Abstract
OBJECTIVE
To measure how often a breast-related concern was documented in medical records after screening mammography according to the mammogram result (normal, or true-negative vs false-positive) and to measure changes in health care utilization in the year after the mammogram.
DESIGN
Cohort study.
SETTING
Large health maintenance organization in New England.
PATIENTS
Group of 496 women with false-positive screening mammograms and a comparison group of 496 women with normal screening mammograms, matched for location and year of mammogram.
MEASUREMENTS AND MAIN RESULTS
1) Documentation in clinicians' notes of patient concern about the breast and 2) ambulatory health care utilization, both breast-related and non–breast-related, in the year after the mammogram. Fifty (10%) of 496 women with false-positive mammograms had documentation of breast-related concern during the 12 months after the mammogram, compared to 1 (0.2%) woman with a normal mammogram (P = .001). Documented concern increased with the intensity of recommended follow-up (P = .009). Subsequent ambulatory visits, not related to the screening mammogram, increased in the year after the mammogram among women with false-positive mammograms, both in terms of breast-related visits (incidence ratio, 3.07; 95% confidence interval [CI], 1.69 to 5.93) and non-breast-related visits (incidence ratio, 1.14; 95% CI, 1.03 to 1.25).
CONCLUSIONS
Clinicians document concern about breast cancer in 10% of women who have false-positive mammograms, and subsequent use of health care services are increased among women with false-positive mammogram results.
Keywords: mammography, screening, false positive, anxiety, health care utilization
Although screening mammography decreases breast cancer mortality by about a third, the procedure is not without hazards. Mammography is not 100% specific, and approximately 90%–95% of mammograms requiring follow-up investigations do not result in breast cancer diagnoses. Nationwide, 10% of screening mammograms result in false-positives.1 After 10 mammograms, the estimated cumulative risk of a false-positive is nearly 50%.2
Studies from the United States,3 Norway,4 and Great Britain5,6 have found increased anxiety among women who have abnormal mammograms, in some cases even after further evaluation has ruled out cancer.3 However, the extent to which patients express this concern to clinicians, and whether clinicians document patient concern in their medical records, is unknown.
Patient concern that may be raised by screening for disease has been shown to result in “labeling” effects. Untoward effects on daily functioning and increased absenteeism from work have been identified among people diagnosed with hypertension.7,8 Evidence of labeling effects has been found in other conditions.9 These effects have not been documented after breast cancer screening, although concern about breast cancer among women is high.10 While it is clear that recommended follow-up after a positive screening mammogram entails increased utilization of health care services,2,11 whether false-positive mammograms affect health care utilization in other ways has not been reported.
We wondered if women with false-positive mammograms would be more likely than women with normal mammograms to have concern about breast cancer that is documented by their clinicians, and whether such concern was associated with increased numbers of ambulatory visits beyond those required for the work-up of the false-positive mammogram. We therefore studied the frequency of clinician documentation of patient concern and the number of ambulatory visits in a group of women with false-positive mammograms, and compared these findings to those in women with normal mammograms.
METHODS
Setting and Study Population
This retrospective cohort study was conducted at a large HMO; the study population has been described in full elsewhere.2 Subjects for this study were drawn from an existing population of 2,400 women who had been identified for a study of breast cancer screening outcomes over the 10-year period from July 1, 1983, to June 30,1993.2 All women were between the ages of 40 and 69 as of July 1, 1983. They were continuously enrolled in the HMO from July 1, 1983, to June 30, 1995, and free of breast cancer as of July 1, 1983.
A screening mammogram is one ordered in the absence of signs or symptoms of breast cancer. A screening mammogram was classified as positive if theradiologist's reading was indeterminate or aroused a suspicion of cancer, or if there was a recommendation for non-routine follow-up, including physical examination, diagnostic mammography within the next 12 months, ultrasound examination, or biopsy. A positive screening mammogram was classified as true-positive if breast cancer (invasive or ductal carcinoma in situ) was histologically diagnosed in the patient within one year of the test, and as false-positive otherwise. This definition of a false-positive screening mammogram was consistent with current recommendations for mammography audits12–14 and reports by other investigators.1,15–17 Negative screening mammograms were those with normal readings and recommended for routine follow-up only.
