Abstract
OBJECTIVE
Recent reports have linked calcium channel blockers (CCBs) with an increased risk of acute myocardial infarction (AMI). We sought to determine to what extent physicians relinquished CCBs following these adverse reports and if there were differences in the use of CCBs and other AMI therapies across 3 levels of specialist involvement: generalist attendings, collaborative care (generalist with cardiologist consultation), and cardiologist attendings.
DESIGN
We measured use of CCbsduring hospitalization for AMI before (1992-1993) and after (1995-1996) the adverse CCb reports, controlling for hospital-, physician-, and patient-level variables. We also examined use of efective medications (aspirin, β-blockers, thrombolhytic therapy) and ineffective AMI treatments (lidocaine).
SETTINGS
Thirty-seven community-based hospitals in Minnesota.
PATIENTS
Population-based sample of 5,347 patients admitted with AMI.
MEASUREMENTS
The primary outcome was prescription of a CCB at the time of discharge from hospital. Secondary outcomes included use of other effective and ineffective AMI therapies during hospitalization and at discharge.
MAIN RESULTS
Compared wit cariologists, generalist attendings were less likely to use aspirin (37% va 68% adusted odds ratio [OR], 0.58; 95% confidence interval [95% CI), 0.42 to 0.80) and thrombolytics (29% vs 64% adjusted OR, adjusted OR, 0.93; 95% CI, 0.66 to 1.31). From 1992–1993 to 1995–1996, the use of CCBs in patients with AMI decresed from 24% to 10%, the net result of physicians starting CCBs less often and discontinuing them more often. In multivariate models, the odds of CCB relinquishment after the adverse reports (adjusted Or, 0.33; 95% CI, 0.27 to 0.39) were independent of, and not modified by, the involvement of a cardiologist.
CONCLUSIONS
Compared with cardiologists, generalist physicians were less likely to adopt some effective AMI therapies, particularly those associated with risk such as thrombolytic therapy. However, generalists were as likely as cardiologists to relinquish CCBs after the adverse reports. This pattern of practice may be the generalist physicians' response to an expanding, but increasingly risky and uncertain, pharmacopoeia.
Keywords: acute myocardial infarction, drug utilization, calcim channel blockers, physician specialty, prescribing, adoption, relinquishment
In recent years, a growing number of studies have compared the performance of generalist and specialist physicians in the management of common conditions,1–5 such as acute myocardial infarction [AMI].6–11 Typically, these studies have documented the underuse of proven effective therapies by generalists. Usually, generalists are thought to have less knowledge of recent advances or less condition-specific experience than specialists.1–12 Alternately, generalists may be more cautious about change and innovation, or more concerned about risks and complications than benefits.1–4,6,13–17
Two assumptions are common to many of the above studies. The first is that superior knowledge translates into superior practice.4,9,12,17–19 However, a great deal of evidence to the contrary exists.14,17–23 A second assumption is that rapid adoption of new advances is an unqualified good.4,13–16,24,25 The history of medicine, however, is punctuated with examples of innovations that were rapidly adopted and later found to be ineffective or harmful.16,24,25 Specialists may adopt emerging therapies more aggressively, independent of the available evidence, while generalists may deliberately delay adoption until there is more evidence of benefit or the long-term risks are better understood.1,4,15,25 This delayed adoption is variously attributed to caution,1,4,6 skepticism,14,23 inertia,26 a “wait-and-see attitude,”4,27 or conservatism.4,16,17 Rather than studying adoption, some researchers have suggested that a more informative approach for understanding decision making might be studying relinquishment.25,27–29 Relinquishment is the abandoning of a therapy that is considered ineffective or harmful, and for which an alternative exists.25 Unlike adoption, relinquishment may be a “rational clinical decision, rather than a response to nonclinical pressures.”25
We studied the relinquishment of calcium channel blocker (CCB) therapy for patients with AMI. Introduced in the mid-1980s, CCBs were new, expensive, heavily marketed, well tolerated, presumed safe, and capable of decreasing both blood pressure and angina.24,30,31 Within a decade, physicians had adopted CCBs for 30% to 40% of all patients with AMI discharged from hospital, although these drugs had never been convincingly demonstrated to prolong life or prevent adverse cardiovascular events.30–33 Beginning in March 1995, a number of adverse reports appeared in the literature, linking the use of short-acting CCBs with an increased risk of AMI.34–36 Associated with these reports were editorials urging cautious interpretation and a need for further study,37–39 and waves of intense media scrutiny.37,40,41 Regardless of the validity of these adverse reports, CCBs, as a class, became associated with potential risk. Furthermore, evidence of benefit in the AMI setting was still lacking.30,31
We hypothesized that after the adverse reports there would be a decrease in the use of CCBs in patients with AMI, and generalists would be as likely to decrease the use of CCBs as specialists. To test these hypotheses, we performed cross-sectional analyses of population-based data on the treatment of AMI by generalists and specialists, collected before (1992–1993) and after (1995–1996) the widespread scientific and media reports concerning the safety of CCBs. We also studied the use of other AMI treatments, both effective (aspirin, β-blockers, and thrombolytic therapy) and ineffective (lidocaine), and examined differences in the use of these agents across physician specialty as well as changes over time.
METHODS
Patients and Setting
The data for the present study are from the medical records of patients admitted with AMI to 37 Minnesota hospitals. These hospitals were involved in a controlled quality improvement intervention for the early treatment of AMI.42 The intervention itself was not directed at CCBs and did not affect their use. Data were collected for 10 months before (October 1992 to August 1993) and 10 months after (July 1995 to May 1996) the first adverse reports about CCBs. Our study hospitals represented more than 80% of all community hospital beds and more than half of all AMIs statewide. Two of the hospitals were public teaching hospitals, and 17 were located in rural communities.
