Abstract
OBJECTIVE
To determine if a visual intervention (medication grid) delivered to physicians can reduce medication regimen complexity.
DESIGN
Nonrandomized, controlled trial.
SETTING
Veterans Affairs medical center.
PATIENTS/PARTICIPANTS
Eight hundred thirty-six patients taking at least 5 medications at the time of admission and the 48 teams of physicians and students on the general medicine inpatient service.
INTERVENTION
For intervention patients, a medication grid was created that displayed all of the patients' medicines and the times of administration for 1 week. This grid was delivered to the admitting resident soon after admission.
MEASUREMENTS AND MAIN RESULTS
For the patients of each team of physicians, we calculated the change in the average number of medications and doses from admission to discharge. The number of medications in the intervention group decreased by 0.92 per patient, and increased by 1.65 in the control group (P < .001). The mean number of doses per day in the intervention group decreased by 2.47 per patient and increased by 3.83 in the control group (P < .001).
CONCLUSIONS
This simple intervention had a significant impact on medication regimen complexity in this population. Apparently, physicians were able to address polypharmacy when the issue was brought to their attention.
Keywords: polypharmacy, drug therapy, medical education, veterans
Physicians today provide care to patients with multiple medical problems at a time when the development and use of medications is increasing. These trends, combined with recommendations from clinical practice guidelines and consumer demand for drugs, often result in complex medication regimens for many patients. The complexity of a medication regimen can be defined by the number of medications (polypharmacy) and the number of times per day or “doses” that the patient takes a medication (multiple dosing schedules). Complex medication regimens are troublesome to patients and physicians due to the resulting problems of nonadherence, therapeutic failure, and adverse drug reactions.1–3 In addition, complex medication regimens contribute to the estimated costs of $20 to $70 billion dollars per year due to drug-related morbidity and mortality.4,5
Previous interventions directed at physicians to impact polypharmacy have met with variable success. While chart review followed by detailed recommendations from physicians have decreased medications,6,7 attempts with geriatric consultation teams, clinical pharmacists, and monthly computerized summaries generally have not reduced numbers of medications.8–10 Even when successful, the time and expense required by these interventions would make implementation impractical. In addition, these studies have focused on the number of medications only, largely ignoring dosing schedule, which is a critical factor determining regimen complexity.11
To address the problem of medication regimen complexity, we designed a simple visual tool in the form of a 7-day medication grid depicting the number of medications and doses each patient was supposed to take during each week. The grid was designed to quickly illustrate regimen complexity to physicians and encourage them to simplify the regimen. The objective of the study was to determine if the medication grid significantly reduced the number of medications and doses prescribed by physicians in an intervention group compared with the control group.
METHODS
Study Design and Setting
The study was a controlled trial of the medication grid versus no intervention among resident physicians and inpatients with polypharmacy (operationally defined as taking at least 5 medications concurrently) who were the patients of resident physicians. The site for the study was the general medicine wards of the Durham Veterans Affairs Medical Center. The project was approved by the Durham VAMC Research and Human Studies Committees.
Patients and Physicians
Patients were eligible if they were admitted to the General Medical Service between July 1997 and May 1998 and were taking at least 5 medications at admission. Patients were ineligible if they were already enrolled in the study, died during the admission, or were discharged by a team other than the admitting team. The control and intervention groups each consisted of 24 general medicine ward teams, which were led by second- and third-year internal medicine residents. Team generally consisted of a resident, an intern, and 1 or 2 medical students or physician assistant students. Attending physicians were general internists and subspecialists, each of whom supervised 2 ward teams. Attending physicians rotated every 4 weeks and did not cross collection periods for the intervention or control groups. Clinical pharmacists and pharmacy students rounded with several, but not all, teams during the various rotations. To avoid contamination bias, the 8 teams in each rotation were all assigned to either the intervention group or to the control group. Intervention and control collection periods alternated throughout the year. Three of 6 rotations included in the trial were selected to constitute the intervention group; the remaining 3 rotations constituted the control group. Interns and residents rotated on different dates, and therefore data collection was suspended until all interns exposed to the intervention left the service. No interns or residents returned to the service later in the year. The duration of data collection for the teams was similar for both the intervention and control groups (5 to 7 weeks).