All women who had at least one false-positive mammogram between July 1, 1983, and June 30, 1993, were identified for the present study. For comparison purposes, the screening mammograms of women who had no false-positive mammograms during the study period were examined. We selected for each woman with a false-positive screening mammogram a comparison woman who had a screening mammogram read as negative that was performed within 365 days of the false-positive mammogram. All mammograms identified for this study were denoted as “index mammograms.”
Data Elements
We calculated the number of screening mammograms prior to the index mammogram, and adjusted this number by available person-time since 1983 (the beginning of the 10-year study period). We also noted whether the woman had a past history of a breast symptom or a breast biopsy before the index mammogram date. Demographic variables, family history of breast cancer, and use of hormone replacement therapy during the study period (but prior to the index mammogram) were also obtained from chart review. The level of intensity of recommended follow-up for screening mammograms that were false-positive included additional views requested, six-month interval mammogram, ultrasound, and biopsy recommended.
For each woman in the study, we reviewed the medical record, including dictations, for all ambulatory visits one year before and one year after the index mammogram. Trained reviewers abstracted the computerized medical records onto standardized forms. The computerized record made it possible to exclude the medical records of the day before, the day of, and the day after the index mammogram date in order to blind abstractors to the mammogram outcome. The twelve-month “before” and “after” portions of the records were separated and all records were abstracted in random order. To test the effectiveness of our blinding strategy, medical record reviewers were asked to guess the time period for each portion abstracted.
For each outpatient visit, we recorded the date and type of visit (for example: scheduled visit, telephone call, urgent care), and the department (internal medicine, surgery, obstetrics/gynecology, or mental health). We coded all visits as either breast-related, non–breast-related, or unknown. We recorded whether the patient or the clinician initiated the visit, or if the initiation could not be determined. A visit was considered patient-initiated if a patient presented because of a new symptom, or if she was seeking follow-up for a previously known problem, for which the prior recommendation had been to follow up “as needed,” or if the patient returned much earlier than the previously documented recommendation. Scheduled clinician-recommended or referral visits and telephone calls reporting test results were considered clinician-initiated. If it could not be determined whether the patient or the clinician initiated the visit, the reason for the visit was coded “unknown.”
Dictations of ambulatory visits were reviewed for content that reflected patient concern or emotional distress related to the breast, or specifically to breast cancer; such records were flagged by the abstractors. We then used a consensus process among the investigators (MB, SM, SP, SF), who were blinded to the mammogram outcome, to determine whether patient concern was evident either from clinician documentation of patient emotional status, including descriptions of patient emotions or documentation of their own actions directed to patient's emotional needs (e.g., “urged patient to call friend,” etc).
One investigator (MB) double reviewed the first 60 charts to assure accuracy and consistency in coding. Thereafter, 2 medical record reviewers reviewed all charts with blind double review of 5% of each others'charts to assure consistency. The Human Studies Committee of Harvard Pilgrim Health Care approved the study protocol.
Analysis
All statistical analyses were performed using SAS statistical software (SAS Institute, Inc., Cary, NC). Analyses were limited to the occurrence of the first false-positive mammogram if a woman had more than one, and the corresponding normal mammograms in the comparison group of women. χ2 tests and t tests were used to compare characteristics of the group of women with false-positive screening mammograms and the group with normal mammograms.
At the woman-level, we compared documentation of concern or clinician response to concern at any visit in the two years of observation among women with false-positive mammograms with those who had normal mammograms, using χ2 tests. The relationship between documentation of concern and the intensity of recommended follow-up of the abnormal mammograms was tested using the Mantel-Haenszel χ2 test for trend.
Visits to internal medicine and surgery departments before and after the mammogram were compared within the two groups (false-positive and normal mammograms) using t tests. Visit counts between the two groups of women were also compared using t tests. The proportion of women in the normal screening mammogram group, and the proportion of those with false-positive screening mammograms with differing levels of intensity of follow-up recommended, with one or more visits to a mental health provider in the year after the mammogram were compared using χ2 tests.