During the 20 months of data collection, 5,347 patients (2,409 patients before; 2,938 patients after) were admitted with a diagnosis of acute or suspected myocardial infarction (MI) and met at least 2 of the following criteria: (1) clinical symptoms typical of AMI, (2) compatible electrocardiographic findings as documented in the medical record, and (3) elevated serum creatine kinase and myocardial band fractions. Patients were excluded if they died before admission, were transferred from a nonstudy hospital, or had suffered an AMI in the previous 2 weeks. We also excluded patients with missing data ( n =210, 3.9%), for a final study sample of 5,138 patients.
Dependent Variable
The primary outcome was the prescription of any CCB at discharge. Decreased use of CCBs between our 2 observation periods represented “relinquishment.” Because the decision not to start a medication may be different from stopping a medication, we also performed separate analyses for the 3,903 patients who were not taking CCBs at admission (potentially eligible for starting) and the 1,235 patients who were already using CCBs at the time of admission (potentially eligible for stopping).
Independent Variables
We described temporal trends by using a binary variable, the prescription of a CCB before (1992–1993) or after (1995–1996) the adverse reports. We collected data at the level of the hospital ( n =37), physician ( n =2,265), and patient ( n =5,138). At the hospital level, we assessed teaching status, location (urban or rural), and relative AMI volume (<50, 50 to 100, or >100 AMIs per year).
We defined 3 levels of involvement by specialist physicians in the care of AMI patients: (1) generalist attending (an internist, family physician, or general practitioner as attending); (2) collaborative care (a generalist attending with a consultation from a cardiologist); and (3) cardiologist attending.9 We also determined attending physician age (<40, 40 to 50, or >50 years) and board certification.
We collected demographic and socioeconomic variables for each patient, including age (<55, 55 to 64, 65 to 74, or >74 years), gender, race (white or nonwhite), and health insurance status (fee for service [Medicaid, Blue Cross, Medicare, other commercial coverage] or managed care [including Medicare HMO]). Variables related to the patient's previous clinical history included the presence of angina, prior MI, revascularization, congestive heart failure, atrial fibrillation, hypertension, hyperlipidemia, diabetes mellitus, peripheral vascular disease, bronchospastic diseases, known β-blocker intolerance, and preadmission medications.
We collected information on the use of AMI therapies and other clinical variables related to the patient's hospital course, including the use of thrombolytic therapy and lidocaine, development of post-AMI angina, congestive heart failure, atrial fibrillation, or hypertension. We assessed noncardiac comorbidity with Greenfield's Index of Co-Existent Disease (ICED).43 This index has components for coexistent diseases and their severity, based on physical impairment and functional status. We graded each component on an ordinal scale, and then combined levels of coexistent diseases and impairment into a final 4-point scale, ranging from no comorbidity (ICED =0) to severe comorbidity (ICED =3).43 Based on the results of our previous work,44 we used ICED score as a binary variable, i.e., the absence (ICED =0, 1, or 2) or presence (ICED =3) of severe comorbidity. Finally, we collected data on all cardiovascular medications prescribed at discharge, including aspirin, β-blockers, nitrates, loop diuretics, angiotensin-converting enzyme (ACE) inhibitors, and CCBs.
Data Collection and Integrity
Nurses experienced in the care of AMI patients collected data from medical records using an abstraction instrument designed for the study. Abstractors were required to demonstrate initial and ongoing interrater agreement with a criterion review of 95% or more.42 We conducted retrospective audits of a random sample of 10% of each abstractor's completed cases to ensure each abstractor met and maintained the data quality standard of 95% all-item agreement with a study auditor.42
Statistical Analysis
We performed all analyses using SAS 6.12 (SAS/STAT User's Guide. Version 6. Vol. 2. 4th ed. SAS Institute, Cary, NC). Using χ2tests for univariate analyses, we compared study variables across the 3 levels of cardiologist involvement, including their use of effective (aspirin, β-blockers, and thrombolytics) and ineffective (CCBs and lidocaine) therapies for AMI. We also studied differences in the use of these 5 AMI therapies between our initial observation period (1992–1993) and our follow-up period (1995–1996).
We used multivariate logistic regression to study the association between the involvement of a cardiologist and the use of CCBs at discharge, controlling for time and all variables significantly associated ( P <.05) with the level of cardiologist involvement in univariate analysis. We included 4 additional terms in these models (prior use of CCBs, bronchospastic disease, prior AMI, and post-AMI angina) because these variables were found to be significantly associated with the use of CCBs at discharge. To examine whether temporal trends were modified by the involvement of a cardiologist, we included pairwise interaction terms (collaborative care by time and generalist attending by time) in models that used cardiologists as the referent group. Neither interaction term was statistically significant ( P >.05), so these terms were excluded from final models. We used similar modeling strategies to examine the multivariate association between the level of cardiologist involvement and the use of aspirin, β-blockers, thrombolytic therapy, and lidocaine.
We used generalized estimating equations (GEE) to account for the possible lack of independence (clustering) of patients treated within the same hospital,45–47 a manifestation of local practice style.20,21 We used robust, model-based GEE estimates for all adjusted odds ratios (OR), 95% confidence intervals (CI), and P values.45,46
RESULTS
Univariate Analysis
Most of the 5,138 patients studied were elderly (mean age, 67±14 years); 62% were male; and most were white (89%). A description of study variables, stratified across different levels of cardiologist involvement, appears in Table 1). Most patients (76%) had a cardiologist involved in their care, either as an attending physician (23%) or a consultant (53%). However, the patients cared for by generalist attendings were substantially older and had more severe comorbidity than the patients for whom a cardiologist was consulted or was the attending.
Table 1.
