Abstract
OBJECTIVE:
To examine initiation of newer antiretroviral treatments across sociodemographic subgroups during the 3 years following the introduction of these treatments, and explore persistence on treatment and its association with patient characteristics.
DESIGN:
Merged Medicaid paid claims and HIV/AIDS surveillance data were used to analyze use of protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) over time. Survival analysis techniques were used to analyze initiation of PI/NNRTI use. Ordinary least squares and logistic regression were used to determine predictors of persistence on PI/NNRTI therapy.
SETTING AND PARTICIPANTS:
The study population consisted of 2,459 New Jersey non-HMO adult Medicaid beneficiaries with AIDS, identified through a match between HIV/AIDS Registry and Medicaid files. Their PI/NNRTI use was followed from March 1996, when the first PI was licensed, to the end of 1998.
MEASUREMENTS AND MAIN RESULTS:
African Americans initiated treatment on average 8 months later than non-Hispanic whites; initiation of treatment was also slower for injection drug users and for those who did not receive case management through a Medicaid waiver program. These bivariate findings were confirmed with a multivariate time-to-treatment analysis using proportional hazards regression. Among those initiating PI/NNRTI use, 35% had discontinued it by the end of follow-up. Bivariate analyses of treated individuals found that PI/NNRTI use as a proportion of follow-up time was lower for African Americans and Hispanics, and higher for older individuals and for those receiving case management through a Medicaid waiver program, while injection drug use history was not associated with persistence. These findings were confirmed by a regression analysis, which found that controlling for other characteristics, African-American race, and Hispanic ethnicity were each associated with a significant 8% reduction in the proportion of time on PI/NNRTIs following initiation of treatment. Alternative approaches for modeling persistence produced similar results.
CONCLUSIONS:
Results suggest that consistent longitudinal use is difficult for many patients. Persistence of use was lower for minority beneficiaries despite comparable coverage for pharmacy and other health services through Medicaid. Our findings suggest the need to examine nonfinancial barriers to appropriate use of highly active antiretroviral therapy, and to develop and test programmatic strategies for supporting patients in remaining on these regimens consistently.
Keywords: Medicaid, HIV/AIDS, access, initiation, persistence, protease inhibitors
Highly active antiretroviral therapy (HAART) regimens, incorporating protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) in combination with older antiretroviral agents, have proven effective in lowering mortality and delaying the onset of AIDS.1–5 These therapies have emerged as the standard of care for the treatment of HIV infection since early 1996, when the first of the protease inhibitors were licensed.3,6 While these newer treatments have extended life for many patients, they are costly, have significant side effects, and demand careful management on the part of both medical personnel and patients, who must strictly adhere to a regimen that involves multiple medications on a complicated dosing schedule.7,8 Inconsistent use of or dropout from combination therapy involving PI/NNRTIs can cause a loss of effectiveness at the individual level and can also lead to the transmission of drug-resistant HIV strains.9–11 There are many potential financial and nonfinancial barriers to initial access to these treatments and to continuation of treatment consistently on a long-term basis, and such barriers may disproportionately affect members of disadvantaged subgroups.
Several studies, notably the HIV Cost and Service Utilization Study (HCSUS), a nationally representative study of persons in care for HIV disease, have reported that a majority of HIV-infected individuals initiated these new treatments within 2 years of their introduction.2,12–15 In these studies, estimates of treatment rates ranged from 66% to nearly 90% by the beginning of 1998. However, studies examining long-term consistency of use, once therapy has been initiated, have been rare. Difficult decisions are now required of clinicians to determine when and how to provide combination therapies to individuals with characteristics that they may feel threaten persistent use.16–18 The studies that examine factors affecting adherence to combination therapies have shown that patient characteristics such as gender, race/ethnicity, and injection drug use history; physician opinions; and the presence of or the anticipation of side effects may be associated with adherence.19–24 These studies have typically involved examination of adherence over a relatively short time period25–28; HCSUS for example, used a self-report measure with a 1-week recall period.