Medications
The first author obtained admission medication lists by reviewing the resident's computerized admission note during evening rounds. We did not rely solely on the Durham VAMC pharmacy records as many patients received their medications from other sources. We included medications that were regularly scheduled or pro re nata (PRN), prescription or over-the-counter (OTC), and taken at least once per week. We included PRN and OTC medications because they clearly factor into the complexity of the regimen. We excluded short-term medications such as antibiotics and short courses of steroid, preferring to focus on the long-term regimen. Medications taken every other day or even weekly counted as 1 daily dose. Admission doses included all regularly scheduled medications as well as the maximum number of potential doses for PRN medications (e.g., every 6 hours PRN = 4 doses). Medications taken PRN without a dosing schedule (e.g., sublingual nitroglycerin) were not included in the number of doses but were included in the number of medications. As all patients receive their discharge medications from the Durham VAMC pharmacy, we obtained discharge medications and doses from pharmacy computer records. If necessary, we verified duration of therapy (to exclude short-term medications) on the discharge summary, since discharge summaries include a list of discharge medications with anticipated duration of therapy.
Intervention
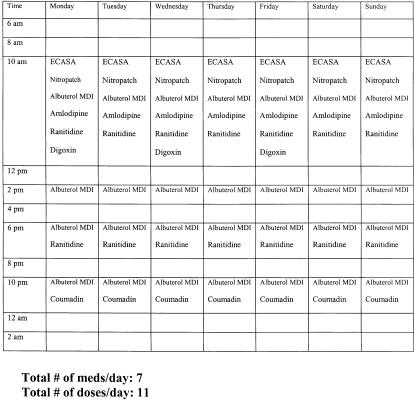
Using the above medication and dose information, we created a medication grid and delivered it to the admitting resident for each team in the intervention group (Fig. 1). The grid contained the times of day for administration, and the columns listed the days of administration. Administration times were based the schedule of administration of the Durham VAMC inpatient pharmacy. The residents received a hard copy of the grid at morning report the day following admission. The residents were instructed to review the grid with the other team members. To assist feasibility, the intervention was minimal, and no explicit instructions were given to the residents regarding the medication regimens.
FIGURE 1.
Sample of medication grid used in an intervention to reduce medication complexity. The first column lists times of administration, and the first row lists the days of the week. The other rows list the prescribed medications.
Statistical Analysis
We used the team of physicians as the unit of analysis. For each team, we calculated the difference between the number of medications at admission and discharge. These change scores were compared between the two groups using a 2-sample t test. Potential confounders included the hospital length of stay, average number of discharge diagnoses, number of admission medications, and the presence of a pharmacist on rounds. To determine the effect on the relationship between group status and medication change, potential confounders were added one at a time to regression models containing medication change score as the dependent variable and group as the independent variable. The above analysis was repeated for the number of doses.
For descriptive purposes, we also compared changes in specific medications between the intervention and control groups. For each team, we found the proportion of patients on each medication at admission and at discharge, and the difference between these proportions was calculated. Since the data were skewed, and we used the Wilcoxon rank sum test to compare these differences between the intervention and control groups. We were also interested in comparing the change in medication classes between the intervention and control groups. Medication classes were defined by the Veterans Affairs classification codes.12 Medication classes were examined in a slightly different manner than individual medications. The total number of medications taken in each medication class was determined for each patient at both admission and discharge. Team averages were then calculated for each medication class. The difference between the admission and discharge team averages was compared between the intervention and control groups using the Wilcoxon rank sum test.
In addition, descriptive statistics for patient level data and physician characteristics were reported. Continuous variables were summarized in terms of medians and interquartile ranges. Numbers and percents were reported for categorical variables.
RESULTS
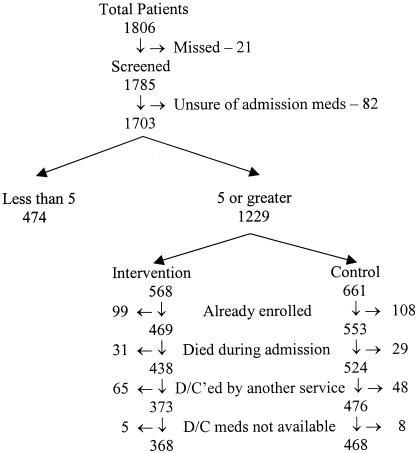
During the period of data collection, 1,806 patients were admitted to the Durham VAMC General Medical Service. Figure 2 outlines the trial profile. Table 1 summarizes the baseline characteristics of the patients. The patients were almost entirely men with a mean age of 65 years, a typical veteran population. Only 6% of the patients in each group were admitted from a long-term care facility. The intervention was principally directed at the residents. The intervention group residents had a median age of 28 years, and 59% were men. The control group residents had a median age of 28 years, and 67% were men. Although the ages were similar, the control group residents were less experienced; while 15 (62%) of the residents in the control group were in their second year of residency, only 11 (46%) of the intervention group were in their second year. Pharmacists rounded with 12 (50%) of the intervention teams and 10 (42%) of the control teams.
