Abstract
Humans are the only natural reservoir of measles virus (MV), one of the most contagious viruses known. MV infection and the profound immunosuppression it causes are currently responsible for nearly one million deaths annually. Human signaling lymphocytic activation molecule (hSLAM) was identified as a receptor for wild-type MV as well as for MV strains prepared as vaccines. To better evaluate the role of hSLAM in MV pathogenesis and MV-induced immunosuppression, we created transgenic (tg) mice that expressed the hSLAM molecule under the control of the lck proximal promoter. hSLAM was expressed on CD4+ and CD8+ T cells in the blood and spleen and also on CD4+, CD8+, CD4+ CD8+, and CD4− CD8− thymocytes. Wild-type MV, after limited passage on B95-8 marmoset B cells, and the Edmonston laboratory strain of MV infected hSLAM-expressing cells. There was a direct correlation between the amount of hSLAM expressed on the cells' surface and the degree of viral infection. Additionally, MV infection induced downregulation of receptor hSLAM and inhibited cell division and proliferation of hSLAM+ but not hSLAM− T cells. Therefore, these tg mice provide the opportunity for analyzing and comparing MV-T cell interactions and MV pathogenesis in cells expressing only the hSLAM MV receptor with those of tg mice whose T cells selectively express another MV receptor, CD46.
Since measles virus (MV) was isolated and attenuated to produce a successful vaccine (7, 20), it was shown to cause a progressive central nervous system (CNS) disease (subacute sclerosing panencephalitis) and was found to be capable of suppressing immune responses (28, 53).
More recently, two developments have aroused interest in the nature of MV. The first was the discovery of two cell surface receptors for MV, namely, the CD46 molecule, which is a member of the complement regulatory cascade of proteins (6, 26, 33), and signaling lymphocytic activation molecule (SLAM), a T-cell costimulatory molecule (9, 18, 52). Whereas the CD46 molecule is ubiquitously expressed on all nucleated cells, SLAM is expressed only on immature thymocytes, activated and memory T cells, B cells, and activated monocytes and dendritic cells (4, 29, 30, 40, 49). The second development was a better understanding of mechanisms of MV-induced immunosuppression. Although the route of infection by MV is respiratory, and despite its widespread dissemination to the skin, the intestinal tract, and the nervous system, the virus has a strong predilection for lymphoid tissues in the early as well as late stages of the disease. Furthermore, lymphoid tissues and cells provide not only a replication site but also a means of transporting the virus within the body. Since both CD46, constitutively, and SLAM, inducibly, are concomitantly present on cells of the human immune system, the relative individual contribution of either of the two MV receptors in MV-induced immunosuppression has been difficult to sort out. MV has been known to induce mitogen unresponsiveness of T cells by direct infection and contact with infected cells (12, 43, 56). However, the lack of a suitable small-animal model has impeded progress toward understanding the pathogenic effects of MV, especially its ability to induce immunosuppression, a CNS disease, and virus-immune cell and virus-neuron interactions. Following the identification of MV receptor CD46, investigators in several laboratories have attempted to express human CD46 in transgenic (tg) mice as models that could be infected by MV (16, 36, 41, 57). The human CD46 protein has a 45% homology with mouse CD46 (54). Mice are not infected by MV unless the virus has been adapted to the murine cells by multiple passages (24) or unless human CD46 is expressed in the mouse. In terms of understanding and solving the puzzle of how MV suppresses the immune responses, recent studies have suggested that MV infects and alters functions of T cells (11, 32, 34) and antigen-presenting cells (APC) (14, 45, 46) and that infection skews the T-cell response to a Th2 phenotype (13).
SLAM is a glycoprotein ligand found on the surface of immature thymocytes, activated and memory T cells, B cells, and activated APC (4, 29, 30, 40, 49). Sequence analyses and gene mapping place SLAM in the CD2 immunoglobulin (Ig) superfamily along with related genes, such as those for 2B4 and SF 2001 (5, 10). SLAM and SLAM-related cell surface receptors are thought to play an important role in adhesion and signaling at the immune synapse between the APC and the T cell. The homology between human and murine SLAM is 58%, and while human SLAM (hSLAM) serves as a receptor for MV, murine SLAM does not (38).
Owing to its expression on cells of the immune system and its role in T-cell biology, hSLAM might be involved in the immunosuppression associated with MV infection. However, distinguishing its activity from that of the other MV receptor, CD46, is difficult because CD46 is also expressed on T cells and APC and influences T-cell biology. We have previously tested a line of tg mice, YAC-CD46, in which expression of the MV receptor CD46 closely mimicked the location and amount of CD46 found in humans. MV replicated in and was recovered from CD46+ immune cells and was associated with suppression of humoral and cell-mediated immune responses (36).
The binding of wild-type (wt) MV from clinical specimens or wt MV passaged on B95-8 marmoset cells to hSLAM was proposed to be much stronger than its binding to CD46 (8, 25, 37, 44). Thus, we generated novel tg mice that exclusively expressed hSLAM behind the lck proximal promoter. In this paper, we record our findings from experiments with tg mice expressing hSLAM protein on T lymphocytes in the blood, spleen, and thymus. We note here four main observations. First, T lymphocytes that expressed hSLAM were permissive both to a laboratory strain of MV and to a wt MV passaged on B95-8 cells. Second, the degree of hSLAM expression correlated directly with the ability of MV to infect CD4+ and CD8+ T cells. Third, after MV infection of such cells, hSLAM was downregulated from the cell surface. Fourth, MV infection of hSLAM-expressing murine T cells inhibited their division and proliferation. Therefore, this tg model reproduces important aspects of MV infection of human T cells and, as such, should be useful for dissecting the molecular basis of MV-induced immunosuppression and the role of T cells expressing hSLAM in this process compared to that of T cells from tg mice expressing only the CD46 MV receptor.
MATERIALS AND METHODS
Construction of expression plasmid.
The hSLAM cDNA (pCAGSLAM) was provided by Yusuke Yanagi (Kyushu University). To amplify the hSLAM cDNA, PCR was performed by using pCAGSLAM as a template and Sl-1 (5′-GAAGATCTACCATGGATCCCAAGGGGCTCC-3′) and Sl-2 (5′-GAAGATCTCCTTCAGAAAGTCCCTTTGTTGG-3′) as the primers. For the construction of plck-SLAM, amplified hSLAM cDNA was digested with BglII and then cloned into BamHI-treated p1017 vector, which was obtained from Jenny Tian (Merck Research Laboratories). The p1017 vector contains the lck proximal promoter and a human growth hormone (hGH) minigene that have a transcription terminator for efficient gene expression in tg mice (3). The entire hSLAM gene of 1,040 bp was sequenced to ensure that it was intact. The sequences of junction sites between the promoter and the inserted gene were also verified by sequencing analysis to confirm that the fusion gene was properly constructed.
Generation of tg mice.
