | 285 ± 284 | 205 ± 233 | 364 ± 334 |
| Crude number of events per 1,000 person-y | 20.8 | 50.6 | 19.9 |
| Adjusted rate ratio† (95% CI) | 1.03 (0.93 to 1.14) | 2.31 (2.11 to 2.54) | — |
Comparisons of all variables reach statistical significance (P < .01). Between-group differences may reflect large sample sizes rather than clinically meaningful differences.
Adjusted for age, gender, and use of β-blockers, lipid-lowering drugs, diuretics, lithium, atypical antipsychotics, antiepileptics, nasal corticosteroids, intravenous corticosteroids, rectal corticosteroids, transplant medications, hospitalizations, and clinic visits.
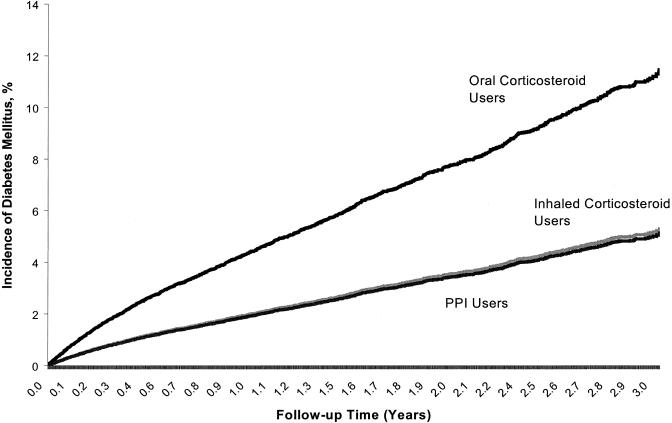
The mean observation period was 299 (SD 302) days, totaling 101,576 person-years of follow-up. For every 1,000 person-years, the crude incidence of diabetes was 50.6 in the oral corticosteroid group, 20.8 in the inhaled corticosteroid group, and 19.9 in the PPI group. Oral corticosteroid users were significantly more likely than PPI users to develop diabetes mellitus (adjusted rate ratio [aRR], 2.31; 95% confidence interval [95% CI], 2.11 to 2.54), while the risk of inhaled corticosteroid users was not significantly different from that of PPI users (aRR, 1.03; 95% CI, 0.93 to 1.14). Figure 1 outlines the estimated incidence of diabetes over time associated with each of the 3 study groups. The NNH for continuous use of oral corticosteroids relative to PPIs over 1, 2, and 3 years of use based on the Cox proportional hazards estimates were 41, 23, and 16, respectively (Table 2).
FIGURE 1.
Incidence of diabetes over time among study groups.
Table 2.
Estimated Number Needed to Harm for Oral Corticosteroid Users as a Function of Duration of Use*
| Incidence of Diabetes Mellitus | ||||
|---|---|---|---|---|
| Years of Use | Oral Corticosteroid Users, % | PPI Users, % | Absolute Risk Difference, % | Number Needed to Harm |
| 1 | 4.3 | 1.9 | 2.4 | 41 |
| 2 | 7.7 | 3.4 | 4.3 | 23 |
| 3 | 11.0 | 4.9 | 6.1 | 16 |
Based on Cox proportional hazards estimates adjusting for use of β-blockers, lipid-lowering drugs, diuretics, lithium, atypical antipsychotics, antiepileptics, nasal corticosteroids, intravenous corticosteroids, rectal corticosteroids, transplant medications, hospitalizations, and clinic visits.
Four sensitivity analyses, examining only “healthier” users void of the comorbidities and hospitalizations previously accounted for (N = 44,505), those 75 years of age or younger (N = 74,633), only males (N = 51,157), and only females (N = 72,993), revealed a consistently increased risk of diabetes among only the oral corticosteroid users.
DISCUSSION
To our knowledge, this is the largest study ever to examine the quantitative relationship between corticosteroid use and the development of diabetes mellitus. We have shown that the risk of developing diabetes more than doubles in elderly patients who are newly initiated on an oral corticosteroid, similar to a previous case-control study in the outpatient setting.6 This association is also consistent with other smaller studies.2–5 It is unlikely that these findings result only from an unmasking of latent diabetes early in treatment, because the incidence curves continue to diverge throughout the observation period. While this association may be intuitive to most clinicians, we failed to observe an association between inhaled corticosteroid use and the development of diabetes mellitus. These results were found despite a very conservative study design. For example, we studied only new users of corticosteroids and restricted follow-up to the period of active administration. Our findings were robust across a range of relevant subgroups.