We limited the multivariate analyses to those visits that we could specify as patient-initiated in order to focus on utilization that was not part of the appropriate clinician-directed follow up to an abnormal mammogram. Breast-related visits were analyzed separately from non-breast-related visits to provide specific information on the types of visits occurring after an abnormal test result, e.g., unresolved concern about the breast or visits initiated by patients for other clinical contact. We performed Poisson regression analysis (by using PROC GENMOD with option DISTRIBUTION = POISSON) to examine the relationship between having a false-positive mammogram and the number of post-mammogram patient-initiated visits to internal medicine and surgery, adjusting for the number of visits before the mammogram and patient characteristics. We expected these distributions to be asymmetric, due to the large number of women who would have few or no visits. Poisson regression is an appropriate choice for count data regression models and has been previously used with applications to health care utilization.18,19 The sample variance and mean for each model were checked for evidence of overdispersion, and adjustment made for overdispersion as needed.
The following variables were included in the models: age, estrogen use, past history of a breast symptom, past history of a breast biopsy, positive family history of breast cancer, number of previous mammograms, and intensity of recommended follow-up of abnormal mammograms. Parameter estimates were exponentiated to obtain incidence density ratios (IR) and 95% confidence intervals, an exercise similar to exponentiating parameter estimates in logistic regression to obtain odds ratios.20 The incidence ratios represent the ratio of the number of visits associated with one level of a parameter compared to the referent level.
RESULTS
Four hundred and ninety-six women had at least one false-positive screening mammogram during the ten-year period. Women in the false-positive group were significantly younger and were more likely than women with normal mammograms to have had a previous breast symptom or a previous breast biopsy in the study period (Table 1). They had fewer previous screening mammograms, and were more likely to have a positive family history of breast cancer. The recommendations for further evaluation of the false-positive mammograms are shown in Table 1.
Table 1.
Selected Characteristics in Women with False Positive and Normal Mammograms
| Study Group | ||
|---|---|---|
| Characteristic | False-Positive Mammogram (n = 496) | Normal Mammogram (n = 496) |
| Age*, y | 50.2 | 52.4† |
| Current or previous estrogen use, % | 21.6 | 20.2 |
| Previous breast symptom, % | 12.7 | 7.9† |
| History of breast biopsy, % | 6.5 | 2.2† |
| Positive family history of breast cancer, % | 24.4 | 19.2† |
| Number of previous mammograms, per year‡ | 0.24 | 0.29† |
| Recommended follow-up to mammogram, %§ | ||
| Additional mammographic views | 16.8 | |
| Follow-up mammography in 6 months | 41.1 | |
| Ultrasound | 24.0 | |
| Biopsy | 18.2 | |
Age as of 7/1/83.
P < .05.
The ratio of number of screening mammograms between 7/1/83 and the index mammogram date, divided by observation time (in years) between those two dates.
Of those for which specific recommendations were available from radiology report (n = 380).
Documentation of concern
Fifty women (10%) with false-positive mammograms had documentation in their medical records of concern about breast cancer or a clinician response to the patient's concern in the year following the mammogram, compared to 1 woman (0.2%) with a normal mammogram (P = .001). The median time between the mammogram and the first visit with a notation of concern was 37 days (mean time 65 days, range 1–336 days). Concern was documented in more than one visit in 16 women (3%). There was no difference in breast-related concern documented between the two groups prior to the index mammogram (0.5% vs 0.3%, P = .5).
Examples of clinical notations in three different patients follow: “The patient was reassured that this represented nothing more than a breast cyst in my opinion and that of the radiologist here. She nonetheless has considerable concern and is herself apparently in favor of open excisional biopsy”; “Examination today shows her to look somewhat anxious”; and “Approp[riately] upset. Dis[cusse]d possible dx's. Urged to call friend who had similar experience.”
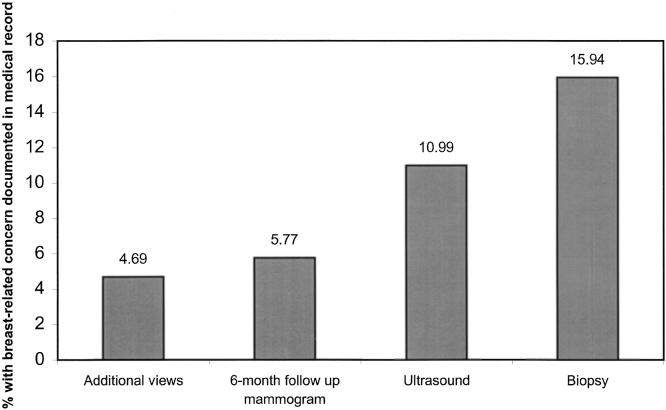
Documentation of patient concern or a clinician's response increased with the intensity of recommended follow-up (Figure 1). Nearly 16% of women for whom breast biopsies were recommended had concern documented in their medical records, compared to 4.7% of women for whom additional mammographic views were the only recommended follow-up (P = .009).