The full-length lck proximal promoter-hSLAM fragment of 6.3 kb was isolated by digestion of plck-SLAM with NotI and was microinjected into fertilized oocytes of FVB/N, C57BL/6, and FVB/N × C57BL/6 mice. The oocytes were then implanted into pseudopregnant CD1 female mice at the The Scripps Research Institute transgenic facility. Tail biopsy specimens were collected from pups, and DNA was isolated for detection of hSLAM cDNA. Several tg mouse founders were identified by using a slot blot hybridization assay with a random-primed 32P-labeled probe for the entire hSLAM gene. Tg mice founders and their offspring were confirmed by using gene-specific PCR analysis. Tail genomic DNA (500 ng) was used as a template with primers 5′-AGTTCAGCGCTTTTGCCTGC-3′ and 5′-CTGTTGGCTGGGTTCAGTG-3′ to amplify the internal region of hSLAM or with another set of primers (Sl-1 and Sl-2) to amplify the entire hSLAM gene. All founders were shown to have intact full-length hSLAM gene by PCR analysis.
Reverse transcriptase (RT) PCR analysis.
Various organs, namely, brain, kidney, liver, lung, heart, bone marrow, spleen, and thymus, were removed from saline-perfused mice and homogenized. Tri-reagent (Molecular Research Center) was used to purify total RNA from these tissues in accordance with the manufacturer's description. RNAs were treated with DNase I to remove possible contaminating genomic DNA in the total RNA. hSLAM RNA was detected via RT PCR assay with the hSLAM gene-specific primers (Sl-1 and Sl-2) described above. One hundred nanograms of total RNA was mixed with primers and SuperScript II RT/Platinum Taq mix (Invitrogen) in the buffer supplied. The RT reaction was processed for 30 min at 50°C followed by amplification of cDNA through 30 cycles of 95°C for 30s, 55°C for 30 s, and 72°C for 1 min. As an internal control, the transcript of β-actin was detected with the primers 5′-TCATGAAGTGTGACGTTGACATCCGT-3′ and 5′-CTTAGAAGCATTTGCGGTGCACGATG-3′. RT PCR products were separated by electrophoresis through a 1% agarose gel.
Fluorescence-activated cell sorter (FACS) analysis.
To detect hSLAM protein expression, blood samples were collected from the retro-orbital venous plexus, diluted 1/100 in a lysis buffer of red blood cells (140 mM NH4Cl, 17 mM Tris-HCl, pH 7.2) and incubated on ice for 30 to 45 min to remove red blood cells. The white blood cells were stained with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD4 antibody or anti-CD8 antibody (BD PharMingen). Staining for hSLAM was performed with an anti-hSLAM antibody (Advanced ImmunoChemical Inc.) conjugated to Cy5 by using a monoclonal antibody labeling kit (Amersham Biosciences). Incubation of cells with various fluorochrome antibodies was carried out for 20 to 30 min on ice in a phosphate-buffered saline (PBS) solution containing 1% fetal bovine serum (FBS) and 0.1% sodium azide. After being washed three times, the cells were fixed in 1% formaldehyde in PBS. To screen for expression of hSLAM protein, two to three non-tg littermates (hSLAM negative in PCR analysis) and two to six tg mice (hSLAM positive in PCR analysis) of F1 mice per founder were used in each FACS analysis. To check the expression of hSLAM protein on various immune cells, splenocytes prepared as described below were stained with either anti-B220, anti-CD11b, anti-CD11c, or anti-NK1.1 antibody (BD PharMingen) along with antibody to hSLAM. For the detection of MV proteins on cell surface, cells were incubated with a human polyclonal serum to MV (41) for 20 min, washed three times, and then stained with PE-conjugated donkey anti-human IgG antibody (Jackson Immunoresearch Laboratories) along with directly conjugated fluorochrome antibodies to mouse CD4 and CD8 and to hSLAM. Acquisition of fluorescent cells was performed on a FACSCalibur flow cytometer (Becton Dickinson). Acquired cells were analyzed by CellQuest (Becton Dickinson) or FlowJo (Treestar) software.
Analysis of in vitro MV infection.
Edmonston MV (MV-Ed) was passaged and titered on Vero cells. wt MV of the JW strain, which was originally obtained from PBMCs of a patient with acute MV infection, was passaged less than 7 times in, and titered on, B95-8 cells as described previously (25). This was necessary to increase the titer of MV for subsequent experimental studies, and this virus is referred to as MV-JWB. B95-8 cells were maintained in RPMI 1640 medium containing 7% FBS, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. For in vitro viral infection analysis, single-cell suspensions of spleen and thymus from tg or non-tg littermates were obtained by passage through a 70-μm (pore size) sterile mesh. Splenocytes were treated with 0.83% ammonium chloride for 4 min to remove erythrocytes. Purified cells were cultured in RPMI complete medium containing 7% FBS, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 10 mM HEPES, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 0.05 mM β-mercaptoethanol, and 50 U of interleukin-2 (IL-2)/ml. To stimulate T lymphocytes in vitro, 20 ng of phorbol myristate acetate (PMA)/ml and 1 μg of ionomycin/ml were added to the culture for 15 to 20 h. Cells (106) from tg or non-tg mice were mock infected or infected with MV-Ed or MV-JWB at a multiplicity of infection (MOI) of 0.8 to 1.0 at 37°C for 2.5 h. After being washed, infected cells were cultured in the complete medium containing IL-2. Infectious virus was quantitated by infectious center assay (16), while expression of viral proteins was determined by flow cytometry. To detect viral transcription or replication products, RT-PCR analysis was performed with total RNA extracted from infected T cells and MV N-specific primers.
Analysis of in vivo MV infection.
Newborn 1- to 2-day-old tg or non-tg mice were injected intraperitoneally (i.p.) with MV-JWB at a dose of 4 × 104 50% tissue culture infective doses (TCID50) in a volume of 50 μl. Mice were sacrificed 2 days after inoculation, and thymi were harvested. Single-cell suspension of thymocytes was obtained by passage through a 70-μm sterile mesh. Cells were tested for surface expression of hSLAM and MV proteins by flow cytometry as described above. As a negative control, B95-8 cell supernatants in the absence of MV inoculation were prepared identically to the JW virus stock and were used for mock in vivo infection analysis.
Proliferation assays.
Splenocytes from tg mice and their non-tg littermates processed as mentioned above were labeled with carboxyfluorescein succidimyl ester (CFSE) (Molecular Probes, Eugene, Oreg.). Cells (3 × 106) were resuspended with PBS containing 2.5 μM CFSE and incubated at 37°C for 10 min. Unincorporated CFSE was removed by washing with PBS three times, and 106 lymphocytes from tg or non-tg mice were either mock infected or infected with MV at an MOI of 1.0. After 2.5 h at 37°C, half of each sample was washed and stained with human polyclonal antibody to MV followed by an antibody to human IgG conjugated to PE and an antibody to murine CD4 conjugated to allophycocyanin (day 0). The remaining half of the cells was treated with 20 ng of PMA/ml and 1 μg of ionomycin/ml, cultured in complete medium containing IL-2, and at 2 days after MV infection, prepared and treated as described above for day 0 samples. Detection of CFSE and surface expression of MV proteins on CD4+ cells was performed by antibodies and FACS analysis as described previously (32).
RESULTS
Generation of hSLAM-expressing tg mice.
To produce tg mice expressing hSLAM on T lymphocytes, a fusion gene containing the lck proximal promoter and the hSLAM gene was constructed (Fig. 1A). Embryos of FVB/N, FVB/N × C57BL/6, and C57BL/6 mouse strains were microinjected with a DNA fragment containing the lck proximal promoter-hSLAM fusion gene. Eleven lines of lck-SLAM tg mice, consisting of two lines of FVB/N mice, seven lines of FVB/N × C57BL/6 mice, and two lines of C57BL/6 mice, have incorporated hSLAM cDNA in their genome, as shown by screening by using gene-specific PCR analysis and/or slot blot hybridization (data not shown). All these founders were bred to ensure that they passed the hSLAM transgene to F1 progeny. All except one line of FVB/N × C57BL/6 mice did so.