Variables Stratified by the Level of Cardiologist Involvement*
| Variable | Overall, %N =5138 | Generalist Attending, %N =1247 | Collaborative Care, %N =2727 | Cardiologist Attending, %N =1164 | P Value |
|---|---|---|---|---|---|
| Hospital-level | |||||
| Urban | 82 | 47 | 91 | 97 | <.001 |
| High volume | 66 | 37 | 76 | 73 | <.001 |
| Physician-level | |||||
| Board-certified | 82 | 86 | 74 | 97 | <.001 |
| Age >50 y | 21 | 17 | 23 | 21 | .008 |
| Patient-level Sociodemographic | |||||
| Age >74 years | 32 | 46 | 31 | 20 | <.001 |
| Female | 38 | 44 | 38 | 28 | <.001 |
| White | 89 | 89 | 91 | 88 | >.5 |
| Managed care | 30 | 19 | 34 | 33 | <.001 |
| Previous medical history | |||||
| Prior use of CCBs | 24 | 25 | 24 | 24 | >.5 |
| Prior hypertension | 49 | 47 | 52 | 44 | <.001 |
| Prior atrial fibrillation | 8 | 10 | 7 | 6 | .002 |
| Hyperlipidemia | 33 | 22 | 36 | 38 | <.001 |
| Diabetes mellitus | 21 | 23 | 22 | 16 | <.001 |
| Peripheral vascular disease | 9 | 8 | 11 | 7 | <.001 |
| Bronchospastic disease | 15 | 15 | 16 | 14 | >.5 |
| Prior CHF | 13 | 19 | 12 | 8 | <.001 |
| Prior AMI | 26 | 28 | 25 | 25 | .08 |
| Prior PTCA or CABG | 14 | 10 | 14 | 18 | <.001 |
| Severe comorbidity† | 24 | 29 | 24 | 18 | <.001 |
| Hospital course | |||||
| Post-AMI angina | 63 | 61 | 63 | 66 | .07 |
| Post-AMI CHF | 23 | 27 | 24 | 20 | <.001 |
| Thrombolytic use | 44 | 29 | 42 | 64 | <.001 |
| Lidocaine use | 28 | 28 | 29 | 27 | >.5 |
| Discharge medications | |||||
| Aspirin | 57 | 37 | 60 | 68 | <.001 |
| β Blockers | 36 | 20 | 40 | 46 | <.001 |
| Nitrates | 44 | 34 | 46 | 50 | <.001 |
| ACE inhibitors | 18 | 13 | 20 | 19 | <.001 |
| Loop diuretics | 18 | 20 | 18 | 15 | <.001 |
| CCBs | 16 | 14 | 16 | 19 | .008 |
From 5138 patients hospitalized with acute myocardial infarction (AMI). Collaborative care refers to a generalist attending with cardiologist consultation. CHF indicates congestive heart failure; PTCA, percutaneous transluminal coronary angioplasty; CABG, coronary artery bypass grafting; CCB, calcium channel blockers; ACE, angiotensin converting enzyme.
Based on Greenfield's Index of Co-existent Disease.
In general, there appeared to be a graded relationship between the increased use of effective AMI therapies and the greater involvement of a cardiologist in providing care ( Table 1 and Table 2). For example, thrombolytic therapy was used in 29% of patients of generalist attendings, 42% of patients when a generalist consulted a cardiologist, and 64% of patients of cardiologists. Over time, there was an increase in the use of all effective AMI therapies (Table 3). In unadjusted analyses, we observed that the more directly involved a cardiologist was in providing AMI care, the greater was the adoption of effective therapies over time.
Table 2.
Overall Use of Therapies for Acute Myocardial Infarction According to the Level of Cardiologist Involvement*
| Generalist Attending vs Cardiologist Attending | Collaborative Care vs Cardiologist Attending | |||
|---|---|---|---|---|
| Therapy | Odds Ratio (95% Cl) | Adjusted Odds Ratio†(95% Cl) | Odds Ratio (95% Cl) | Adjusted Odds Ratio†(95% Cl) |
| Aspirin | 0.28 (0.24 to 0.33) | 0.58 (0.42 to 0.80) | 0.71 (0.61 to 0.82) | 1.10 (0.90 to 1.34) |
| β Blockers | 0.29 (0.25 to 0.35) | 0.93 (0.66 to 1.31) | 0.76 (0.66 to 0.87) | 0.93 (0.84 to 1.40) |
| Thrombolytic | 0.23 (0.19 to 0.27) | 0.18 (0.13 to 0.25) | 0.41 (0.35 to 0.47) | 0.45 (0.38 to 0.53) |
| CCBs | 0.71 (0.57 to 0.88) | 0.83 (0.57 to 1.21) | 0.86 (0.72 to 1.02) | 0.88 (0.71 to 1.09) |
| Lidocaine | 0.79 (0.66 to 0.94) | 0.78 (0.55 to 1.09) | 0.76 (0.66 to 0.88) | 0.86 (0.72 to 1.03) |
*From 5138 patients hospitalized with acute myocardial infarction (AMI). Cardiologist attendings cared for 1164 patients, and were consulted for 2727 patients (collaborative care); generalist attendings cared for 1247 patients. CCBs indicates calcium channel blockers, CI, confidence interval.
Separate multivariate models for each therapy with cardiologist attendings as the reference group; adjusted for all significant variables presented in Table 1, using multiple logistic regression and generalized estimating equations (see Methods).
Table 3.