Although zidovudine adherence has been measured in earlier studies over relatively long periods of time,29–31 there is a need for longitudinal data on the experience of patients taking newer antiretrovirals over time. There is also a need for studies that focus on nonfinancial barriers to care by holding payer status constant. Insurance status is often included as a covariate in studies of samples with diverse health coverage; however, even with statistical controls for the main effect of insurance status, results from such samples tell us little about variations in use within major coverage groups such as those whose care is paid for by Medicaid, the most important single payer for HIV/AIDS care.32 There is a need for studies of the newer antiviral treatments in large populations with comparable third-party coverage. Medicaid beneficiaries are particularly important because the poverty and complex care needs of these patients may make them vulnerable to inconsistent use of the new therapies.33
In the present paper, we analyze initiation of antiretroviral treatment regimens that include PI/NNRTI drugs among Medicaid beneficiaries with AIDS in New Jersey during the 1996–1998 period, representing approximately the first 3 years following the introduction of these new classes of antiretrovirals. Among those who initiated PI/NNRTI therapy, we also examine the extent to which treatment was continued over time.
METHODS
Data
This study utilizes data from a statewide population of patients with AIDS participating in the New Jersey Medicaid program. The study population was identified through a file match between the New Jersey HIV/AIDS Registry and the Medicaid eligibility file. The file match was accomplished through a cooperative agreement between 2 state agencies: the New Jersey Department of Health and Senior Services (DHSS), which administers the HIV/AIDS registry, and the Division of Medical Assistance and Health Services (DMAHS)in the Department of Human Services, which administers the Medicaid system. The file link was done by DHSS, using identifying fields common to both files such as name, birth date, gender, and social security number. Once the link was established, the researchers were provided with client-level files containing claims, and Medicaid eligibility and Registry information such as exposure category and dates of diagnosis, in which individuals were identified only with scrambled Medicaid ID numbers.
DMAHS provided the research team with files consisting of Medicaid claims for services and pharmacy prescriptions provided from January 1988 through April 1999 and Medicaid eligibility information. The claims file provided information on claim type, diagnosis, category of service, dates of service, and actual amount paid by Medicaid for each of the services. The pharmacy file contained National Drug Codes (NDCs), dispensing date, amount paid by Medicaid, and therapeutic class category. The eligibility file contained the Medicaid enrollment and termination dates for each individual.
Study Population
Controversy continues to exist about the best time to initiate therapy with highly active agents in patients with early-stage HIV disease.34 For this reason, we focus in this paper on treatment among individuals with AIDS, defined as those who had an AIDS diagnosis in the Registry or who had at least 1 paid claim with a diagnosis of an AIDS-defining opportunistic infection (OI) during the period of observation. We also restricted the analysis to adults surviving the observation period with uninterrupted Medicaid eligibility, whose health care utilization could be observed continuously. Focusing the analysis on survivors avoids the complications of end-of-life treatment issues (although, as the sensitivity analysis results reported below indicate, results were similar whether or not decedents were included). Thus, our final selection criteria included age of 18 or over at the time of AIDS diagnosis, with enrollment in Medicaid for at least 90 days during the study period, who survived the observation period and had no interruptions in Medicaid coverage after March 1996 and who therefore provided uninterrupted information on pharmaceutical use. New Jersey did not mandate managed care participation during the observation period for the Medicaid disabled population, and relatively few persons with AIDS participated in such plans.35 We excluded any individuals who did participate in managed care plans, since paid claims are not generated for participants enrolled in managed care. Of the 2,678 individuals who were eligible to be included in the study, 219 (8%) were excluded because of interruptions in Medicaid eligibility, leaving 2,459. Seventy percent of the study population (n = 1,739) survived all of the observation period.