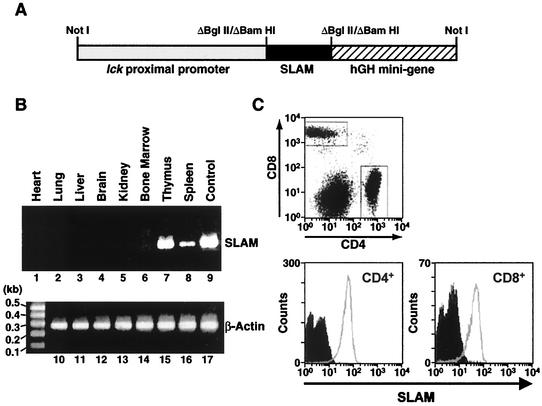
FIG. 1.
Tg construct of lck-SLAM fusion gene and analysis of tg mice expressing hSLAM. (A) Fusion gene construct used to generate tg mice. Briefly, hSLAM cDNA (black box) was amplified by PCR and engineered to have BglII sites on both ends; it was introduced into BamHI-treated p1017 vector between the lck proximal promoter (gray box) and the hGH minigene (striped box). By treatment of NotI enzyme, the lck-SLAM fusion gene was purified for microinjection to produce hSLAM tg mice. The scheme depicts the relative sizes of each component (lck proximal promoter:hSLAM:hGH minigene size ratio, 3:1:2). (B) RT PCR analysis to detect hSLAM RNA transcripts from diverse murine organs. Total RNAs were extracted from various organs of saline-perfused lck-SLAM tg mice from line 2 of the FVB/N strain. Total RNAs (100 ng) from heart (lanes 1 and 10), lung (lanes 2 and 11), liver (lanes 3 and 12), brain (lanes 4 and 13), kidney (lanes 5 and 14), bone marrow (lanes 6 and 16), thymus (lanes 7 and 15), and spleen (lanes 8 and 17) were used in the RT PCR assay with full-length hSLAM gene-specific primers (lanes 1 through 8) or β-actin primers (lanes 10 through 17). As a control, hSLAM DNA was amplified by PCR with hSLAM cDNA as a template (lane 9). (C) Expression of hSLAM protein on T lymphocytes obtained from the blood of lck-SLAM tg mice. White cells from line 2 tg mouse of the FVB/N strain and its non-tg littermate were stained with FITC-labeled anti-CD4 antibody, PE-labeled anti-CD8 antibody, and Cy5-conjugated anti-hSLAM antibody. By FACS analysis, CD4+ cells and CD8+ cells were gated, and the expression levels of hSLAM protein by non-tg mice (filled histogram) and tg mice (open histogram) were compared.
To investigate the expression of hSLAM in several organs, total RNAs were extracted from various organs of lck-SLAM F2 mice (data from line 2 of the FVB/N strain are shown in Fig. 1), and RT PCR analysis was performed. Prior to removal of organs, mice were perfused with 25 ml of sterile PBS via intracardiac inoculation to ensure removal of endogenous peripheral murine lymphocytes. From thymus and spleen of tg mice, RT PCR products corresponding to full-length hSLAM gene were obtained (Fig. 1B, lanes 7 and 8). In contrast, no mRNAs encoding hSLAM were detected from either liver, lung, brain, kidney, heart, or bone marrow of tg mice (Fig. 1B, lanes 1 to 6) or from any organ of non-tg mice (data not shown), although RNAs prepared from those organs were intact, as confirmed by another RT PCR analysis to detect the mRNAs of β-actin (Fig. 1B, lanes 10 to 17). Data from the RT PCR analysis matched the results from Northern blot analysis (data not shown). The distribution profile of hSLAM RNA directed by the control of the lck proximal promoter correlated with the presence of T cells in the spleen and thymus.
Several F1 mice per mouse line were tested for the expression of hSLAM protein. White blood cells were prepared from peripheral blood and tested for the expression of hSLAM protein. Since the lck proximal promoter was reported to be specific for T cells (47), CD4+ or CD8+ T cells from blood were tested for the surface expression of hSLAM protein. By FACS analysis, most T cells of tg mice, as shown for line 2 FVB/N (Fig. 1C) mice, expressed hSLAM protein on the surfaces of CD4+ and CD8+ cells, while none was detected on either CD4+ or CD8+ T cells obtained from non-tg mice (Fig. 1C).
hSLAM protein expression on splenocytes and thymocytes from lck-SLAM tg mice.
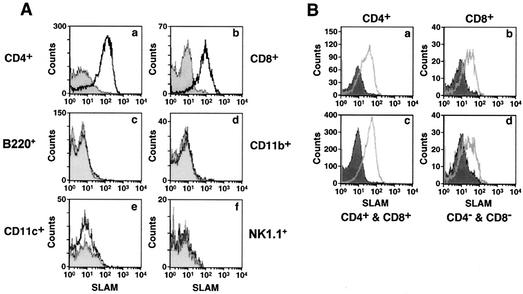
To examine the expression pattern of hSLAM protein on various cell types of the immune system, splenocytes were analyzed by FACS by using antibodies against specific cell marker proteins CD4, CD8, B220, CD11b, CD11c, or NK1.1. Again, hSLAM protein was easily detected on CD4+ or CD8+ cells from spleen, but not on B cells, NK cells, CD11c+ or CD11b+ cells (Fig. 2A). Thymocytes from tg mice, including CD4 and CD8 double-negative and double-positive cells as well as CD4 or CD8 single-positive cells, expressed hSLAM protein (Fig. 2B). These studies indicate that the expression of hSLAM protein is restricted to immature and mature T lymphocytes in lck-SLAM tg mice, as would be expected for the lck proximal promoter (47).
FIG. 2.
Expression of hSLAM on various cells of the immune system. (A) hSLAM expression on different cell types from murine spleen cells. Splenocytes were stained with antibody to murine CD4 (graph a), CD8 (graph b), B220 (graph c), CD11b (graph d), CD11c (graph e), or NK1.1 molecules (graph f) combined with antibody to hSLAM. The histograms represent the expression levels of hSLAM on specific cells from tg (open histogram) or non-tg (filled histogram) mice. (B) hSLAM expression on thymocytes obtained from non-tg and tg mice. Histograms show the expression of hSLAM on CD4+ (graph a), CD8+ (graph b), CD4+ CD8+ (graph c), and CD4− CD8− (graph d) cell types from tg (open histogram) or non-tg (filled histogram) mice.
Susceptibility of splenic T cells from lck-SLAM tg mice to MV infection in vitro.