FIGURE 1.
Proportion of women with concern documented in the medical record within 12 months after a false-positive mammogram according to intensity of follow-up recommendation.
The κ statistic for agreement between the abstractors' guess as to the time period of the chart section under review and the actual time period was 0.482, indicating poor to moderate ability to discern whether the record under review represented the time period before or after the index mammogram.
Ambulatory Visits
In the year before the index mammogram, women who later had a false-positive mammogram had more breast-related visits to internal medicine and surgery departments than women with normal mammograms; this difference was of borderline significance (mean of 0.15 visits vs 0.09 visits,P = .051). After the index mammogram, overall utilization in internal medicine and surgery was higher among women with false-positive mammograms than among women with normal mammograms (8.18 visits vs 6.00 visits, P < .0001) (Table 2). As expected, clinicians initiated many more breast-related visits among women with false-positive mammograms than among women with normal mammograms (1.93 visits vs 0.07 visits, P < .0001). Women with false-positive mammograms initiated 3 times as many breast-related visits as women with normal mammograms (0.16 visits vs 0.05 visits, P = .0006). Among women with false-positive mammograms, utilization unrelated to the breast increased slightly for patient-initiated (3.68 vs 3.58), but not for clinician-initiated visits (1.52 vs 1.66) though neither of these differences reached statistical significance.
Table 2.
Mean Unadjusted Number of Ambulatory Visits* in Twelve-Month Period After a Mammogram
| Study Group | |||
|---|---|---|---|
| Visit Type | False-Positive Mammogram (n = 496) | Normal Mammogram (n = 496) | P Value |
| All ambulatory visits† | 8.18 | 6.00 | <.0001 |
| Breast-related visits | 2.34 | 0.16 | <.0001 |
| Clinician-initiated | 1.93 | 0.07 | <.0001 |
| Patient-initiated | 0.16 | 0.05 | .0006 |
| Unknown | 0.25 | 0.04 | <.0001 |
Includes visits to internal medicine and surgery.
Includes visits that are breast-related, non–breast-related, and visits in which breast-relatedness could not be determined.
Increases in health care utilization were not limited to departments of surgery and internal medicine. Among the women with false-positive mammograms, those with recommendation for breast biopsies were more likely to visit mental health providers at least once after the mammogram, compared to women with normal mammograms (20.3% vs 9.3%, P = .005).
Using multivariate models, we analyzed the number of patient-initiated visits, i.e., excluding scheduled follow-up requested by clinicians, in the year after the index mammogram. In the models we controlled for age, number of patient-initiated visits in the year before the mammogram, use of estrogen replacement therapy, family history of breast cancer, annualized number of previous mammograms, previous breast symptom, history of breast biopsy, and intensity of recommended follow-up. The result of the index mammogram was the most powerful single predictor of patient-initiated, breast-related visits in the year after the index mammogram, with an incidence ratio of 4.03(95% CI, 2.97 to 5.47) (Table 3); a previous history of breast symptoms and estrogen use also were associated with a significant increase in the number of breast-related visits. For non–breast-related visits initiated by patients, the outcome of the index mammogram was again a significant independent predictor of utilization, withan incidence ratio of 1.18 (95% CI, 1.09 to 1.28) (Table 4), corresponding to an 18% increase in number of visits after a false-positive mammogram. The number of previous mammograms and estrogen use were also associated with increased utilization in the year after a false-positive mammogram.
Table 3.