FIGURE 2.
Trial profile.
Table 1.
Patient Characteristics
| Characteristic | Intervention (n = 368) | Control (n = 468) |
|---|---|---|
| Mean, age, y | 65.2 | 65.6 |
| Men, % | 99.2 | 98.5 |
| African, % | 27.2 | 25.6 |
| Skilled nursing facility resident, % | 6.0 | 5.6 |
| American Discharge diagnoses, mean N | 7.8 | 8.4 |
| Mean length of stay, d | 6.0 | 6.4 |
| Admit mean, median (IQR) | 9 (7–11) | 8 (6–10) |
| Admit doses, median (IQR) | 17 (11–23) | 15 (10–20) |
IQR indicates interqauntile ranges.
Table 2 shows the mean changes in medications and in doses from admission to discharge for the 2 groups. Both of these differences were statistically significant. These differences remained significant after controlling for the number of discharge diagnoses, number of admission medications, length of stay, and the presence of a clinical pharmacist.
Table 2.
Change (mean±sd) in Medications and Doses over the Follow-up Period.
| Control (n = 24) | Intervention (n = 24) | P Value* | |
|---|---|---|---|
| Medications | 1.65 ± (0.60) | −0.92 (0.71) | <.001 |
| Doses | 3.83 ± (1.34) | −2.47 (1.55) | <.001 |
Two-sample t test.
The analysis of the medications are shown by class and by individual medication. Table 3 shows the change in medications using the VA formulary classification. In almost all of the classes, the number of medications in the control group increased, while the number of medications in the intervention group decreased. In all but three classes (blood products, ophthalmic agents, antimicrobials), these differences were statistically significant.
Table 3.
Median Number of Admission and Discharge Medications by drug class.
| Control (n = 24) | Intervention (n = 24) | ||||
|---|---|---|---|---|---|
| Drug Class | Admission | Discharge | Admission | Discharge | Change Score P Value* |
| Cardiovascular | 2.77 | 3.00 | 2.80 | 2.87 | <.001 |
| Central nervous system | 1.74 | 2.02 | 1.89 | 1.75 | <.001 |
| Gastrointestinal | 0.96 | 1.27 | 1.05 | 1.00 | <.001 |
| Respiratory | 0.75 | 0.87 | 1.00 | 0.81 | <.001 |
| Hormones/synthetics | 0.51 | 0.59 | 0.60 | 0.58 | <.001 |
| Vitamins | 0.36 | 0.43 | 0.44 | 0.41 | .006 |
| Nutrients/minerals | 0.35 | 0.37 | 0.35 | 0.32 | .002 |
| Musculoskeletal | 0.27 | 0.27 | 0.34 | 0.24 | .005 |
| Blood products | 0.16 | 0.22 | 0.20 | 0.17 | .17 |
| Antihistamines | 0.12 | 0.15 | 0.12 | 0.05 | <.001 |
| Ophthalmic agents | 0.078 | 0.076 | 0.065 | 0.054 | .34 |
| Antimicrobials | 0.077 | 0.101 | 0.080 | 0.045 | .08 |
| Dermatological | 0.057 | 0.077 | 0.063 | 0.031 | .01 |
Wilcoxon rank sum test.
Table 4 4 lists the 20 most common medications for which there was a significant difference in the change score between the intervention and control groups. The values reported are the proportion of patients in each group taking each medication at admission and discharge. The P values represent the results of the Wilcoxon rank sum tests of the change scores between the two groups. With most of the medicines, the change score was positive in the control group (reflecting an increase in the proportion of patients prescribed a medication) and negative in the intervention group. Even when the change score for a medication was positive in both groups, the increase was greater in the control group. The 20 medications not impacted by the intervention were also examined. These medications included lisinopril, atenolol, metoprolol, amlodipine, ipratropium inhaler, warfarin, digoxin, and insulin.