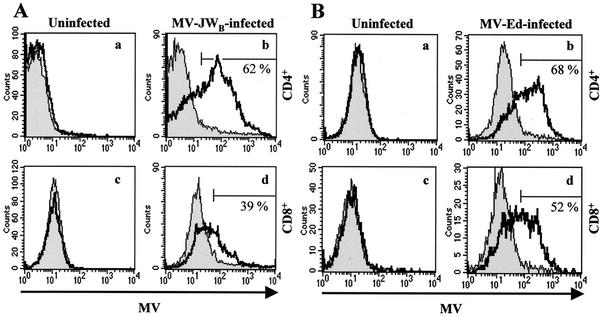
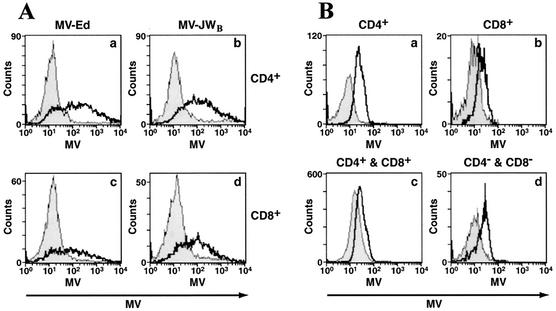
Since hSLAM protein was first identified as an immune cell-specific receptor for wt MV and for MV passaged on B95-8 marmoset cells, we assayed the susceptibility of mouse T cells expressing hSLAM to in vitro infection by the MV-JWB strain. The primary human isolate MV-JW, which was maintained in our laboratory by passage through primary human lymphocytes (25), was passaged on marmoset B95-8 cells in order to enhance its titer from 103 to 106 TCID50/ml. MV-JW and other human clinical isolates of wt MV passaged on primary human cells had titers too low to reach an appropriate MOI needed for our studies; hence, wt isolates were passaged for limited times (less than 7 passages) on, and were easily recovered from, B95-8 cells. Like wt MV and unlike laboratory strains of MV such as MV-Ed passaged on epithelial cells, B95-8-passaged wt MV has been shown to retain pathogenicity for monkeys (21). Therefore, B95-8 cell-adapted wt MV has been widely used to produce wt MV-like stocks that mirror many aspects of the clinical MV biology (21, 25, 52). Since stimulation of T cells with mitogens is required for efficient MV replication and production of infectious MV in human lymphoid cells (19, 27, 50, 55), splenic T lymphocytes from tg mice and non-tg littermates were incubated with T-cell mitogens PMA and ionomycin (see Materials and Methods) before infection with B95-8-passaged wt MV-JW strain. Once activated, lymphocytes were infected with MV-JWB at an MOI of 0.8, and the appearance of the two viral surface glycoproteins, hemagglutinin (HA) and fusion (F) proteins, on inoculated cells' surfaces was detected with a MV-specific antiserum. As a result of infection with MV-JWB, CD4+ T lymphocytes (62%) studied at 2 days postinoculation were infected and expressed viral proteins (Fig. 3A, graphs a and b). CD8+ T cells (39%) from tg mice were also infected with MV-JWB at day 2 (Fig. 3A, graphs c and d). In contrast, the background was less than an average of 3% (range, 2 to 6%) for splenic hSLAM− T cells from non-tg littermates, supporting the idea that MV is a human-specific pathogen and that mouse SLAM on T cells does not act as a MV receptor. When cells were mock infected (control supernatant from B95-8 cells), no fluorescence was detected on tg or non-tg cells. Therefore, hSLAM expression on mouse primary T lymphocytes renders them permissive to wt MV.
FIG. 3.
CD4+ and CD8+ murine T cells expressing hSLAM on their surfaces can be infected by both a wt MV after low passage in B95-8 marmoset cells (MV-JWB) and a laboratory (MV-Ed) strain of MV. (A) Infection of splenic T lymphocytes with MV-JWB. Activated CD4+ (graphs a and b) and CD8+ (graphs c and d) cells were mock infected (graphs a and c) or infected (graphs b and d) with MV-JWB. Two days postinfection, MV proteins were detected by flow cytometry for tg (open histogram) and non-tg (filled histogram) mice. (B) Infection of T lymphocytes by the MV-Ed laboratory strain. Activated CD4+ (graphs a and b) and CD8+ (graphs c and d) lymphocytes were mock infected (graphs a and c) or infected (graphs b and d) with MV-Ed strain. Three days postinfection, cells were analyzed. An area of MV-positive cells was arbitrarily selected and compared to uninfected cells. The percentage of infected cells from non-tg mice was subtracted from the percentage of infected cells from tg mice; the results are shown in graphs b and d.
hSLAM protein has also been reported to be a receptor for laboratory strains of MV passaged on epithelial cells. To determine whether T lymphocytes from hSLAM tg mice were also susceptible to a laboratory strain of MV infection, cells were infected with MV-Ed at an MOI of 0.8. At day 3, the majority of CD4+ (68%) and CD8+ (52%) T lymphocytes from hSLAM tg mice expressed the MV surface glycoproteins (Fig. 3B). Again, less than 4% of non-tg hSLAM− CD8+ or CD4+ T cells were similarly infected by MV. Confirmation of T cell infection was obtained by documenting the presence of newly generated viral positive-strand RNA by using RT PCR to demonstrate the presence of the MV N gene. MV N is a product of viral transcription and/or replication. In addition, infectious viruses were produced from in vitro-infected spleen cells of hSLAM+ tg mice, as detected by infectious center assay when MV-infected T cells cocultured on a Vero cell monolayer caused cytopathic effects on Vero cells (data not shown).
The above experiments used PMA and ionomycin stimulation, but similar results were obtained with stimulation provided by allogeneic lymphocytes or antibodies against CD3 and CD28 (data not shown). Overall, these results clearly demonstrate that hSLAM protein on murine T cells can act as a receptor for MV and that its expression alone is sufficient to allow MV entry into mouse T cells. This process grants MV the opportunity for viral replication and production of infectious MV progeny in mouse T lymphocytes.
Parallel association of the amount of hSLAM expression and the degree of MV infection in lymphocytes.