Temporal Trends in the Use of Therapies for Acute Myocardial Infarction, Stratified by the Level of Cardiologist Involvement*†
| Variable | 1992–1993 N =2,265 (%) | 1995–1996 N =2,873 (%) | Adjusted Odds Ratio†(95% Cl) 1995–1996 vs. 1992–1993 |
|---|---|---|---|
| Aspirin | 1203 (53) | 1707 (59) | 1.48 (1.25 to 1.73) |
| Cardiologist attending | 335/564 (59) | 460/600 (77) | 1.97 (1.43 to 2.74) |
| Collaborative care | 639/1091 (59) | 1009/1636 (62) | 1.40 (1.16 to 1.71) |
| Generalist attending | 229/610 (38) | 238/637 (37) | 1.20 (0.90 to 1.61) |
| β Blockers | 697 (31) | 1178 (41) | 1.32 (1.12 to 1.55) |
| Cardiologist attending | 196/564 (35) | 344/600 (57) | 1.65 (1.21 to 2.27) |
| Collaborative care | 385/1091 (35) | 697/1636 (43) | 1.23 (0.94 to 1.62) |
| Generalist attending | 116/610 (19) | 137/637 (22) | 1.29 (0.90 to 1.87) |
| Thrombolytic | 930 (41) | 1330 (46) | 1.71 (1.48 to 1.97) |
| Cardiologist attending | 337/564 (60) | 410/600 (68) | 1.59 (1.18 to 2.14) |
| Collaborative care | 431/1091 (40) | 719/1636 (44) | 1.60 (1.33 to 1.92) |
| Generalist attending | 162/610 (27) | 201/637 (32) | 2.10 (1.51 to 2.93) |
| CCBs | 536 (24) | 298 (10) | 0.33 (0.27 to 0.39) |
| Cardiologist attending | 155/564 (28) | 61/600 (10) | 0.23 (0.15 to 0.36) |
| Collaborative care | 266/1091 (24) | 179/1636 (11) | 0.35 (0.28 to 0.45) |
| Generalist attending | 115/610 (19) | 58/637 (9) | 0.30 (0.18 to 0.48) |
| Lidocaine | 846 (37) | 582 (20) | 0.31 (0.26 to 0.37) |
| Cardiologist attending | 255/564 (45) | 117/600 (20) | 0.21 (0.15 to 0.30) |
| Collaborative care | 368/1091 (34) | 350/1636 (21) | 0.41 (0.33 to 0.50) |
| Generalist attending | 223/610 (37) | 115/637 (18) | 0.26 (0.19 to 0.37) |
*From 5138 patients hospitalized with acute myocardial infarction (AMI). Cardiologist attendings cared for 1,164 patients, and were consulted for 2,727 patients (collaborative care); generalist attendings cared for 1,247 patients. CCBs indicates calcium channel blockers; CI, confidence intervals.
Separate multivariate models for each therapy, stratified by the level of cardiologist involvement, and then adjusted for all significant variables presented in Table 1, using multiple logistic regression and generalized estimating equations (see Methods).
The use of CCBs decreased somewhat during the 10 months of the 1992–1993 observation period, from 26% (October/November 1992) to 22% (July/August 1993; P =.065 for linear trend) of all AMI patients. This is consistent with the 3% to 4% decrease per year described in the literature for this same time period.32,33 During this initial period, which was before the adverse reports, the patients of cardiologists were more likely to use CCBs than collaborative care patients or the patients of generalists (28% vs 24% vs 19%, P =.002).
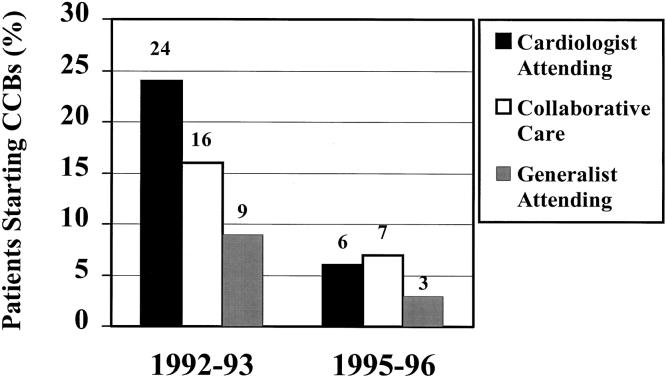
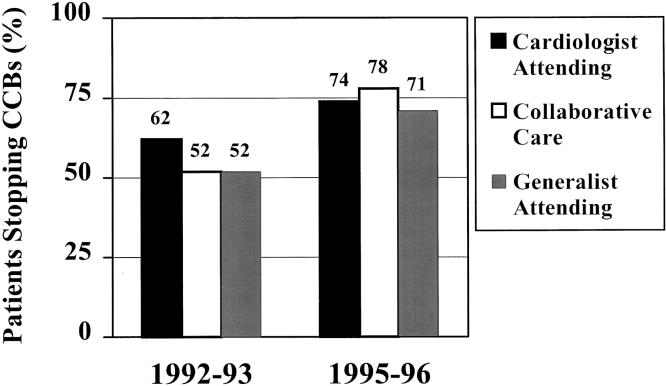
The use of CCBs decreased an absolute 14%, from 24% of patients with AMI before the adverse reports to only 10% after the reports. The proportion of patients starting CCBs decreased over time (from 16% to 6%), while the proportion stopping CCBs increased over time (from 55% to 76%). Figure 1Figure 2
FIGURE 1.
Starting calcium channel blockers in patients after acute myocardial infarction (AMI). In 1992–1993, 272 (16%) of the 1,683 patients not taking a calcium channel blocker (CCB) at admission were started on one following AMI; in 1995–1996, 139 (6%) of the 2,220 patients not taking a CCB at admission were started on one following AMI.
FIGURE 2.
Stopping calcium channel blockers in patients after acute myocardial infarction (AMI). In 1992–1993, 318 (55%) of the 582 patients who were taking a calcium channel blocker (CCB) at admission had it discontinued following AMI; in 1995–1996, 494 (76%) of the 653 patients who were taking a CCB had it discontinued following AMI.
display the temporal trends for starting and stopping CCBs, stratified by the level of cardiologist involvement. Patients who were prescribed CCBs after AMI were more likely to be elderly, female, and have preexisting indications such as hypertension or atrial fibrillation (Table 4) Patients already using a CCB at the time of admission or who had a specific contraindication to a β-blockers such as bronchospastic disease were also more likely to be discharged with a CCB.
Table 4.