Measures
Dependent Variables
Time to initiation of protease inhibitor/non-nucleoside reverse transcriptase inhibitor therapy. We used NDCs recorded in pharmacy claims to identify PI or NNRTI use. We focused on these key drugs rather than a more elaborate measure of HAART use (i.e., PI/NNRTI plus appropriate other antiretroviral drugs) since operationalizing such definitions is difficult and there is not clear consensus on the combinations that constitute appropriate HAART therapy. PI drugs available during the observation period included ritonavir, indinavir, saquinavir, and nelfinavir, while NNRTIs included nevirapine, delavirdine and efavirenz. On the basis of the NDCs, we constructed an indicator variable for PI/NNRTI use. For individuals with at least 1 paid prescription for PI/NNRTIs, time to PI/NNRTI was defined as time from January 1996 to the date of first prescription of PI/NNRTIs. Patients who never used PI/NNRTIs during the follow-up period also contributed person-time to the survival analyses. For these individuals, time from January 1996 to the end of the study period was calculated.
>Persistence of PI/NNRTI use. This measure was defined only for users of PI/NNRTIs. On the basis of dispensing dates of PI or NNRTI claims, we derived total number of days on PI/NNRTI drugs. Follow-up was calculated as the number of days from first prescription date to the end of the study. By dividing total number of days on PI/NNRTI by follow-up time as defined above, we computed proportion of time on PI/NNRTI drugs. This measure varies from just above 0 for those with brief use to 1.0 for those with claims covering the entire period of observation after initiation. A similar method has been successfully used in published studies on persistence of zidovudine treatment.30,36
Independent Variables
Demographics. These data were based on information in the Registry. Race/ethnicity was characterized as African American, Hispanic, and non-Hispanic white. In multivariate analyses, non-Hispanic white and female gender were used as the comparison group. We created an indicator variable identifying beneficiaries with histories of injection drug use. We collapsed age at diagnosis into categorical groups—18 to 29 years (the reference group in multivariate models), 30 to 39 years, 40 to 49 years, and 50+ years—anticipating its nonlinear effects on outcomes. Since patterns of HIV health care in geographical areas where there is a high concentration of HIV patients may differ from those in areas where patients are less concentrated, we included geographical location in the analysis, contrasting patterns of PI/NNRTI use in the high-HIV-prevalence areas of the state (the counties nearest to New York City and to Philadelphia, comprising Essex, Hudson, Passaic, Bergen, Union and Camden) and elsewhere.
Illness stage To control for disease stage, indicator variables for year of diagnosis and presence of OIs were included in the regression as control variables. Opportunistic infections were identified on the basis of diagnostic codes conforming to the International Classification of Diseases, 9th Revision, Clinical Modification for medical care episodes contained in the claims data (list of codes is available from the senior author on request). Year of diagnosis was categorized into 3 time periods: prior to 1992, 1993–1994, and 1995–1996.
Medicare participation. Recently, Medicaid beneficiaries with HIV have more frequently survived the waiting period for Medicare eligibility and become dually eligible for both Medicare and Medicaid.37 On the basis of our previous research, we expect Medicare participants to have greater access to outpatient medical treatments.38 Therefore, we included Medicare coverage as a covariate in our study. Medicare coverage was assessed on the basis of information in the Medicaid claims files.
>Waiver status Some of New Jersey's Medicaid population is enrolled in the AIDS Community Care Alternatives Program (ACCAP), an HIV-specific Medicaid home- and community-based care waiver program that offers a variety of services, including case management and private-duty nursing. Access to the case management and other community-based services provided by ACCAP could affect treatment patterns.39 Therefore, we used waiver status as a covariate in all our analyses. Participation in the waiver program was determined by using the procedure codes in Medicaid claims for waivered services.