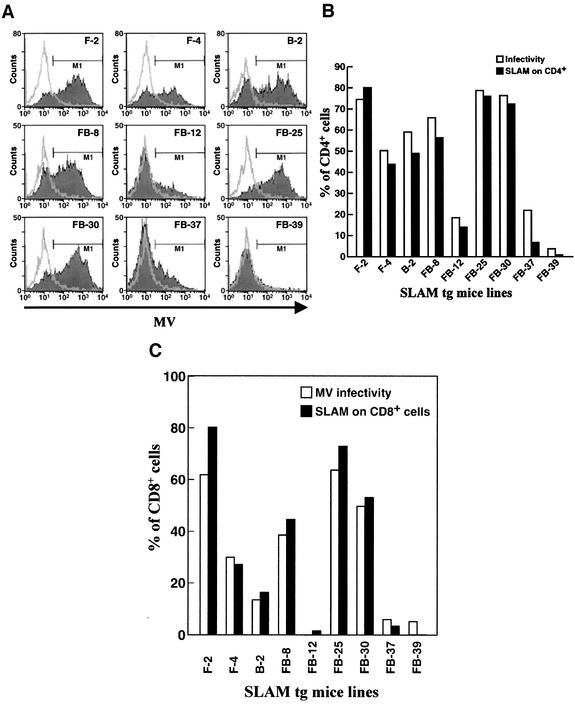
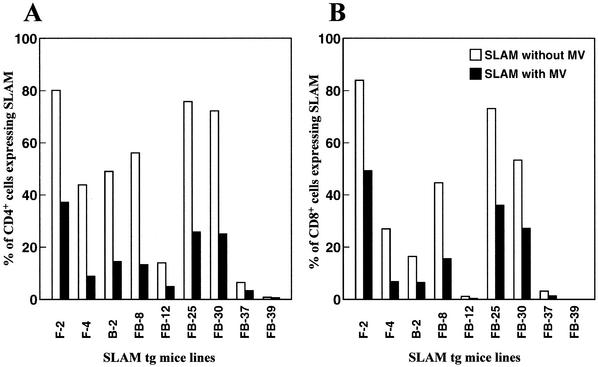
Lymphocytes from each of the nine tg mouse lines expressing hSLAM and from three non-tg mouse littermates were assayed for the expression level of hSLAM and the susceptibility to MV infection. While CD4+ T cells from non-tg mice littermates did not express hSLAM and were resistant to MV infection, CD4+ T cells from tg mice expressing hSLAM protein, as expected, were susceptible to MV infection (Fig. 4A). CD4+ T cells from those lines expressing the greatest amount of hSLAM on their surfaces, i.e., line 2 of FVB/N mice (F-2) and lines 25 (FB-25) and 30 (FB-30) of FVB/N × C57BL/6 mice, were the most susceptible to MV infection (Fig. 4). CD4+ T cells from a line expressing no hSLAM, namely, tg mice of the FVB/N × C57BL/6 line (FB-39), or from lines expressing the least amount of hSLAM, namely, tg mice of FVB/N × C57BL/6 lines (FB-12 and FB-37), were either not infected or minimally infected by MV, respectively. Finally, CD4+ T cells from those lines that expressed intermediate levels of hSLAM, i.e., FVB/N (F-4), C57BL/6 (B-2), and FVB/N × C57BL/6 (FB-8), showed less infectivity by MV than CD4+ T cells from lines F-2, FB-25, and FB-30 but more than lines FB-12, FB-37, and FB-39. Figure 4C shows equivalent results with CD8+ T cells from the various tg lines. CD8+ T cells from mouse lines expressing the greatest amount of hSLAM displayed the greatest susceptibility to MV, while CD8+ T cells from lines expressing the least amount of hSLAM were the least susceptible to MV infection.
FIG. 4.
Correlation between viral infectivity and the expression level of hSLAM on murine CD4+ and CD8+ T cells. (A) Nine lck-SLAM tg mouse lines were tested for susceptibility to MV infection. Lines 2 (F-2) and 4 (F-4) of the FVB/N strain, line 2 (B-2) of the C57BL/6 strain, and lines 8 (FB-8), 12 (FB-12), 25 (FB-25), 30 (FB-30), 37 (FB-37), and 39 (FB-39) of the FVB/N × C57BL/6 strain are tg mouse lines (filled histogram). Three non-tg mice from three strains (FVB/N, C57BL/6, and FVB/N × C57BL/6) were used as negative controls (open histogram). After infection of splenocytes with MV-Ed at an MOI of 1.0, the amount of MV surface proteins on CD4+ T lymphocytes was detected by FACS analysis. (B) Graphs of results from panel A, with the percentage of infected cells (M1) (open bars) compared to expression levels of hSLAM (filled bars). The percentages of background infection of each non-tg mouse were subtracted from the percentages of infected CD4+ T cells of tg mice, and the expression levels of hSLAM protein on CD4+ T cells were surveyed in all tg mouse lines and compared to those of non-tg mice. (C) Correlation between the amount of hSLAM expressed on murine CD8+ T cells and their infectibility by MV. This histogram shows the correlation of MV infectivity (open bars) to surface expression level of hSLAM (filled bars) on CD8+ T lymphocytes. Studies were conducted in the same manner as described in panel B for CD4+ cells.
Next we analyzed the fate of hSLAM receptor expression on T cells after MV infection. SLAM has been reported as a costimulatory molecule affecting Th1/Th2 balance (2, 4), and the modulation of SLAM expression by MV infection might play a role in the suppression of the immune system. T cells from the nine lines of tg mice were infected with MV-Ed, and the degree of hSLAM expression before and after virus infection was analyzed. After infection with MV-Ed, hSLAM was downregulated on T cells from all lines, reaching from 50 to 80% loss of hSLAM expression in several instances. Downregulation of hSLAM was observed in both CD4+ (Fig. 5A) and CD8+ (Fig. 5B) T cells in all tg mice lines ordinarily expressing hSLAM protein. Similar to infection with MV-Ed, infection with MV-JWB down-modulated MV receptor hSLAM (data not shown).
FIG. 5.
MV infection downregulates the hSLAM receptor. (A) Downregulation of hSLAM on CD4+ splenic T cells from nine tg lines and three non-tg mice, described in Fig. 4, following MV infection. Results were obtained 3 days after MV-Ed infection in vitro. Expression of hSLAM following MV infection (filled bars) and hSLAM expression in the absence of MV infection (open bars) are shown. Data were obtained by subtraction of percentages of expression in non-tg mouse lines from those in tg mouse lines. (B) hSLAM expression profile from CD8+ T lymphocytes with (filled bars) or without (open bars) MV infection.
MV infects thymocytes from lck-SLAM tg mice in vitro and in vivo.
High levels of hSLAM expression occur on thymocytes from tg mice, as shown at both the RNA and protein levels (Fig. 1B and 2B). As anticipated, incubation of such thymocytes with either MV-Ed or MV-JWB led to productive MV infection. Figure 6A shows that at 2 days postinfection, MV HA and F proteins were detected on the surface of CD4+ T cells (Fig. 6A, graphs a and b) and CD8+ T cells (Fig. 6A, graphs c and d).
FIG. 6.
MV infects thymocytes from lck-SLAM tg mice. (A) In vitro MV infection of thymocytes from tg (open histogram) and non-tg (filled histogram) mice. Nonstimulated thymocytes were infected with MV-Ed (graphs a and c) or MV-JWB (graphs b and d) at an MOI of 1.0. CD4+ T cells (graphs a and b) and CD8+ T cells (graphs c and d) were stained for MV to detect viral proteins 2 days postinfection. (B) In vivo infection of hSLAM tg mice. Neonate animals of both non-tg (filled histogram) and tg (open histogram) mice were infected i.p. with MV-JWB at 4 × 104 TCID50. Two days postinfection, CD4+ (graph a), CD8+ (graph b), CD4+ CD8+ (graph c), and CD4− CD8− (graph d) lymphocytes were analyzed for expression of MV proteins. Data shown are representative of three independent experiments.
To evaluate whether hSLAM-expressing tg mice were permissive to MV infection in vivo, newborn mice were injected i.p. with 4 × 104 TCID50 of wt MV-JWB, since previous reports showed that newborn CD46 tg mice were more susceptible than adult mice to MV infection (36, 41) because they lacked the mature immune system possessed by adults (23, 39). After 2 days, MV-inoculated mice were sacrificed and thymocytes obtained were analyzed for the expression of MV proteins. Thymocytes obtained from MV-inoculated tg mice expressed MV proteins on CD4, CD8, double-positive (CD4+ CD8+), or double-negative (CD4− CD8−) T cells. In contrast, thymocytes from non-tg mice infected with MV did not have any MV protein expression (Fig. 6B). MV infection of tg mice in vivo was confirmed in three independent experiments with five to nine mice for each experiment. As an additional negative control, hSLAM tg mice were injected with supernatants from uninfected B95-8 cells. Surface expression of MV proteins was not detected on thymocytes of such tg mice after inoculation with these control supernatants (data not shown). Thus, MV-JWB infects those tg mice in vivo that express hSLAM protein as a MV receptor.