Variables Associated with the Use of Calcium Channel Blockers (CCBs) After Acute Myocardial Infarction*
| Variables | No. Using CCBs/Total No. (%) | Odds Ratio (95% Cl) | Adjusted Odds Ratio*(95% Cl) |
|---|---|---|---|
| CCBs at discharge Time | 834/5,138 (16) | ||
| 1992–1993 (referent) | 536/2265 (24) | 1.0 | 1.0 |
| 1995–1996 | 298/2,873 (10) | 0.37 (0.32 to 0.43) | 0.33 (0.27 to 0.39) |
| Hospital-level | |||
| Urban | 723/4,200 (17) | 1.55 (1.25 to 1.92) | 1.02 (0.67 to 1.55) |
| High volume† | 606/3,374 (18) | 1.48 (1.25 to 1.74) | 1.18 (0.96 to 1.45) |
| Physician-level | |||
| Cardiologist (referent) | 216/1,164 (19) | 1.0 | 1.0 |
| Collaborative care | 445/2,727 (16) | 0.86 (0.72 to 1.02) | 0.93 (0.75 to 1.15) |
| Generalist attending | 173/1,247 (14) | 0.71 (0.57 to 0.88) | 0.92 (0.68 to 1.24) |
| Board-certified | 706/4,223 (17) | 1.23 (1.01 to 1.51) | 1.00 (0.80 to 1.27) |
| Age >50 y | 205/1,082 (19) | 1.27 (1.07 to 1.52) | 1.29 (1.06 to 1.58) |
| Patient-level Sociodemographic | |||
| Age >74 y | 294/1,648 (18) | 1.19 (1.02 to 1.39) | 1.30 (1.06 to 1.59) |
| Female | 385/1,933 (20) | 1.52 (1.32 to 1.77) | 1.41 (1.18 to 1.68) |
| Managed care | 223/1,553 (14) | 0.82 (0.70 to 0.96) | 0.97 (0.80 to 1.18) |
| Previous medical history | |||
| Prior use of CCBs | 423/1,235 (34) | 4.43 (3.82 to 5.13) | 4.76 (3.88 to 5.75) |
| Prior hypertension | 501/2,511 (20) | 1.71 (1.48 to 2.00) | 1.25 (1.05 to 1.50) |
| Prior atrial fibrillation | 96/392 (25) | 1.76 (1.39 to 2.24) | 1.78 (1.34 to 2.39) |
| Hyperlipidemia | 284/1,696 (17) | 1.06 (0.90 to 1.24) | 1.09 (0.91 to 1.30) |
| Diabetes mellitus | 196/1,067 (18) | 1.21 (1.02 to 1.44) | 1.13 (0.92 to 1.39) |
| Peripheral vascular disease | 100/473 (21) | 1.44 (1.14 to 1.81) | 1.08 (0.83 to 1.42) |
| Bronchospastic disease | 173/792 (22) | 1.56 (1.29 to 1.88) | 1.25 (1.01 to 1.55) |
| Prior CHF | 134/658 (20) | 1.38 (1.12 to 1.70) | 0.99 (0.76 to 1.31) |
| Prior AMI | 298/1,312 (23) | 1.80 (1.54 to 2.11) | 1.20 (0.97 to 1.47) |
| Prior PTCA or CABG | 57/706 (22) | 1.59 (1.31 to 1.93) | 0.99 (0.77 to 1.27) |
| Severe comorbidity‡ | 214/1,217 (18) | 1.14 (0.96 to 1.35) | 1.11 (0.91 to 1.37) |
| Hospital course | |||
| Post-AMI angina | 560/3,263 (17) | 1.21 (1.04 to 1.42) | 1.05 (0.88 to 1.26) |
| Post-AMI CHF | 156/1,203 (13) | 0.72 (0.59 to 0.86) | 0.49 (0.39 to 0.62) |
| Thrombolytic use | 255/2,260 (11) | 0.51 (0.43 to 0.59) | 0.55 (0.46 to 0.66) |
| Discharge medications | |||
| Aspirin | 652/2,910 (22) | 3.24 (2.74 to 3.84) | 5.00 (3.97 to 6.36) |
| β Blockers | 246/1,875 (13) | 0.69 (0.59 to 0.81) | 0.35 (0.28 to 0.42) |
| Nitrates | 542/2,257 (24) | 2.80 (2.41 to 3.26) | 2.15 (1.78 to 2.60) |
| ACE inhibitors | 141/929 (15) | 0.91 (0.75 to 1.11) | 0.50 (0.40 to 0.64) |
| Loop diuretics | 205/910 (23) | 1.66 (1.39 to 1.99) | 1.04 (0.82 to 1.34) |
*Multivariate analysis with multiple logistic regression, using generalized estimating equations, and adjusted for all variables presented above (see Methods). CCBs indicates calcium channel blockers; AMI, acute myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; CABG, coronary artery bypass grafting; CHF, congestive heart failure; ACE, angiotensin converting enzyme; CI, confidence intervals.
High volume is >100 AMIs per year.
Based on Greenfield's Index of Co-existent Disease.
Multivariate Analysis
The seemingly large overall differences in the use of effective therapies between cardiologists and generalists that we observed in unadjusted comparisons were largely explained by differences in patient characteristics (Table 2). For example, only 20% of patients of generalists received β-blockers, compared with 46% of patients of cardiologists (OR, 0.29; 95% CI, 0.25 to 0.35); after multivariate adjustment, this 26% difference was no longer significant (adjusted OR, 0.93; 95% CI, 0.66 to 1.31). However, compared with cardiologists, generalist attendings were less likely to use aspirin and thrombolytic therapy. Collaborative care patients tended to have intermediate levels of use; however, compared with patients of cardiologists, the only significant difference we observed was in the use of thrombolytic therapy.
Between the 2 observation periods, physicians increased their use of effective AMI therapies (Table 3). However, the only difference we found in temporal trends according to the level of cardiologist involvement was a greater increase over time in the use of aspirin by cardiologists (adjusted OR, 1.97) than generalists (adjusted OR, 1.20; P =.035 for the difference).
Between the 2 observation periods, physicians decreased their use of ineffective therapies such as CCBs and lidocaine (Tables 3 and Table 4). We observed a decrease in the use of CCBs from 1992–1993 to 1995–1996 (adjusted OR, 0.33; 95% CI, 0.27 to 0.39). This represents the adjusted odds of CCB relinquishment, and it was independent of physician specialty (Tables 3 and Table 4). Furthermore, CCB relinquishment was not significantly modified by physician specialty. The adjusted odds of CCB relinquishment did not differ between cardiologists (adjusted OR, 0.23) and generalists (adjusted OR, 0.30: P =.12 for the difference). However, a number of other variables, particularly other medications, were associated with the use of CCBs at discharge (Table 4). There was also a decrease in the use of lidocaine from 1992–1993 to 1995–1996 (Table 3). The adjusted odds of lidocaine relinquishment did not differ between cardiologists and generalists ( P =.24 for the difference).