The Relationship Between Patient-initiated Breast-related Visits in the Year After a Mammogram and Certain Variables*
| Parameter | β | SE | Incidence Ratio† | 95% Confidence Interval |
|---|---|---|---|---|
| False-positive outcome | 1.3939 | 0.3051 | 4.03 | 2.97 to 5.47 |
| Previous breast symptom | 1.0573 | 0.2540 | 2.88 | 2.23 to 3.71 |
| Number of visits before mammogram | 0.6969 | 0.2567 | 2.01 | 1.55 to 2.60 |
| Estrogen use | 0.6493 | 0.2209 | 1.91 | 1.53 to 2.39 |
| Age at mammogram | −0.0052 | 0.0134 | 0.99 | 0.98 to1.01 |
Poisson regression analysis model also includes family history of breast cancer, previous breast symptom, severity of follow-up recommendation, and history of breast biopsy (none significant).
For example, the incidence ratio gives the ratio of the number of visits in one year after the event by women with false-positive mammogram outcome relative to the number of visits by women with normal mammograms.
Table 4.
The Relationship Between Patient-initiated Non-breast-related Visits in the Year After a Mammogram and Certain Variables*
| Parameter | β | SE | Incidence Ratio | 95% Confidence Interval |
|---|---|---|---|---|
| Number of previous mammograms | 0.4746 | 0.1267 | 1.61 | 1.42 to 1.83 |
| Estrogen use | 0.2530 | 0.0554 | 1.29 | 1.22 to 1.36 |
| False-positive outcome | 0.1662 | 0.0817 | 1.18 | 1.09 to 1.28 |
| Number of visits before the mammogram | 0.0612 | 0.0034 | 1.06 | 1.06 to 1.07 |
| Age at mammogram | 0.0067 | 0.0029 | 1.01 | 1.00 to 1.01 |
Poisson regression analysis model also includes family history of breast cancer, previous breast symptom, severity of follow-up recommendation, and history of breast biopsy (none significant).
DISCUSSION
We found that clinicians noted concern among 10% of women who experienced false-positive mammograms. Because medical record review is an insensitive tool for detecting patient concern, it is likely that 10% underestimates the true frequency of clinician awareness of concern in women after abnormal screening mammograms. These concerns were not limited to the time period immediately following the mammogram: the median time between the mammogram and the visit in which concern is documented was 37 days.
We also found a significant increase in health care utilization in the year after a false-positive mammogram compared to the previous year, using multivariate analysis to take account of systematic differences between the women with false-positive and normal screening mammograms. Increased numbers of visits initiated by clinicians is in keeping with the findings of others,2,11 who have described significant numbers of procedures and follow-up visits after positive mammograms. While increased utilization for workup of an abnormality is expected, we found that in addition, patients themselves initiated a significantly higher number of visits, both related to breast issues, and unrelated to the breast, in the period after a false-positive mammogram. Our finding of an 18% increase in ambulatory visits not related to the breast among women with false-positive mammograms is the first demonstration of this phenomenon occurring after mammography that we could find in the literature and deserves further study.
The increase we found in health care utilization in the year after a false-positive mammogram is small, but could translate into large effects at the national level. Our previous work has shown that approximately50% of women undergoing annual mammography screening over a ten-year period will experience at least one false-positive mammogram.2 Therefore, the increase in visits we found after a false-positive mammogram may apply to 16 million women in the United States over a decade, and could result in 9.7 million breast-related visits (Excess in utilization is represented by incidence ratio − 1; the estimated number of excess visits is calculated by the product of the excess incidence ratio × number of women affected × number of visits per year at baseline or [4.03 − 1.0] × 16 million women × .2 breast-related visits per woman per year = 9.7 million visits.) and 14.4 million non–breast-related visits (Excess ulilization in non–breast-related visits represented by [1.18−1.00] × 16 million women × 5 non-breast-related visits per woman per year = 14.4 million visits.).
Since Fentiman initially described “iatrogenic anxiety” due to mammography recalls,21 several studies have confirmed its existence. The majority of the published data regarding women's psychological responses to screening and to false-positive mammograms (all gathered with telephone interviews and mailed surveys) have found that women with false-positive mammograms report more anxiety or concern about breast cancer compared with women whose mammograms were normal.3–5,22–25 The percentage of women with concern in previous studies3,22,26 varied from 33%26 to 63%.22 Although we found a lower prevalence of concern than these studies, our method of detecting concern (examination of clinician records) has a lower sensitivity than do survey methods, both because women may not always share this concern, and clinicians may not always record it. Despite its lower sensitivity, record review is a valuable adjunct to survey research because it is free from the biases inherent in survey research such as response bias or social desirability bias.