Table 4.
Median Proportions of Patients Taking Individual Medications that changed significantly.
| Control (n = 24) | Intervention (n = 24) | ||||
|---|---|---|---|---|---|
| Medication | Admission | Discharge | Admission | Discharge | Change Score P Value* |
| Aspirin | 0.53 | 0.64 | 0.64 | 0.65 | <.001 |
| Furosemide | 0.32 | 0.33 | 0.39 | 0.32 | .01 |
| Albuterol inhaler | 0.28 | 0.32 | 0.35 | 0.32 | .01 |
| Cimetidine | 0.22 | 0.27 | 0.25 | 0.20 | <.001 |
| Sublingual nitroglycerin | 0.20 | 0.30 | 0.28 | 0.30 | .001 |
| Nitroglycerin path | 0.19 | 0.26 | 0.22 | 0.28 | .02 |
| Docusate | 0.19 | 0.22 | 0.17 | 0.17 | .04 |
| Simvastatin | 0.15 | 0.22 | 0.14 | 0.16 | .003 |
| Multivitamin | 0.15 | 0.19 | 0.21 | 0.19 | .01 |
| Acetominophen | 0.13 | 0.18 | 0.15 | 0.12 | <.001 |
| Isosorbide dinitrate | 0.12 | 0.14 | 0.13 | 0.13 | .004 |
| Beclomethasone inhaler | 0.12 | 0.14 | 0.13 | 0.13 | .004 |
| Iron sulfate | 0.10 | 0.16 | 0.13 | 0.14 | .03 |
| Percocet | 0.10 | 0.10 | 0.14 | 0.13 | .02 |
| Ibuprofen | 0.077 | 0.070 | 0.085 | 0.062 | .02 |
| Amitriptyline | 0.071 | 0.091 | 0.10 | 0.056 | <.001 |
| Diltiazem | 0.062 | 0.070 | 0.076 | 0.050 | .01 |
| Folate | 0.054 | 0.060 | 0.071 | 0.057 | .01 |
| Calcium carbonate | 0.042 | 0.078 | 0.051 | 0.044 | .04 |
| Guaifenesin | 0.041 | 0.056 | 0.058 | 0.00 | <.001 |
Wilcoxon rank sum test. Only indicators with statistically significant changes are listed.
DISCUSSION
Our study found that the intervention of the simple medication grid led to a statistically and clinically significant decrease in the number of medications and doses. We excluded antibiotics and other short-term medications and examined only those medications prescribed indefinitely. In fact, the number of medications and doses in the intervention group decreased, while the number of medications and doses in the control group increased. These differences persisted after controlling for factors such as the presence of a clinical pharmacist, length of stay, and the number of discharge diagnoses. The median number of medications in several therapeutic classes, including central nervous system, gastrointestinal, and respiratory drugs, hormones/synthetics, vitamins, antihistamines, and antimicrobials, decreased in the intervention group and increased in the control group. Several individual medications significantly changed between the intervention and control groups. The list included medications found in an earlier study to be prescribed inappropriately due to duplication (bronchodilators, antidepressants), indication (multivitamins), and duration of treatment (laxatives, multivitamins).13 Cimetidine, which has previously been associated with overuse,14,15 was reduced only in the intervention group. In addition, this list also included amitriptyline, a drug that should generally be avoided in elderly individuals.16 At the same time, we were encouraged by the list of medications that did not change with our intervention. For safety reasons, we would have been concerned if medications such as β-blockers, angiotensin converting enzyme inhibitors, digoxin, or warfarin were withdrawn in significant numbers.