Analytic Procedures
Initiation of PI/NNRTI: Product-Limit Estimation and Proportional Hazards Regression
In modeling time to initiation of PI therapy, we need to take into account the fact that for some individuals, initiation had not yet occurred by the end of the follow-up period. We used survival analysis techniques to include these “censored” cases in the analysis. In the descriptive analyses, we applied product-limit (Kaplan-Meier) estimation techniques. The log rank statistic was used to assess significant group differences in months to PI/NNRTI initiation. In multivariate analyses, effects of patient characteristics on time to initiation of PI therapy were ascertained using proportional hazards regression. A point estimate and confidence interval (CI) for the relative hazard were computed for the effect of each covariate. The relative hazard estimate can be interpreted as the multiplicative effect of the covariate on the hazard of PI/NNRTI use at a particular time, given that PI/NNRTIs were not used up to that time. Therefore, a negative coefficient, implying a relative hazard below 1.0, represents a lower hazard of PI use at a given level of time and thus a higher expected time to initiation of therapy, implying less-favorable access to care.
Persistence of PI/NNRTIS: Ordinary Least Squares and Logistic Regressions
These analyses were restricted to users of PI/NNRTI drugs. Persistence was defined as proportion of follow-up time during which PI/NNRTI drugs were used following initiation of therapy. Group differences in persistence were analyzed with t tests. Ordinary least square regressions were used to isolate the effects of covariates on persistence of PI/NNRTI use.
Quarterly Use of Protease Inhibitors: Longitudinal Logistic Regressions
We also tested the robustness of our results by analyzing quarterly use of PI/NNRTIs after initiation, using longitudinal logistic regressions. Person-quarter files were created by organizing PI/NNRTI use data into quarters counting forward from date of first prescription; the unit of analysis was the person-quarter, with the number of quarterly observations varying across individuals depending on the time of initiation and end of study. Because repeated measurements from the same persons were used to measure persistence, we applied robust covariance methods.40 The odds of use of PI/NNRTIs by person i in quarter t were estimated in a logistic regression model. This model is an extension of the general linear model with a complex error structure, and was estimated with the generalized estimating equation (GEE) technique for binary outcome variables using STATA's xtgee procedure (STATA Corp., College Station, Tex).
RESULTS
Of the 1,739 patients, 62% were male; 58% were African American, 19% Hispanic, and 23% non-Hispanic white. A majority (79%) were aged 30 to 49 years at the time of initial diagnosis. Injection drug use history was the most common exposure category (60%). Seventy percent of the study cohort lived in the high-prevalence urban areas of the state (near New York City or Philadelphia) at the time of initial diagnosis. A minority (15%) were enrolled in the waiver program, and 37% were on Medicare during part or all of the follow-up period.
Delays in PI/NNRTI Use
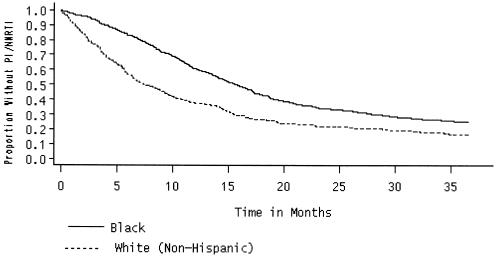
Of the 1,739 fully observed patients (who survived the full observation period), 1,261 (67%) had at least 1 paid claim for PI/NNRTIs between January of 1996 and December of 1998. Median months (Kaplan-Meier product-limit estimates) to PI/NNRTI initiation by subgroups are presented in Table 1). Overall, the median time to PI/NNRTI initiation was 14 months. On the basis of the log-rank statistic, we found significant differences in median months to initiation of therapy by race/ethnicity, mode of transmission, waiver program participation, and Medicare coverage. African Americans waited 8 months longer than whites (15.2 months vs 7.5 months) to initiate therapy (Fig. 1). Similarly, injection drug users (IDUs) experienced a longer waiting period than did nonIDUs (14.5 months vs 12.3 months). Median months to initiation was only 6.6 months for waiver participants, while traditional Medicaid beneficiaries waited almost 15 months to initiate therapy. Those with dual eligibility waited only 10 months. No differences in PI/NNRTI initiation by gender, age at diagnosis, year of diagnosis, and county of residence were noted.
Table 1.