The length of time patient concern persists after a false-positive mammogram has varied in studies,4,5,23,24,27,28 levels of anxiety in women with false-positive mammograms became comparable to those with normal mammograms within a period of 6 weeks27 to 18 months.4 We limited our medical record review to a twelve-month period after the index mammogram, and found that clinicians documented concern in the medical records of women with false-positive mammograms up to 11 months after the mammogram date.
We found only one other study reporting on overall health care utilization after mammograms. Gram et al. found no difference in women's self-reported frequency of visits to clinicians between those with false-positive mammograms and those with normal mammograms.4 Our method (chart review) for measuring utilization was more accurate. Our findings suggest that, beyond recommended follow-up, false-positive mammograms are associated with higher numbers of patient-initiated visits for both breast-related and non–breast-related reasons, including increased use of mental health services. Severe mental distress has been alluded to previously in a case report of suicide after a false-positive mammogram.29 Whether an increase in mental health utilization was due to anxiety caused by the abnormal screening test, or an exacerbation of an underlying psychiatric disorder by the event, is not possible to know because visit records for mental health were not reviewed in detail. This finding should be sought in other settings and if persistent, is worthy of further study.
Studies of other screening modalities have described how labeling due to abnormal test results may affect behavior,8 and that the assumption of a “sick role” may entail more frequent visits to a health professional.9 On the other hand, women in our study may have been motivated by the experience to seek treatment for other, previously neglected concerns, such as hypertension or diabetes. While the effects of labeling were initially described after screening for hypertension,7,8 few other screening efforts have been investigated as to their social and psychological consequences.30–32 The increasing numbers of traditional and genetic screening tests, and the increasing participation in these tests means that small effects on an individual level may have a large cumulative impact. We believe our finding may be relevant to clinicians counseling patients after screening procedures.
The computerized medical records allowed us to shuffle medical records and blind the abstractors as to the mammography outcome. Because visits to surgeons and internists for follow-up procedures were part of the medical record for women with false-positive mammograms, an abstractor could have been potentially influenced in her documentation of concern if she suspected the woman to be from a particular study group. However, the low κ (0.482) for the abstractors' ability to guess the time period of the woman's chart that was being reviewed suggests that this bias, if present, was small. The number of ambulatory visits before and after the mammograms should not be subject to similar bias because they are simple counts.
Baseline differences in the study and comparison groups on several clinical variables are expected because of the increased chance of a false-positive mammogram occurring in women with a particular history.33 We were able to control for these variables in our multivariate analysis, however, the existence of other unmeasured variables cannot be ruled out.
This study took advantage of utilization data for a known population in an ambulatory care setting that used an automated medical record system containing full text and dictation for almost all ambulatory visits. While likely less sensitive than prospectively collected survey data for detection of patient anxiety, our study method provides valuable support to those findings because it is not subject to response bias, which affected previous studies done in this field. Reliance on clinician's notes is an insensitive measure, which would bias our findings toward the null; in addition clinicians may be more likely to document concern in women with positive mammograms. Evidence against the latter causing significant bias comes from other studies that collected data directly from women3–5,22–25 and documented findings similar to ours.
Mammography is a valuable tool for the early detection of breast cancer, but like all screening procedures, it has both benefits and harms.34 While some concern about breast cancer may be appropriate and encourage women to continue screening, it is not known how much is too much. Excess concern about breast cancer caused by false-positive mammograms, and the potential excess utilization of health care services associated with a false-positive result suggest that health care providers need to find ways to reassure patients after abnormal mammograms. Current efforts by radiology practices to decrease the proportion of examinations recommended for follow-up,35 to provide immediate review of mammograms,36 and to expedite the completion of follow-up are important steps toward these goals. Best practices in communicating with women prior to undergoing screening need to be established, to be sure that women understand the possibility of false-positive results when undergoing mammography.
Acknowledgments
We gratefully acknowledge the contributions of Nana Amma Twum-Danso, MD, who assisted in the conceptualization of the study questions, and collected pilot data for this project. This study was supported by the Thomas O. Pyle fellowship and a project grant from the Harvard Pilgrim Health Care Foundation and by the American Cancer Society. Dr. Elmore was the recipient of a Robert Wood Johnson Generalist Faculty Award.