Previous interventions aimed at impacting polypharmacy have had variable results. Several studies suggested, however, that an intervention aimed at physicians could be successful. Kroenke and Pinholt made recommendations to physicians caring for patients on 5 or more medications, and they decreased the number of medications per patient from 5.9 to 5.4.6 Meyer et al. compared a simple intervention with a more intensive intervention in patients taking 10 or more medications. In addition to a control group, one group's providers received a letter asking them to address polypharmacy, and the third group received chart review and detailed recommendations. The intensive and simple notifications both led to significant reductions at 4 months, but there was no difference between the intensive and simple notification groups. Of note, these differences with the control group no longer existed at 12 months.7 Hamdy et al. addressed the issue of polypharmacy in the extended care setting with a straightforward intervention. If a patient was taking more than 10 medications, the physician for that patient was notified and asked to review the medications. During the 5-year study period, the number of patients taking 10 or more medications decreased from 67 to 9, and the mean number of medications per patient decreased from 5.5 to 4.6.17 Hanlon and colleagues evaluated the effect of a 12-month clinical pharmacist intervention involving elderly VA outpatients and their primary physicians in a randomized, controlled trial. The number of medications per patient in the intervention group decreased from 7.6 to 6.9, although this result was not statistically different from the control group.9
Drawing from these previous studies, we theorized that our simple yet visually compelling intervention would encourage the physicians to address polypharmacy. The medication grid was meant to call the issue to their attention. We wanted to impress upon the physicians what the patients were trying to accomplish in taking their medications each day. Intentionally, this was the extent of our intervention. We felt that recommendations from chart review would be time consuming and thus impractical. At the same time, we wanted to allow the physicians the autonomy to make only those changes with which they were comfortable. To aid practical implementation of the medication grid, we also wanted to limit the expense of the intervention. Therefore, the residents in the study did not receive additional education. With rather basic software, even generation of the grid could be automated.
A particularly encouraging aspect of this study was the response of the physicians to the intervention. The physicians were generally receptive to the notion of targeting polypharmacy, and they seemed impressed by the difficulty of some patient regimens. One persistent concern is that time would decrease the novelty of the grid and minimize the impact. As our intervention lasted only 5 to 7 weeks, we were unlikely to see this problem. For this reason, we did not provide the grid for all patients. With the inclusion criteria of at least 5 medications, we highlighted these patients. The choice of at least 5 medications is rather arbitrary, and perhaps focusing on patients with more medications or doses would increase the staying power of the intervention.
To sustain the impact of the intervention following discharge would require efforts other than the medication grid. Major changes in the medication regimen during an admission may not be communicated well to the primary care provider. In our hospital, patients receive a typed summary of the discharge medications and are instructed to take this summary to their next appointment with their primary care provider. In addition, patient education at the time of discharge is essential if significant medication changes occur. Otherwise, patients may take previously prescribed medications along with or instead of the discharge medications. Omori et al. found that these errors were more likely with a greater number of changes in the regimen during the admission.18 The greatest concern from these errors is the risk of adverse drug reactions. These outcomes were beyond the scope of this study, although they would be an important aspect of further work in this area. With appropriate patient education and outpatient contact, the simplified regimen has the potential to decrease these errors. Several previous studies have found that multiple medication use is strongly associated with the development of adverse drug reactions, and the risk increases with increasing drug use.19–21
In addition to those mentioned above, there are several potential limitations to this study. Due to logistical reasons associated with resident scheduling, we did not randomize the residents. With limited resources, the data collection could not be performed in a blinded manner. In addition, given that our study involved male veterans and house staff, the generalizability of our results may be limited.
Physicians, patients, and the pharmaceutical industry have all contributed to the development of polypharmacy. With advances in the pharmaceutical industry and changes in health insurance benefits, drug consumption is increasing. As the population ages and lives with more chronic illnesses, patients may require and demand multiple medications. In addition, physician groups have developed clinical practice guidelines that often involve multiple medications. Combating polypharmacy will clearly require interventions aimed at all parties involved, including physicians, pharmacists, and patients. Our study targeted physicians caring for inpatients, and our rather simple intervention reduced medication regimen complexity in this population. This result did not require an extensive educational intervention. When the problem was brought to their attention, physicians were effective in the reduction of medication regimen complexity.
Acknowledgments
This work was supported by the Geriatric Research Education and Clinical Center (GRECC) of the Durham VAMC, and by the Duke Claude D. Pepper Older Americans Independence Center, no. 1P60AG11268-02.