Initiation of PI/NNRTIs among HIV-infected New Jersey Medicaid Participants (N = 1,739)
| Proportional Hazards Regression | ||||
|---|---|---|---|---|
| n | Censored, % | Median Months to Initiation† | Hazards Ratio (95% Confidence Interval) | |
| All | 1,739 | 27.5 | 14.1 | — — |
| Gender | ||||
| Male | 1,070 | 28.2 | 13.9 | — — |
| Female | 669 | 26.3 | 14.6 | 1.07 (0.95 to 1.20) |
| Race/ethnicity | ||||
| White | 391 | 19.9 | 7.5w | — — |
| African American | 1,005 | 37.3 | 15.2 | 0.63*(0.55 to 0.72) |
| Hispanic | 333 | 31.2 | 16.8 | 0.65*(0.54 to 0.78) |
| Age at diagnosis, y | ||||
| 18–29 | 250 | 34.0 | 14.0 | — — |
| 30–39 | 860 | 26.1 | 13.1 | 1.17 (0.98 to 1.39) |
| 40–49 | 523 | 26.4 | 13.6 | 1.18 (0.97 to 1.43) |
| >50 | 106 | 28.3 | 14.5 | 1.09 (0.83 to 1.44) |
| Mode of transmission | ||||
| Injection drug users | 1,048 | 27.1 | 14.5w | 0.88*(0.77 to 1.00) |
| NonIDUs | 467 | 26.8 | 12.3 | — — |
| County of residence | ||||
| High-prevalence area | 1,252 | 27.1 | 14.7 | 1.16*(1.02 to 1.33) |
| Elsewhere | 487 | 28.3 | 12.1 | — — |
| Year of diagnosis | ||||
| 1991–92 | 423 | 27.2 | 15.0 | — — |
| 1993–94 | 897 | 28.3 | 14.3 | 0.94 (0.84 to 1.05) |
| 1995–96 | 419 | 26.0 | 12.5 | 0.79 (0.44 to 1.40) |
| Waiver status | ||||
| NonACCAP | 1,486 | 29.5 | 15.1w | — — |
| ACCAP | 253 | 15.4 | 6.6 | 1.27*(1.08 to 1.49) |
| Medicare coverage | ||||
| No Medicare | 1,089 | 33.7 | 16.4w | — — |
| Medicare | 650 | 17.1 | 10.2 | 1.50*(1.33 to 1.69) |
| Opportunistic infection | ||||
| No | 741 | 35.1 | 17.8w | — — |
| Yes | 998 | 21.8 | 11.0 | 1.48*(1.32 to 1.67) |
* P ≤ .05.
Based on Kaplan-Meier estimates of months to PI/NNRTI use.
PI/NNRTI, protease inhibitors/non-nucleoside reverse transcriptase inhibitors. The study population is based on continuously eligible adult Medicaid participants with AIDS and alive as of December 1998, age 18 or older. Utilization was observed between January 1996 and December 1998. The superscript w denotes significant group differences in PI/NNRTI initiation at the 5% significance level based on log-rank statistic. Odds ratios are estimated from Cox proportional hazards regression on time to PI/NNRTI use. The regression also includes an intercept term. Asterisks indicate the statistical significance of estimated effects, relative to the omitted reference category.
IDUs, injection drug users; ACCAP, AIDS Community Care Alternatives Program.
FIGURE 1.
Time to PI/NNRTI initiation.