Effect of MV infection on proliferation of mouse T cells expressing hSLAM.
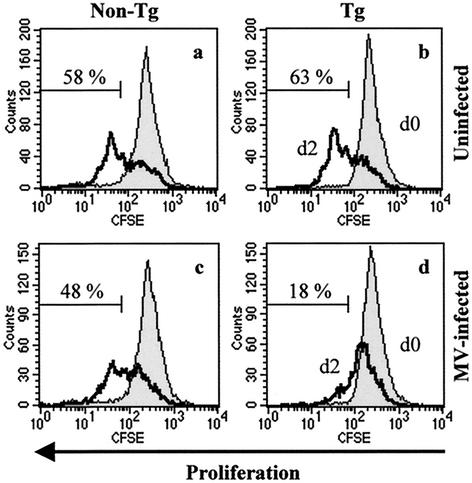
The last series of experiments studied the biologic consequences of MV infection on T cells expressing the hSLAM receptor. hSLAM-bearing splenic T cells were labeled with CFSE, infected with MV, and then stimulated with PMA and ionomycin. MV infection inhibited the mitogen-induced proliferation and division of CD4+ T cells from tg mice, as observed 2 days postinfection (Fig. 7d). By contrast, mock-infected T cells from tg or non-tg mice or non-hSLAM-expressing T cells incubated with MV were able to proliferate and divide after mitogen stimulation (Fig. 7a to c). FACS analysis enabled us to distinguish activated cells (large size) from unactivated cells in forward scatter-side scatter profile. Thymocytes and splenic T cells from non-tg and tg mice reacted to mitogens similarly, becoming bigger in size in the absence of MV inoculation. However, most MV-infected splenic CD4+ T cells (90%) and thymocytes from tg mice remained unactivated (as reflected in the small size of the cells) in forward scatter-side scatter profile (data not shown). These results indicate that MV infects mouse T cells expressing hSLAM, inhibits their proliferation, and makes them unresponsive to mitogenic stimulation.
FIG. 7.
Infection by MV aborts the proliferation of T lymphocytes. CFSE-labeled splenocytes from tg mice (graphs b and d) and non-tg littermates (graphs a and c) were either mock infected (graphs a and b) or infected with MV-Ed strain (graphs c and d) at an MOI of 1.0. CD4+ cells were then analyzed for proliferation by FACS. Histograms show baseline CFSE-labeled at day 0 (d0) (filled histogram) and proliferation at day 2 (d2) (open histogram).
DISCUSSION
By generation of tg mice that expressed hSLAM, our studies showed that the hSLAM protein could be used as a cellular receptor by wt MV passaged on B95-8 marmoset cells (MV-JWB) as well as by laboratory strain MV-Ed on mouse T lymphocytes in vitro and in vivo. Expression of hSLAM on murine T lymphocytes made otherwise-resistant T cells susceptible to MV infection. This allowed us to probe the relevance of MV infection via the hSLAM receptor and its effect on the biological function of T cells. The cellular susceptibility to MV infection directly coincided with the amounts of hSLAM expressed on T cells. Furthermore, MV infection of T lymphocytes from hSLAM tg mice induced downregulation of hSLAM receptor and inhibited the proliferation of infected T cells. Therefore, the hSLAM tg mouse model offers the opportunity to use MV in a small-animal model to dissect the role of SLAM modulation and evaluate MV-T-cell interaction and MV-induced immunosuppression in the absence of the other known MV receptor, CD46. Such analysis is not possible on human T cells, as they bear both CD46 and SLAM molecules.
The use of the lck proximal promoter in producing tg mice resulted in specific expression of hSLAM protein on immature and mature T lymphocytes in blood, spleen, and thymus. This mimicked, in part, SLAM expression in human T cells, although in the tg mice, SLAM is constitutively expressed, while in human T cells, SLAM is induced upon activation and is consistently expressed in immature thymocytes and memory T cells (2, 4, 49). The amount of hSLAM protein in most of our tg lines was slightly higher on CD4+ cells than on CD8+ cells. This might be due to the activity of lck proximal promoter used for generation of hSLAM tg mice or to differential stability of the SLAM protein in CD4+ and CD8+ cells. By using nine tg mice lines, each expressing different amounts of hSLAM protein, a significant and direct correlation between hSLAM expression and MV infectivity was observed (Fig. 4). Since activated T cells are known to express a high amount of SLAM protein (2, 4, 49), they may be good target cells for MV infection and virus amplification in lymphoid cells. Supporting this idea, it was reported that stimulation of human T cells increased the efficiency of RNA replication and production of infectious MV (19, 27, 50, 55). Also, SLAM mRNA has been shown to be expressed at 7- to 25-fold higher levels in human Th1 cells than in Th2 cells (15). Preferential expression of SLAM protein on Th1 cells over Th2 cells may allow MV to efficiently infect Th1 cells, thereby disrupting cell-mediated immune responses in favor of a Th2 response. Interestingly, a skewed Th2 response has been reported in patients infected with MV and argued as a likely factor in the pathogenesis of the virus-induced immunosuppression (13). The lck-SLAM tg mice should be of great value in further testing this hypothesis.
We observed downregulation of hSLAM protein on mouse T cells from lck-SLAM tg mice when CD4+ or CD8+ cells were infected with MV. Moreover, we found the hSLAM protein on uninfected T lymphocytes in the same culture with MV-infected cells to be downregulated (data not shown), indicating an indirect contact-mediated downregulation of SLAM. This indirect effect has been described (9, 51) and presumed to occur by contact of MV HA on MV-infected cells with SLAM on uninfected cells. Since engagement of SLAM has been shown to induce the synthesis of gamma interferon, thus skewing the immune response toward a Th1 phenotype, MV-induced downregulation of hSLAM may explain in part the biased Th2 cytokine phenotype in measles patients (13). Several molecules in immune cells, such as SLAM-associated protein (SAP), SH2-containing inositol phosphatase (SHIP), and Src-like kinases Lck and Fyn, are thought to interact with and modulate the function of SLAM (17, 22, 42, 48). Changes in the signaling cascade initiated by SLAM modulation could be another mechanism for MV-induced immunosuppression. hSLAM tg mice should be helpful to investigate the association of MV infection with SLAM-mediated signaling and the effect on modifying host gene expression.
Human thymus hosts T-cell development and is infectible by MV (31, 35). However, it has been difficult to examine MV infection of thymocytes in vivo. One attempt has been the use of SCID-hu mice, where thymic epithelium cells, but not thymocytes, were shown to be infectible with MV, resulting in apoptosis of uninfected thymocytes (1). However, our data show that thymocytes from hSLAM tg mice were susceptible to infection with MV in vivo as well as in vitro, and infected thymocytes did not undergo enhanced apoptosis over their normal baseline (data not shown). The lck-SLAM tg mice described here should also be of value in sorting out MV-thymocyte interaction. Interestingly, we showed that constitutive expression of hSLAM protein on immature and mature murine T cells allowed MV infection, leading to an inhibition of proliferation. By this means, MV could potentially contribute to the disruption of host immune system by MV. In addition, SLAM may play a role in MV-induced T-cell nonresponsiveness to other stimuli, and infected T cells may not react normally to risk signaling induced by other microbial secondary infections. Such events are currently under investigation.