REFERENCES
- 1.Grymonpre RE, Mitenko PA, Sitar DS, Aoki FY, Montgomery PR. Drug-associated hospital admissions in older medical patients. J Am Geriatr Soc. 1988;36:1092–8. doi: 10.1111/j.1532-5415.1988.tb04395.x. [DOI] [PubMed] [Google Scholar]
- 2.Montamat SC, Cusack B. Overcoming problems with polypharmacy and drug misuse in the elderly. Clin Geriatr Med. 1992;8:143–58. [PubMed] [Google Scholar]
- 3.Stewart RB, Cooper JW. Polypharmacy in the aged. Practical solutions. Drugs Aging. 1994;4:449–61. doi: 10.2165/00002512-199404060-00002. [DOI] [PubMed] [Google Scholar]
- 4.Prescription Drugs and the Elderly. Report to the Honorable Ron Wyden, House of Representatives. Washington, DC: United States General Accounting Office; 1995. [Google Scholar]
- 5.Johnson JA, Bootman JL. Drug-related morbidity and mortality: a cost of illness model. Arch Intern Med. 1995;155:1949–56. [PubMed] [Google Scholar]
- 6.Kroenke K, Pinholt EM. Reducing polypharmacy in the elderly. A controlled trial of physician feedback. J Am Geriatr Soc. 1990;38:31–6. doi: 10.1111/j.1532-5415.1990.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 7.Meyer TJ, Van Kooten D, Marsh S, Prochazka AV. Reduction of polypharmacy by feedback to clinicians. J Gen Intern Med. 1991;6:133–6. doi: 10.1007/BF02598309. [DOI] [PubMed] [Google Scholar]
- 8.Allen CM, Becker PM, McVey LJ, Saltz C, Feussner JR, Cohen HJ. A randomized, controlled clinical trial of a geriatric consultation team. Compliance with recommendations. JAMA. 1998;255:2617–21. [PubMed] [Google Scholar]
- 9.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100:428–37. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 10.Hershey CO, Porter DK, Breslau D, Cohen DI. Influence of simple computerized feedback on prescription changes in an ambulatory clinic. A randomized clinical trial. Med Care. 1986;24:472–81. doi: 10.1097/00005650-198606000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045–53. doi: 10.1016/0895-4356(92)90144-c. [DOI] [PubMed] [Google Scholar]
- 12.United States Pharmacopeia Dispensing Information (USP DI), Appendix IV . Veterans Affairs Medication Classification System. Rockville, Md: United States Phamacopeial Convention; 1997. pp. 3070–88. [Google Scholar]
- 13.Schmader K, Hanlon JT, Weinberger M, et al. Appropriateness of medication prescribing in ambulatory elderly patients. J Am Geriatr Soc. 1994;42:1241–7. doi: 10.1111/j.1532-5415.1994.tb06504.x. [DOI] [PubMed] [Google Scholar]
- 14.Schade RR, Donaldson Rm., Jr How physicians use cimetidine: a survey of hospital patients and published cases. N Engl J Med. 1981;304:1281–4. doi: 10.1056/NEJM198105213042109. [DOI] [PubMed] [Google Scholar]
- 15.Sherman DS, Avorn J, Campion EW. Cimetidine use in nursing homes: prolonged therapy and excessive doses. J Am Geriatr Soc. 1987;35:1023–7. doi: 10.1111/j.1532-5415.1987.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 16.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997;157:1531–6. [PubMed] [Google Scholar]
- 17.Hamdy RC, Moore SW, Whalen K, et al. Reducing polypharmacy in extended care. South Med J. 1995;88:534–8. doi: 10.1097/00007611-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Omori M, Potyk RP, Kroenke K. The adverse effects of hospitalization on drug regimens. Arch Intern Med. 1991;151:1562–4. [PubMed] [Google Scholar]
- 19.Chrischilles EA, Segar ET, Wallace RB. Self-reported adverse drug reactions and related resource use. A study of community-dwelling persons 65 years of age and older. Ann Intern Med. 1992;117:634–40. doi: 10.7326/0003-4819-117-8-634. [DOI] [PubMed] [Google Scholar]
- 20.Col N, Fanale JE, Kronholm P. The role of medication noncompliance and adverse drug reactions in hospitalizations in the elderly. Arch Intern Med. 1990;150:841–5. [PubMed] [Google Scholar]
- 21.Mannesse CK, Derkx FH, de Ridder MA, Man in 'T Veld AJ, van der Cammen TJ. Adverse drug reactions in elderly patients as contributing factor for hospital admission: cross-sectional study. BMJ. 1997;315:1057–8. doi: 10.1136/bmj.315.7115.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]