Table 1 also reports results from a multivariate survival analysis of time to initiation, using a Cox proportional hazards model. Hazard ratios with 95% CIs are displayed (hazard ratios above 1.0 imply more rapid initiation of therapy). The validity of the proportional hazards assumption was tested by visual comparison of the log-minus-log distribution functions for each level of the independent variable.41 We did not observe any crossing hazard functions in these plots that would indicate violation of the proportional hazard assumption. Findings from multivariate analyses were similar to bivariate findings. African Americans and Hispanics as well as IDUs had significantly greater delays in use relative to whites and nonIDUs. The hazard ratio was 0.63 (95% CI, 0.55 to 0.72) for African Americans, 0.65 (95% CI, 0.54 to 0.78) for Hispanics, and 0.88 (0.77 to 1.00) for IDUs. Waiver program participation was associated with more rapid initiation of PI/NNRTIs, with a hazard ratio of 1.27 (95% CI, 1.08 to 1.49). Similar results were found for Medicare coverage, which was associated with a hazard ratio of 1.50 (95% CI, 1.33 to 1.69). Significant geographic variation was apparent, with patients living in high-prevalence areas experiencing more rapid initiation of PI/NNRTIs, with a hazard ratio of 1.15 (95% CI, 1.02 to 1.33).
Sensitivity to Exclusion/Inclusion of Decedents
To test that these results are robust and generalizable to patients with more advanced disease, we repeated our analyses on the whole sample (n = 2,459), including patients who did not survive all of the observation period. Results of these analyses were similar to those for nondecedents.
Persistence of PI/NNRTI Use
Among those initiating PI/NNRTI use, 35% had discontinued it by the end of follow-up in December of 1998. On average, beneficiaries initiating PI/NNRTI therapy received such therapy for 66% of the follow-up time after their first prescription (Table 2). In bivariate analyses, significant differences were observed in the proportion of follow-up time on the new drug therapies by race/ethnicity, age at diagnosis, waiver program participation, and Medicare coverage. Minorities were less persistent in the use of PI/NNRTIs than were non-Hispanic whites. On average, African Americans used PI/NNRTIs for 64% of observation time following initiation, compared to 72% of the time by whites. Similarly, Hispanics remained on therapy for only 64% of the time after the first prescription. Patients who were 18 to 29 years of age at the time of diagnosis were the least persistent users of PI/NNRTIs, using these medications only 61% of the time as compared to 66%, 69% and 68% of the time for patients who were older when diagnosed with HIV/AIDS (30–39, 40–49 and 50+ years, respectively).
Table 2.
Persistence on PI/NNRTIs among HIV-infected Medicaid Beneficiaries (N = 1,261)
| Mean | Model 1, β | Model 2, β | Model 3, β | |
|---|---|---|---|---|
| All | 0.66 | — | — | — |
| Gender | ||||
| Male | 0.66 | — | — | — |
| Female | 0.66 | 0.01 | 0.02 | 0.02 |
| Race/ethnicity | ||||
| White | 0.72w | — | — | — |
| African American | 0.64 | −0.09* | −0.08* | −0.08* |
| Hispanic | 0.64 | −0.09* | −0.08* | −0.08* |
| Age at diagnosis, y | ||||
| 18–29 | 0.61w | — | — | — |
| 30–39 | 0.66 | 0.06* | 0.05* | 0.05* |
| 40–49 | 0.69 | 0.09* | 0.08* | 0.08* |
| >50 | 0.68 | 0.09* | 0.08* | 0.08† |
| Mode of transmission | ||||
| Injection drug users | 0.66 | −0.03 | −0.02 | −0.02 |
| NonIDUs | 0.68 | — | — | — |
| County of residence | ||||
| High-prevalence area | 0.67 | 0.04* | 0.05* | 0.05* |
| Elsewhere | 0.65 | — | — | — |
| Waiver status | ||||
| NonACCAP | 0.65w | — | — | — |
| ACCAP | 0.73 | — | 0.06* | 0.06* |
| Medicare coverage | ||||
| No Medicare | 0.64w | — | — | — |
| Medicare | 0.69 | — | 0.03† | 0.04* |
| Year of diagnosis | ||||
| 1991–92 | 0.66 | — | — | — |
| 1993–94 | 0.67 | — | — | 0.02 |
| 1995–96 | 0.64 | — | — | 0.01 |
| Opportunistic infection | ||||
| No | 0.67 | — | — | — |
| Yes | 0.66 | — | — | −0.03 |
P < .05.
05 < P < .10.