The selection of cellular receptors by viruses determines their initial cellular tropism and ultimately plays a role in viral pathogenesis. In addition to T cells, SLAM is expressed on other cells of the immune system, primarily APC such as dendritic cells and monocytes. We have successfully used the CD11c promoter to express SLAM protein on mouse APC (unpublished data). It will be useful to study the interactions between MV, T cells, and APC in vivo and in vitro. The other known MV receptor, CD46, has also been expressed in tg mice (16, 36, 41, 57). The profile of hCD46 expression in these mice mimicked CD46 expression in humans. The initial route of natural MV infection of humans is believed to occur through the respiratory tract, and such respiratory epithelia express CD46 but not SLAM (29). Thereafter, MV infects lymphoid tissues, where secondary infection and replication amplifies virus. Dendritic cells, monocytes, and T cells express both CD46 and SLAM molecules on their surface. MV then spreads to multiple sites, including the digestive tract, skin, and CNS, where primary cells in these organs express CD46 and not SLAM. The availability of cell type-specific CD46- or SLAM-expressing tg mice and the ability to infect these tg mice with MV suggests that the single tg mice and the CD46 × SLAM double tg mice might be useful for studying various aspects of virus spread, virus-induced immunosuppression, and pathogenesis caused by MV infection.
Acknowledgments
This work was supported by NIH grants AI36222 to M.B.A.O. and AI46441 to M.M.; N.A. was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research.
We thank Sam H. Shin, Mayra Estrada, and Phi Truong for technical assistance and Sung Key Jang (Pohang University of Science and Technology, Pohang, Korea) for academic cooperation. We also thank Yusuke Yanagi (Kyushu University, Fukuoka, Japan) for graciously supplying the hSLAM gene used in our studies.
REFERENCES
- 1.Auwaerter, P. G., H. Kaneshima, J. M. McCune, G. Wiegand, and D. E. Griffin. 1996. Measles virus infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J. Virol. 70:3734-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aversa, G., J. Carballido, J. Punnonen, C.-C. J. Chang, T. Hauser, B. G. Cocks, and J. E. de Vries. 1997. SLAM and its role in T cell activation and Th cell responses. Immunol. Cell Biol. 75:202-205. [DOI] [PubMed] [Google Scholar]
- 3.Chaffin, K. E., C. R. Beals, T. M. Wilkie, K. A. Forbush, M. I. Simon, and R. M. Perlmutter. 1990. Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. EMBO J. 9:3821-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocks, B. G., C.-C. J. Chang, J. M. Carballido, H. Yssel, J. E. de Vries, and G. Aversa. 1995. A novel receptor involved in T-cell activation. Nature 376:260-263. [DOI] [PubMed] [Google Scholar]
- 5.Davis, S. J., S. Ikemizu, M. K. Wild, and P. A. van der Merwe. 1998. CD2 and the nature of protein interactions mediating cell-cell recognition. Immunol. Rev. 163:217-236. [DOI] [PubMed] [Google Scholar]
- 6.Dorig, R., A. Marcel, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 7.Enders, J. F., and T. C. Peebles. 1954. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc. Soc. Exp. Biol. Med. 86:277-286. [DOI] [PubMed] [Google Scholar]
- 8.Erlenhofer, C., W. P. Duprex, B. K. Rima, V. ter Meulen, and J. Schneider-Schaulies. 2002. Analysis of receptor (CD46, CD150) usage by measles virus. J. Gen. Virol. 83:1431-1436. [DOI] [PubMed] [Google Scholar]
- 9.Erlenhoefer, C., W. J. Wurzer, S. Löffler, S. Schneider-Schaulies, V. ter Meulen, and J. Schneider-Schaulies. 2001. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J. Virol. 75:4499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser, C. C., D. Howie, M. Morra, Y. Qiu, C. Murphy, Q. Shen, J. C. Gutierrez-Ramos, A. Coyle, G. A. Kingsbury, and C. Terhorst. 2002. Identification and characterization of SF2000 and SF2001, two new members of the immune receptor SLAM/CD2 family. Immunogenetics 53:843-850. [DOI] [PubMed] [Google Scholar]
- 11.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin, D. E. 1995. Immune responses during measles virus infection, p. 117-134. In M. Billeter and V. ter Meulen (ed.), Measles virus. Springer, Berlin, Germany.
- 13.Griffin, D. E., and B. J. Ward. 1993. Differential CD4 T cell activation in measles. J. Infect. Dis. 168:275-281. [DOI] [PubMed] [Google Scholar]
- 14.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamalainen, H., S. Meissner, and R. Lahesmaa. 2000. Signaling lymphocytic activation molecule is differentially expressed in human Th1 and Th2 cells. J. Immunol. Methods 242:9-19. [DOI] [PubMed] [Google Scholar]
- 16.Horvat, B., P. Rivailler, G. Varior-Krishnan, A. Cardoso, D. Gerlier, and C. Rabourdin-Combe. 1996. Transgenic mice expressing human measles virus (MV) receptor provide cells exhibiting different permissivities to MV infection. J. Virol. 70:6673-6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howie, D., M. Simarro, J. Sayos, M. Guirado, J. Sancho, and C. Terhorst. 2002. Molecular dissection of the signaling and costimulatory functions of CD150 (SLAM): CD150/SAP binding and CD150-mediated costimulation. Blood 99:957-965. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, E. C., C. Iorio, F. Sarangi, A. A. Khine, and C. D. Richardson. 2001. CDw150 (SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology 279:9-21. [DOI] [PubMed] [Google Scholar]
- 19.Hyypia, T., P. Korkiamaki, and R. Vainionpaa. 1985. Replication of measles virus in human lymphocytes. J. Exp. Med. 161:1261-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz, S. L., M. V. Milovanovic, and J. F. Enders. 1958. Propagation of measles virus in cultures of chicken embryo cells. Proc. Soc. Exp. Biol. Med. 97:23-29. [DOI] [PubMed] [Google Scholar]
- 21.Kobune, F., H. Sakata, and A. Sugiura. 1990. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J. Virol. 64:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latour, S., G. Gish, C. D. Helgason, R. K. Humphries, T. Pawson, and A. Veillette. 2001. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat. Immunol. 2:681-690. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence, D. M. P., M. M. Vaughn, A. R. Belman, J. S. Cole, and G. F. Rall. 1999. Immune response-mediated protection of adult but not neonatal mice from neuron-restricted measles virus infection and central nervous system disease. J. Virol. 73:1795-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebert, U. G., and D. Finke. 1995. Measles virus infections in rodents. Curr. Top. Microbiol. Immunol. 191:149-166. [DOI] [PubMed] [Google Scholar]
- 25.Manchester, M., D. S. Eto, A. Valsamakis, P. B. Liton, R. Fernandez-Munoz, P. A. Rota, W. J. Bellini, D. N. Forthal, and M. B. A. Oldstone. 2000. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 74:3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manchester, M., M. K. Liszewski, J. P. Atkinson, and M. B. A. Oldstone. 1994. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc. Natl. Acad. Sci. USA 91:2161-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McChesney, M. B., A. Altman, and M. B. A. Oldstone. 1988. Suppression of T lymphocyte function by measles virus is due to cell cycle arrest in G1. J. Immunol. 140:1269-1273. [PubMed] [Google Scholar]