REFERENCES
- 1.Brown ML, Houn F, Sickles EA, Kessler LG. Screening mammography in community practice: positive predictive value of abnormal findings and yield of follow-up procedures. AJR Am J Roentgenol. 1995;165:1373–7. doi: 10.2214/ajr.165.6.7484568. [DOI] [PubMed] [Google Scholar]
- 2.Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false-positive screening mammograms and clinical breast examinations. N Engl J Med. 1998;338:1089–96. doi: 10.1056/NEJM199804163381601. [DOI] [PubMed] [Google Scholar]
- 3.Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med. 1991;114:657–61. doi: 10.7326/0003-4819-114-8-657. [DOI] [PubMed] [Google Scholar]
- 4.Gram IT, Lund E, Slenker SE. Quality of life following a false positive mammogram. Br J Cancer. 1990;62:1018–22. doi: 10.1038/bjc.1990.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellman R, Angeli N, Christians A, Moss S, Chamberlain J, Maguire P. Psychiatric morbidity associated with screening for breast cancer. Br J Cancer. 1989;60:781–4. doi: 10.1038/bjc.1989.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanson V, McIntosh IB, Power KG, Dobson H. The psychological effects of breast screening in terms of patients' perceived health anxieties. Br J Clin Pract. 1996;50:129–35. [PubMed] [Google Scholar]
- 7.Johnston ME, Gibson ES, Terry CW, et al. Effects of labelling on income, work and social function among hypertensive employees. J Chronic Dis. 1984;37:417–23. doi: 10.1016/0021-9681(84)90025-0. [DOI] [PubMed] [Google Scholar]
- 8.Haynes RB, Sackett DL, Taylor DW, Gibson ES, Johnson AL. Increased absenteeism from work after detection and labeling of hypertensive patients. N Engl J Med. 1978;299:741–4. doi: 10.1056/NEJM197810052991403. [DOI] [PubMed] [Google Scholar]
- 9.Reid MC, Schoen RT, Evan J, Rosenberg JC, Horwitz RI. The consequences of overdiagnosis and overtreatment of Lyme disease: an observational study. Ann Intern Med. 1998;128:354–62. doi: 10.7326/0003-4819-128-5-199803010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Healy BP. Breast cancer in the news: the rise of consumer power in medical care [editorial] J Womens Health. 1997;6:141–2. doi: 10.1089/jwh.1997.6.141. [DOI] [PubMed] [Google Scholar]
- 11.Welch HG, Fisher ES. Diagnostic testing following screening mammography in the elderly. J Natl Cancer Inst. 1998;90:1389–92. doi: 10.1093/jnci/90.18.1389. [DOI] [PubMed] [Google Scholar]
- 12.Linver M, Osuch J, Brenner R, Smith R. The mammography audit: a primer for the Mammography Quality Standards Act (MQSA) AJR Am J Roentgenol. 1995;165:19–25. doi: 10.2214/ajr.165.1.7785586. [DOI] [PubMed] [Google Scholar]
- 13.Sickles EA. Quality assurance: how to audit your own mammography practice. Radiol Clin North Am. 1992;30:265–75. [PubMed] [Google Scholar]
- 14.Bassett L, Hendrick R, Bassford T, et al. Quality determinants of mammography. Clinical practice guideline no. 13. Rockville, Md: Agency for Health Care Policy and Research; 1994. [Google Scholar]
- 15.Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Likelihood ratios for modern screening mammography: risk of breast cancer based on age and mammographic interpretation. JAMA. 1996;276:39–43. doi: 10.1001/jama.276.1.39. [DOI] [PubMed] [Google Scholar]
- 16.Bird R. Low-cost screening mammography: report on finances and review of 21,716 consecutive cases. Radiology. 1989;171:87–90. doi: 10.1148/radiology.171.1.2494683. [DOI] [PubMed] [Google Scholar]
- 17.Robertson C. A private breast imaging practice: medical audit of 25,788 screenings and 1,077 diagnostic examinations. Radiology. 1993;187:75–9. doi: 10.1148/radiology.187.1.8451440. [DOI] [PubMed] [Google Scholar]
- 18.Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17:59–68. doi: 10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Christiansen CL, Morris CN. Hierarchical poisson regression modeling. J Am Stat Assoc. 1997;92:618–32. [Google Scholar]