PI/NNRTI, protease inhibitors/non-nucleoside reverse transcriptase inhibitors. The study population was based on continuously eligible adult Medicaid participants with AIDS and alive as of December 1998, age 18 or older. Utilization was observed between January 1996 and December 1998. The superscript w refers to significant group differences in proportion of follow-up time based on t tests. βs were estimated from OLS regressions on proportion of follow-up time on PI/NNRTIs. The regression also includes an intercept term. Asterisks indicate the statistical significance of estimated effects, relative to the omitted reference category.
IDUs, injection drug users; ACCAP, AIDS Community Care Alternatives Program.
Table 2 also provides results from a multivariate analysis of persistence, using nested ordinary least squares (OLS) regressions on proportion of follow-up time receiving PI/NNRTIs after treatment was initiated. In the first model, only sociodemographic variables were entered. These included gender, race, age at diagnosis, exposure route, and geographic residence. In model 2, waiver program participation and Medicare coverage were added, and in model 3, the illness stage variables were also included. In general, the multivariate models of persistence confirmed bivariate findings, with African Americans and Hispanics found to be less persistent users, while gender and history of injection drug use were not associated with persistence. Patients over age 40 were more persistent, as were participants in the case-managed waiver program. In the multivariate models, residence in a high-prevalence area was also a significant predictor of more-persistent PI/NNRTI use, with patients living in the high-prevalence counties near New York City and Philadelphia more consistent users of PI/NNRTIs. The effect of ethnicity was substantial: in the final model, among users, controlling for other characteristics, African-American race and Hispanic ethnicity were each associated with a significant 8% reduction in the proportion of time on PI/NNRTIs.
We tested the robustness of these socioeconomic differences in persistence across alternative specifications using several different modeling approaches. All models produced generally similar patterns of results, and the race effect was found consistently. First, we dichotomized persistence into highly persistent (PI/NNRTI use ≥80% of the time) versus not highly persistent. Findings from these analyses were similar to OLS regression results on proportion of time on therapy. We further examined the robustness of the findings with 2 additional model specifications. In a simple logistic regression on discontinuation of PI/NNRTI treatment by the end of the follow-up period among those initiating PI/NNRTIs, African Americans were significantly less likely than others to continue (odds ratio, 0.72; 95% CI, 0.53 to 0.98); 37% of African Americans discontinued treatment as compared with 31% of nonLatino whites (results not shown in tables). Finally, as discussed under Methods above, we further tested the robustness of our analyses with a repeated-measures logistic regression on quarterly use of PI/NNRTIs after initiation, using a GEE model suitable for analyzing repeated binary outcomes. The odds of use during a given quarter following initiation of treatment were significantly lower for African Americans than for others (odds ratio, 0.66; 95% CI, 0.51 to 0.86). Thus, in all analyses, persistence was significantly lower for African Americans as compared with other Medicaid beneficiaries.
Sensitivity to Exclusion/Inclusion of Decedents
We tested the sensitivity of these findings to the exclusion of decedents by estimating a GEE model of persistence for the entire sample, including decedents. Findings were comparable to those based only on nondecedents, replicating the findings that being African American was associated with significantly less persistence, and that waiver participation was associated with significantly more persistence. These results should be interpreted with caution, because duration of follow-up was shorter for decedents (mean follow-up was 11 months), making it more difficult to measure persistence. Furthermore, it is desirable to distinguish between end-of-life care and other care, which arguably involve differing treatment issues. For patients with AIDS in the end-of-life period, some discontinuations of care may reflect treatment failure. Therefore, the results reported in the tables are based on persistence of PI/NNRTI use among those who survived the observation period.