DISCUSSION
Relinquishment itself has rarely been studied,25,27–29 and these data are the first to examine differences in relinquishment according to the degree of specialist involvement. Compared with baseline use in 1992–1993, which was before the adverse reports, we found that CCB use had decreased by more than half in 1995–1996. This decrease occurred because CCBs were started less often and stopped more often, a pattern of practice common to both generalists and specialists. Furthermore, our results imply that generalists were less likely than cardiologists to adopt effective AMI treatments such as aspirin and thrombolytic therapy. Despite differences in the adoption of effective therapies, both generalists and specialists were equally likely to relinquish ineffective therapies associated with potential risk such as lidocaine and CCBs.
By the late 1980s, up to 40% of all patients with AMI were prescribed CCBs,30–33 despite the lack of proven benefit in this setting.30,31 Thereafter, there was a steady decline in CCB use post-AMI, estimated at 3% to 4% per year, until 1992–1993.32,33 In Minnesota, we found an absolute reduction of 14% between 1992–1993 and 1995–1996. Except for the adverse reports documented in 1995,34–36 we are unaware of any other historical forces that might have simultaneously influenced CCB prescribing throughout the state.
This decrease in the use of CCBs is consistent with what we know about certain elements of physician decision making. Physicians are often considered risk-averse, employing the principle that the avoidance of harm to a patient is more important than the doing of good, and typified by the credo, primum non nocere.48 One model of decision making that acknowledges these principles is Regret Theory, which proposes that: (1) physicians compare outcomes for a particular therapeutic decision by thinking about the regret they would feel if they failed to choose the best alternative for their patient, and (2) physicians act to minimize their own regret.48,49 When regret is large (e.g., when a patient dies as a direct result of therapy that they had initiated), that alternative is relatively overweighted.48–50
Viewed within this framework, CCBs might have been widely used in 1992–1993 when they were viewed as innovative, of possible benefit in ischemic injury,30,31 and safe, but were relinquished by physicians in 1995–1996 when CCBs were older, still of unproven benefit, and perhaps, potentially harmful. The perception of risk or harm presented in the adverse reports, coupled with the availability of safe and effective alternatives such as β-blockers, could have led to relinquishment of CCBs in an effort to minimize regret.
We found differences in the adoption of effective therapies for the treatment of AMI according to the level of specialist involvement. In our study, generalist physicians caring for patients with AMI were less likely than cardiologists to use aspirin and thrombolytic therapy, a finding consistent with previous literature.6–9 Underuse of proven effective therapies by generalists has been reported for conditions as disparate as AIDS2,3 and peptic ulcer disease,4,5 and has usually been attributed to less knowledge and less condition-specific experience on their part.1–12 However, there may be a number of competing or alternate explanations for these consistently reported findings.
For example, we found that the substantial differences in case-mix between the patients of generalists and cardiologists explained much, but not all, of the apparent difference in the use of effective therapies. It may also be that cardiologists and other specialists have an inherent bias towards action,17,51 tend to be early adopters of emerging therapies for the sake of being innovative,4,13–16,24,25,29 or have different attitudes toward risk.1,3,48–50 Consistent with these possibilities, we found that cardiologists were more likely than generalists to use thrombolytic therapy but not β-blockers. Although we are unaware of any supporting literature, cardiologists may have also adopted a number of other AMI treatments more rapidly than generalists, such as CCBs, prophylactic lidocaine,9,52 encainide and flecainide,53 and magnesium54—in retrospect, all harmful or ineffective. As Mitchell has observed, the “myocardial infarction battlefield is littered with bad advice, and yet each discarded dogma was initially hailed as ‘the answer.’”13
Despite the fact that generalists were less likely to adopt some effective AMI therapies, we found that they were as likely to relinquish CCBs as cardiologists. If this decreased use of CCBs was a response to the adverse reports, generalists were as responsive to potential risk or harm as specialists. This further supports the possibility that knowledge and experience are not the entire explanation for differences in therapeutic decision making between generalists and specialists. Concerns or attitudes regarding risks, long-term complications, and ultimate value may also be very important,1–4,6,13–17 especially given that the patients of generalist physicians tend to be much older and sicker than the patients of cardiologists.7–10
Although we had a large community-based sample of AMI patients and a detailed clinical database that allowed for the control of case-mix and other potential confounders, our study has at least 5 limitations. First, and most important, we did not have enough information about, nor could we statistically control for, prescribing trends before 1992–1993 or between our data collections. Data from 2 large AMI registries, however, provide evidence for only a small, but continuous, decline in the use of CCBs before the adverse reports.32,33 That decline was reflected in our 1992–1993 data. Second, we considered CCBs as a single therapeutic class. We did not have specific information about the relinquishment of short-acting nifedipine, the agent implicated in most of the adverse reports. Third, we did not have information about the role of patient demand. Because the adverse reports were widely cited in the lay press, patient demand probably played a role in physician decision making.17,23 Fourth, we studied the treatment of only 1 condition, AMI, which was also the putative adverse outcome associated with CCB use. Others have shown that, after the adverse reports, there was little change in prescribing of CCBs for hypertension.40,41 Fifth, these results are based on data from the patients and physicians from only 1 state. We do not know whether we can generalize our findings to other physicians caring for other populations of patients.
In conclusion, compared with cardiologists, we believe that the generalist physicians we studied were “therapeutically conservative.” They were less likely than specialists to adopt some effective therapies for AMI, but just as likely to relinquish ineffective therapies associated with possible harm. This pattern of practice may be a response to an expanding, but increasingly risky and uncertain, pharmacopoeia. If educators, policy makers, and specialists want to accelerate the adoption of an effective therapy by generalist physicians, we believe that they may need to more convincingly address concerns about potential risks and long-term complications.