- 20.Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. Cary, NC: SAS Institute; 1997. p. 465. [Google Scholar]
- 21.Fentiman IS. Pensive women, painful vigils: consequences of delay in assessment of mammographic abnormalities. Lancet. 1988:1041–2. doi: 10.1016/s0140-6736(88)91854-5. [DOI] [PubMed] [Google Scholar]
- 22.Ong G, Austoker J, Brett J. Breast screening: adverse psychological consequences one month after placing women on early recall because of diagnostic uncertainty. A multicentre study. J Am Med Screening. 1997;4:158–68. doi: 10.1177/096914139700400309. [DOI] [PubMed] [Google Scholar]
- 23.Scaf-Klomp W, Sanderman R, van de Wiel HB, Otter R, van den Heuvel WJ. Distressed or relieved? Psychological side effects of breast cancer screening in The Netherlands. J Epidemiol Comm Health. 1997;51:705–10. doi: 10.1136/jech.51.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton S, Saidi G, Bickler G, Hunter J. Does routine screening for breast cancer raise anxiety? Results from a three wave prospective study in England. J Epidemiol Comm Health. 1995;49:413–8. doi: 10.1136/jech.49.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsson P, Armelius K, Nordahl G, Lenner P, Westman G. Women with false positive screening mammograms:how do they cope? J Med Screening. 1999;6:89–93. doi: 10.1136/jms.6.2.89. [DOI] [PubMed] [Google Scholar]
- 26.Lindfors KK, O'Connor J, Acredolo CR, Liston SE. Short-interval follow-up mammography versus immediate core biopsy of benign breast lesions: assessment of patient stress. AJR Am J Roentgenol. 1998;171:55–8. doi: 10.2214/ajr.171.1.9648763. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert FJ, Cordiner CM, Affleck IR, et al. How anxiogenic is recall following breast screening and does a family history of breast cancer make a difference? Psycho-Oncology. 1995;4:88. Abstract. [Google Scholar]
- 28.Brett J, Austoker J, Ong G. Do women who undergo further investigation for breast screening suffer adverse psychological consequences? A multi-centre follow-up study comparing different breast screening result groups five months after their last screening appointment. J Public Health Med. 1998;20:396–403. doi: 10.1093/oxfordjournals.pubmed.a024793. [DOI] [PubMed] [Google Scholar]
- 29.Weil JG, Hawker JI. Positive findings of mammography may lead to suicide (letter) Br Med J. 1997;314:754–4. [PMC free article] [PubMed] [Google Scholar]
- 30.Cadman D, Chambers LW, Walter SD, Ferguson R, Johnston N, McNamee J. Evaluation of public health preschool child developmental screening: the process and outcomes of a community program. Am J Public Health. 1977;77:45–51. doi: 10.2105/ajph.77.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paskett ED, Rimer BK. Psychosocial effects of abnormal pap tests and mammograms: a review. J Womens Health. 1995;4:73–82. [Google Scholar]
- 32.Peshkin BN, Lerman C. Genetic counselling for hereditary breast cancer. Lancet. 1999;353:2176–7. doi: 10.1016/S0140-6736(99)90078-8. [DOI] [PubMed] [Google Scholar]
- 33.Brenner R, Pfaff JM. Mammographic changes after excisional breast biopsy for benign disease. AJR Am J Roentgenol. 1996;167:1047–52. doi: 10.2214/ajr.167.4.8819410. [DOI] [PubMed] [Google Scholar]
- 34.Rimer BK. Putting the “informed” in informed consent about mammography. J Natl Cancer Inst. 1995;87:703–4. doi: 10.1093/jnci/87.10.703. [DOI] [PubMed] [Google Scholar]
- 35.Sickles EA. False positive rate of screening mammography [letter] N Engl J Med. 1998;339:561–2. [PubMed] [Google Scholar]
- 36.Liu S, Bassett LW, Sayre J. Women's attitudes about receiving mammographic results directly from radiologists. Radiology. 1994;193:783–6. doi: 10.1148/radiology.193.3.7972824. [DOI] [PubMed] [Google Scholar]