However, the administrative data we used in our study did not allow us to ascertain certain patient characteristics such as genetic predisposition, activity level, or body mass index. Also, a dose–response relationship could not be accurately ascertained given limitations in data availability. Certainly, our findings may not be generalizable to individuals consuming high doses of inhaled corticosteroids, but rather reflect the average elderly user in the overall population. Furthermore, the generalizability of our findings to younger populations is uncertain. These are major limitations of our study and the impact of these limitations is unknown.
In summary, we report numerical estimates of the association between the use of oral corticosteroids and the development of diabetes mellitus in an elderly population. There was no evidence for an association among users of inhaled corticosteroids. The findings provide a quantitative estimate of the risk of developing diabetes mellitus with chronic corticosteroid use for clinicians who seek to more adequately inform their patients of the risks of corticosteroid therapy.11–13
Acknowledgments
Dr. Hux is a career scientist of the Ontario Ministry of Health and Drs. Hux and Mamdani receive salary support from the Institute for Clinical Evaluative Sciences in Ontario.
REFERENCES
- 1.Delaunay F, Khan A, Cintra A, et al. Pancreatic β cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 1997;100:2094–8. doi: 10.1172/JCI119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conn H, Poynard T. Corticosteroids and peptic ulcer: meta-analysis of adverse events during steroid therapy. J Intern Med. 1994;236:619–32. doi: 10.1111/j.1365-2796.1994.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman P, Patterson R, Kunske R. Complications of long-term steroid therapy for asthma. J Allergy Clin Immunol. 1972;49:329–36. doi: 10.1016/0091-6749(72)90131-5. [DOI] [PubMed] [Google Scholar]
- 4.Smyllie H, Connolly C. Incidence of serious complications of corticosteroid therapy in respiratory disease. Thorax. 1968;23:571–81. doi: 10.1136/thx.23.6.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jindal R, Sidner R, Milgrom M. Post-transplant diabetes mellitus: the role of immunosuppression. Drug Saf. 1997;16:242–57. doi: 10.2165/00002018-199716040-00002. [DOI] [PubMed] [Google Scholar]
- 6.Gurwitz J, Bohn R, Glynn R, Monane M, Mogun H, Avorn J. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med. 1994;154:97–101. [PubMed] [Google Scholar]
- 7.Faul JL, Tormey W, Tormey V, Burke C. High dose inhaled corticosteroids and dose dependent loss of diabetic control. BMJ. 1998;317:1491. doi: 10.1136/bmj.317.7171.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faul JL, Cormican LJ, Tormey VJ, et al. Deteriorating diabetic control associated with high-dose inhaled budesonide. Eur Respir J. 1999;14:242–3. doi: 10.1183/09031936.99.141. [DOI] [PubMed] [Google Scholar]
- 9.Hux J, Bica A, Flintoft V, Ivis F. Population based estimates of the incidence and the prevalence of diabetes in Ontario. Diabetes. 2000;49(Suppl 1):388. [Google Scholar]
- 10.Blanchard JF, Ludwig S, Wajda A, et al. Incidence and prevalence of diabetes in Manitoba, 1986–1991. Diabetes Care. 1996;19:807–11. doi: 10.2337/diacare.19.8.807. [DOI] [PubMed] [Google Scholar]
- 11.Robson J. Information needed to decide about cardiovascular treatment in primary care. BMJ. 1997;314:277–80. doi: 10.1136/bmj.314.7076.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hux JE, Naylor CD. Communicating the benefits of chronic preventive therapy: does the format of efficacy data determine patients' acceptance of treatment. Med Decis Making. 1995;15:152–7. doi: 10.1177/0272989X9501500208. [DOI] [PubMed] [Google Scholar]
- 13.McAlister FA, O'Connor AM, Wells G, Grover SA, Laupacis A. When should hypertension be treated? The different perspectives of Canadian family physicians and patients. CMAJ. 2000;163:403–8. [PMC free article] [PubMed] [Google Scholar]