This work was supported by grants from The Agency for Health Care Policy and Research (HSO7357), The National Institute on Aging (AG14474), The Health Care Education and Research Foundation, and The Harvard Pilgrim Health Care Foundation. Dr. Majumdar was the recipient of a National Research Service Award (PE 11001-10).
REFERENCES
- 1.Donohoe MT. Comparing generalist and specialist care: discrepancies, deficiencies, and excesses. Arch Intern Med. 1998;58:1596–608. doi: 10.1001/archinte.158.15.1596. [DOI] [PubMed] [Google Scholar]
- 2.Turner BJ, McKee L, Fanning T, Markson LE. AIDS specialist versus generalist ambulatory care for advanced HIV infection and impact on hospital use. Med Care. 1994;32:902–16. doi: 10.1097/00005650-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Markson LE, Cosler LE, Turner BJ. Implications of generalists' slow adoption of zidovudine in clinical practice. Arch Intern Med. 1994;154:1497–504. [PubMed] [Google Scholar]
- 4.Hirth RA, Fendrick AM, Chernew ME. Specialist and generalist physicians' adoption of antibiotic therapy to eradicate Helicobacter pylori infection. Med Care. 1996;34:1199–204. doi: 10.1097/00005650-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Thamer M, Ray NF, Henderson SC, Rinehart CS, Sherman CR, Ferguson JH. Influence of the NIH Consensus Conference on Helicobacter pylori on physician prescribing among a Medicaid Population. Med Care. 1998;36:646–60. doi: 10.1097/00005650-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ayanian JZ, Hauptman PJ, Guadagnoli E, Antman EM, Pashos CL, McNeil BJ. Knowledge and practices of generalist and specialist physicians regarding drug therapy for acute myocardial infarction. N Engl J Med. 1994;331:1136–42. doi: 10.1056/NEJM199410273311707. [DOI] [PubMed] [Google Scholar]
- 7.Ayanian JZ, Guadagnoli E, McNeil BJ, Cleary PD. Treatment and outcomes of acute myocardial infarction among patients of cardiologists and generalist physicians. Arch Intern Med. 1997;157:2570–6. [PubMed] [Google Scholar]
- 8.Jollis JG, DeLong ER, Peterson ED, et al. Outcome of acute myocardial infarction according to the specialty of the admitting physician. N Engl J Med. 1996;335:1880–7. doi: 10.1056/NEJM199612193352505. [DOI] [PubMed] [Google Scholar]
- 9.Willison DJ, Soumerai SB, McLaughlin TJ, et al. Consultation between cardiologists and generalists in the management of acute myocardial infarction: implications for quality of care. Arch Intern Med. 1998;158:1778–83. doi: 10.1001/archinte.158.16.1778. [DOI] [PubMed] [Google Scholar]
- 10.Nash IS, Corrato RR, Dlutowski MJ, O'Connor JP, Nash DB. Generalist versus specialist care for acute myocardial infarction. Am J Cardiol. 1999;83:650–4. doi: 10.1016/s0002-9149(98)00961-8. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber TL, Elkhatib A, Grines CL, O'Neill WW. Cardiologist versus internist management of patients with unstable angina: treatment patterns and outcomes. J Am Coll Cardiol. 1995;26:577–82. doi: 10.1016/0735-1097(95)00214-O. [DOI] [PubMed] [Google Scholar]
- 12.Goldman L. The value of cardiology. N Engl J Med. 1996;355:1918–9. doi: 10.1056/NEJM199612193352510. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell JR. But will it help my patients with myocardial infarction? The implications of recent trials for everyday country folk. Br Med J (Clin Res Ed) 1982;285:1140–8. doi: 10.1136/bmj.285.6349.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greer AL. The state of the art versus the state of the science. The diffusion of new medical technologies into practice. Int J Technol Assess Health Care. 1988;4:5–26. doi: 10.1017/s0266462300003202. [DOI] [PubMed] [Google Scholar]
- 15.Dixon AS. The evolution of clinical policies. Med Care. 1990;28:201–20. doi: 10.1097/00005650-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Miao LL, Bunker JP, Barnes BA, Mosteller F. Costs, Risks, and Benefits of Surgery. New York: Oxford University Press; 1977. Gastric freezing: an example of the evaluation of medical therapy by randomized clinical trials. pp. 198–211. [Google Scholar]
- 17.Eisenberg JM. Doctors' Decisions and the Cost of Medical Care. Ann Arbor, Mich: Health Administration Press Perspectives; 1986. [Google Scholar]
- 18.Soumerai SB, Majumdar SR, Lipton HL, Strom BL. Pharmacoepidemiology. 3rd ed. Chichester: John Wiley and Sons; 1999. Evaluating and improving physician prescribing. [Google Scholar]
- 19.Tanenbaum SJ. What physicians know. N Engl J Med. 1993;329:1268–70. doi: 10.1056/NEJM199310213291713. [DOI] [PubMed] [Google Scholar]
- 20.Davis P, Gribben B. Rational prescribing and interpractitioner variation. A multilevel approach. Int J Technol Assess Health Care. 1995;11:428–42. doi: 10.1017/s0266462300008655. [DOI] [PubMed] [Google Scholar]
- 21.Wennberg JE. Dealing with medical practice variations: a proposal for action. Health Aff (Millwood) 1984;3:6–32. doi: 10.1377/hlthaff.3.2.6. [DOI] [PubMed] [Google Scholar]
- 22.Avorn J, Chen M, Hartley R. Scientific versus commercial sources of influence on the prescribing behavior of physicians. Am J Med. 1982;73:4–8. doi: 10.1016/0002-9343(82)90911-1. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz RK, Soumerai SB, Avorn J. Physician motivations for nonscientific drug prescribing. Soc Sci Med. 1989;28:577–82. doi: 10.1016/0277-9536(89)90252-9. [DOI] [PubMed] [Google Scholar]
- 24.Manolio TA, Cutler JA, Furberg CD, Psaty BM, Whelton PK, Applegate WB. Trends in pharmacologic management of hypertension in the United States. Arch Intern Med. 1995;155:829–37. [PubMed] [Google Scholar]