- 28.McChesney, M. B., and M. B. A. Oldstone. 1989. Virus-induced immunosuppression: infections with measles virus and human immunodeficiency virus. Adv. Immunol. 45:335-380. [DOI] [PubMed] [Google Scholar]
- 29.McQuaid, S., and S. L. Cosby. 2002. An immunohistochemical study of the distribution of the measles virus receptors, CD46 and SLAM, in normal human tissues and subacute sclerosing panencephalitis. Lab. Investig. 82:403-409. [DOI] [PubMed] [Google Scholar]
- 30.Minagawa, H., K. Tanaka, N. Ono, H. Tatsuo, and Y. Yanagi. 2001. Induction of the measles virus receptor SLAM (CD150) on monocytes. J. Gen. Virol. 82:2913-2917. [DOI] [PubMed] [Google Scholar]
- 31.Moench, T. R., D. E. Griffin, C. Obriecht, A. Vaisberg, and R. T. Johnson. 1988. Acute measles in patients with and without neurological involvement: distribution of measles virus antigen and RNA. J. Infect. Dis. 158:433-437. [DOI] [PubMed] [Google Scholar]
- 32.Naniche, D., S. I. Reed, and M. B. A. Oldstone. 1999. Cell cycle arrest during measles virus infection: a G0-like block leads to suppression of retinoblastoma protein expression. J. Virol. 73:1894-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naniche, D., G. Varior-Krishnan, F. Cervino, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niewiesk, S., M. Gotzelmann, and V. ter Meulen. 2000. Selective in vivo suppression of T lymphocyte responses in experimental measles virus infection. Proc. Natl. Acad. Sci. USA 97:4251-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nozawa, Y., N. Ono, M. Abe, H. Sakuma, and H. Wakasa. 1994. An immunohistochemical study of Warthlin-Finkeldey cells in measles. Pathol. Int. 44:442-447. [DOI] [PubMed] [Google Scholar]
- 36.Oldstone, M. B. A., H. Lewicki, D. Thomas, A. Tishon, S. Dales, J. Patterson, M. Manchester, D. Homann, D. Naniche, and A. Holz. 1999. Measles virus infection in a transgenic model: virus-induced central nervous system disease and immunosuppression. Cell 98:629-640. [DOI] [PubMed] [Google Scholar]
- 37.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75:4399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ono, N., H. Tatsuo, K. Tanaka, H. Minagawa, and Y. Yanagi. 2001. V domain of human SLAM (CDw150) is essential for its function as a measles virus receptor. J. Virol. 75:1594-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson, C. E., D. M. Lawrence, L. A. Echols, and G. F. Rall. 2002. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 76:4497-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Punnonen, J., B. G. Cocks, J. M. Carballido, B. Bennett, D. Peterson, G. Aversa, and J. E. de Vries. 1997. Soluble and membrane-bound forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J. Exp. Med. 185:993-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rall, G. F., M. Manchester, L. R. Daniels, E. Callahan, A. Belman, and M. B. A. Oldstone. 1997. A transgenic mouse model for measles virus infection of the brain. Proc. Natl. Acad. Sci. USA 94:4659-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayos, J., C. Wu, M. Morra, N. Wang, X. Xang, D. Allen, S. van Schaik, L. Notarangelo, R. Geha, M. G. Roncarolo, H. Ottegen, J. E. De Vries, G. Aversa, and C. Terhorst. 1998. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395:462-469. [DOI] [PubMed] [Google Scholar]
- 43.Schlender, J., J. J. Schnorr, P. Spielhoffer, T. Cathomen, R. Cattaneo, M. A. Billeter, V. ter Meulen, and S. Schneider-Schaulies. 1996. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc. Natl. Acad. Sci. USA 93:13194-13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider, U., V. von Messling, P. Devaux, and R. Cattaneo. 2002. Efficiency of measles virus entry and dissemination through different receptors. J. Virol. 76:7460-7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnorr, J. J., S. Xanthakos, P. Keikavoussi, E. Kampgen, V. ter Meulen, and S. Schneider-Schaulies. 1997. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc. Natl. Acad. Sci. USA 94:5326-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Servet-Delprat, C., P. O. Vidalain, H. Bausinger, S. Manie, F. Le Deist, O. Azocar, D. Hanau, A. Fischer, and C. Rabourdin-Combe. 2000. Measles virus induces abnormal differentiation of CD40L-activated human dendritic cells. J. Immunol. 164:1753-1760. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu, C., H. Kawamoto, M. Yamashita, M. Kimura, E. Kondou, Y. Kaneko, S. Okada, T. Tokuhisa, M. Yokoyama, M. Taniguchi, Y. Katsura, and T. Nakayama. 2001. Progression of T cell lineage restriction in the earliest subpopulation of murine adult thymus visualized by the expression of lck proximal promoter activity. Int. Immunol. 13:105-117. [DOI] [PubMed] [Google Scholar]
- 48.Shlapatska, L. M., S. V. Mikhalap, A. G. Berdova, O. M. Zelensky, T. J. Yun, K. E. Nichols, E. A. Clark, and S. P. Sodorenko. 2001. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J. Immunol. 166:5480-5487. [DOI] [PubMed] [Google Scholar]
- 49.Sidorenko, S. P., and E. A. Clark. 1993. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J. Immunol. 151:4614-4624. [PubMed] [Google Scholar]
- 50.Sullivan, J. L., D. W. Barry, S. J. Lucas, and P. Albrecht. 1975. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J. Exp. Med. 142:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka, K., H. Minagawa, M. F. Xie, and Y. Yanagi. 2002. The measles virus hemagglutinin downregulates the cellular receptor SLAM (CD150). Arch. Virol. 147:195-203. [DOI] [PubMed] [Google Scholar]
- 52.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 53.ter Meulen, V., J. R. Stephenson, and H. W. Kreth. 1983. Subacute sclerosing panencephalitis. In H. Fraenkel-Conrat and R. R. Wagner (ed.), The viruses, vol. 18. Plenum Press, New York, N.Y.
- 54.Tsujimura, A., K. Shida, M. Kitamura, M. Nomura, J. Takeda, H. Tanaka, M. Matsumoto, K. Matsumiya, A. Okuyama, Y. Nishimune, M. Okabe, and T. Seya. 1998. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem. J. 330:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vydelingum, S., K. Suryanarayana, R. G. Marusyk, and A. A. Salmi. 1995. Replication of measles virus in human monocytes and T cells. Can. J. Microbiol. 41:620-623. [DOI] [PubMed] [Google Scholar]
- 56.Weidmann, A., A. Maisner, W. Garten, M. Seufert, V. ter Meulen, and S. Schneider-Schaulies. 2000. Proteolytic cleavage of the fusion protein but not membrane fusion is required for measles virus-induced immunosuppression in vitro. J. Virol. 74:1985-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yannoutsos, N., J. N. Ijzermans, C. Harkes, F. Bonthuis, C. Y. Zhou, D. White, R. L. Marquet, and F. Grosveld. 1996. A membrane cofactor protein transgenic mouse model for the study of discordant xenograft rejection. Genes Cells 1:409-419. [DOI] [PubMed] [Google Scholar]