- 25.Mapes RE. Physicians' drug innovation and relinquishment. Soc Sci Med. 1977;11:619–24. doi: 10.1016/0037-7856(77)90044-0. [DOI] [PubMed] [Google Scholar]
- 26.Eddy DM. Practice policies: where do they come from? JAMA. 1990;263:1265–75. doi: 10.1001/jama.263.9.1265. [DOI] [PubMed] [Google Scholar]
- 27.Leape LL. Unnecessary surgery. Annu Rev Publ Health. 1992;13:363–83. doi: 10.1146/annurev.pu.13.050192.002051. [DOI] [PubMed] [Google Scholar]
- 28.Duffy SQ, Farley DE. The protracted demise of medical technology. The case of intermittent positive pressure breathing. Med Care. 1992;30:718–36. doi: 10.1097/00005650-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Rogers EM. Diffusion of Innovations. 4th ed. New York: The Free Press; 1995. [Google Scholar]
- 30.Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction—evidence from clinical trials. N Engl J Med. 1996;335:1660–7. doi: 10.1056/NEJM199611283352207. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf S. Calcium antagonists in coronary artery disease and hypertension. Time for a reevaluation? Circulation. 1995;92:1079–82. doi: 10.1161/01.cir.92.5.1079. [DOI] [PubMed] [Google Scholar]
- 32.Rogers WJ, Bowlby LJ, Chandra NC, et al. Treatment of myocardial infarction in the United States (1990 to 1993). Observations from the National Registry of Myocardial Infarction. Circulation. 1994;90:2103–14. doi: 10.1161/01.cir.90.4.2103. [DOI] [PubMed] [Google Scholar]
- 33.McCormick D, Gurwitz JH, Savageau J, Yarzebski J, Gore JM, Goldberg RJ. Differences in discharge medication after acute myocardial infarction in patients with HMO and fee-for service medical insurance. J Gen Intern Med. 1999;14:73–81. doi: 10.1046/j.1525-1497.1999.00290.x. [DOI] [PubMed] [Google Scholar]
- 34.Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of incident myocardial infarction associated with antihypertensive drug therapies. Circulation. 1995;91:925. Abstract. [PubMed] [Google Scholar]
- 35.Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–5. [PubMed] [Google Scholar]
- 36.Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326–31. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 37.Parmley WW. Sensationalism and the news media. J Am Coll Card. 1995;26:836–7. doi: 10.1016/0735-1097(95)93247-A. [DOI] [PubMed] [Google Scholar]
- 38.Buring JE, Glynn RJ, Hennekens CH. Calcium channel blockers and myocardial infarction. A hypothesis formulated but not yet tested. JAMA. 1995;274:654–5. [PubMed] [Google Scholar]
- 39.Opie LH, Messerli FH. Nifedipine and mortality. Grave defects in the dossier. Circulation. 1995;92:1068–73. doi: 10.1161/01.cir.92.5.1068. [DOI] [PubMed] [Google Scholar]
- 40.Maclure M, Dormuth C, Naumann T, et al. Influences of educational interventions and adverse news about calcium-channel blockers on first-line prescribing of antihypertensive drugs to elderly people in British Columbia. Lancet. 1998;352:943–8. doi: 10.1016/S0140-6736(97)11390-3. [DOI] [PubMed] [Google Scholar]
- 41.Brunt M, Murray MD, Kesterson J, Tierney WM. Does media coverage of medical research influence prescribing? J Gen Intern Med. 1999;17(suppl. 2):89. doi: 10.1046/j.1525-1497.2003.20502.x. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soumerai SB, McLaughlin TJ, Gurwitz JH, et al. Effect of local opinion leaders on quality of care for acute myocardial infarction: a randomized controlled trial. JAMA. 1998;279:1358–63. doi: 10.1001/jama.279.17.1358. [DOI] [PubMed] [Google Scholar]
- 43.Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Med Care. 1993;31:141–54. doi: 10.1097/00005650-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin TJ, Soumerai SB, Willison DJ, et al. The effect of comorbidity on use of thrombolysis or aspirin in patients with acute myocardial infarction eligible for treatment. J Gen Intern Med. 1997;12:1–6. doi: 10.1046/j.1525-1497.1997.12105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allison JJ, Kiefe CI, Cook EF, Gerrity MS, Orav EJ, Centor R. The association of physician attitudes about uncertainty and risk-taking with resource use in a Medicare HMO. Med Decis Making. 1998;18:320–9. doi: 10.1177/0272989X9801800310. [DOI] [PubMed] [Google Scholar]
- 46.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 1996. [Google Scholar]
- 47.Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. J Gen Intern Med. 1992;7:623–9. doi: 10.1007/BF02599201. [DOI] [PubMed] [Google Scholar]
- 48.Hershey JC, Baron J. Clinical reasoning and cognitive processes. Med Decis Making. 1987;7:203–11. doi: 10.1177/0272989X8700700402. [DOI] [PubMed] [Google Scholar]
- 49.Yates JF. Judgement and Decision Making. Englewood Cliffs, NJ: Prentice Hall; 1990. [Google Scholar]
- 50.Feinstein AR. The “chagrin factor” and qualitative decision analysis. Arch Intern Med. 1985;145:1257–9. [PubMed] [Google Scholar]
- 51.Ayanian JZ, Berwick DM. Do physicians have a bias towards action? A classic study revisited. Med Decis Making. 1991;11:154–8. doi: 10.1177/0272989X9101100302. [DOI] [PubMed] [Google Scholar]
- 52.MacMahon S, Collins R, Peto R, Koster RW, Yusuf S. Effects of prophylactic lidocaine in suspected acute myocardial infarction. An overview of results from randomized controlled trials. JAMA. 1988;260:1910–6. [PubMed] [Google Scholar]
- 53.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 54.ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: a randomized factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet. 1995;345:669–75. [PubMed] [Google Scholar]