DISCUSSION
Many individuals with AIDS in our study either were not started on regimens that included PI/NNRTIs in the 3 years following their introduction, or failed to remain on these regimens consistently once therapy was initiated. By the end of 1998, 33% of our study population had not initiated use of PI/NNRTIs. Among those who did initiate use, 35% discontinued use by the end of the observation period, adding to the evidence that consistent longitudinal use is difficult for many patients.19 All individuals in this study were receiving Medicaid, which in New Jersey includes coverage for physician visits and all PI/NNRTI drugs, as well as testing and other aspects of the medical management of antiviral therapy. However, despite being in the same payer system, racial and ethnic minorities experienced greater delays in the initiation of PI/NNRTI therapy and were less persistent in their use of these therapies. African Americans experienced 8 months more of delay in the initiation of therapy than whites, and used these therapies 64% of the time after the first prescription, as compared to 72% for whites. Hispanics also had more delay in initiation and less persistent use, as compared to whites.
It is difficult to determine from administrative data alone the reasons for less-consistent use of PI/NNRTI drugs among minorities. Minorities have been found in multiple studies to experience more barriers to health care access, with differences in use of appropriate treatments that often remain even when analyses control for differences in health coverage.42,43 For example, studies have documented racial disparities in such areas as cardiac44–46 and cancer care.47–49 Disparities in treatment use in our study population may be influenced by differences in care-seeking, nonfinancial access barriers, or differences in quality or continuity of care. Our finding of less-persistent use by African Americans is particularly troublesome given the rates of HIV disease in this population. Although African Americans made up 13% of the U.S. population, they represented 48% of all AIDS cases in 1998 and accounted for half of all new HIV infections.50
We also found that injection drug users experienced longer delays in initiating PI/NNRTI treatment, as has been found in previous studies.51 Although it is unclear whether some of these delays were clinically appropriate, these data identify slower diffusion of this therapy in this group. Once these therapies were initiated, however, IDUs were no less persistent in use, similar to findings reported in the literature with regard to zidovudine treatment.31 While some providers may hesitate to prescribe PI/NNRTI drugs to individuals with a history of injection drug use because of concerns about persistence, our results do not support such assumptions.
Patients living in high-HIV-prevalence areas initiated therapy earlier and were also more persistent users of PI/NNRTIs, once use was initiated. It is possible that medical care providers in these areas have more experience in treating HIV disease and have improved their ability to initiate and maintain standard treatment.52
Older AIDS patients (over age 50 at the time of initial diagnosis) were more persistent users of these new regimens, as compared to their younger counterparts. This finding may appear counterintuitive in light of the greater medical comorbidity and lack of social support that is often thought to characterize older individuals living with HIV (SC, unpublished data, 2000) and concerns about their ability to handle the side effects of these powerful antiviral drugs. It is, however, consistent with earlier research,53 and may reflect such factors as lower levels of active drug and alcohol abuse, more stable living arrangements, and lower impulsivity and greater life experience of older patients with HIV. Individuals over the age of 50 make up 11% of AIDS cases54 and it is important to develop more knowledge about care and adherence patterns for this group. Our findings provide no support for hesitation in initiating HAART regimens for such individuals based on concerns that they may be less able to remain consistently on the regimens over time; if anything, young adults appear to be more problematic in this respect.
Participants in a case-managed waiver program initiated PI/NNRTI treatment nearly 9 months earlier than nonparticipants and were also more persistent in their use of these medications. It is possible that receiving case management with monthly home visits, typically by a nurse case manager, allowed these patients to overcome barriers to persistence that nonparticipants were not able to overcome. The ability of the case managers to assist patients in receiving therapies has been documented previously, and this may also be affecting persistence.39 These findings suggest the need for further studies of the effectiveness of case management strategies in managing patients on complex antiretroviral regimens, and for expanded efforts to provide support to persons living with HIV in remaining on highly active antiretroviral regimens. Such studies need to further examine the association between waiver participation and persistence on newer HIV medications, and to explore whether these associations represent intervention effects as opposed to selection into case management programs55(SC, unpublished data, 1998).
We found no gender differences in either initiation or persistence for these newer treatments. Previous studies have provided mixed findings on this issue.12 These divergences may reflect differences in study populations, sites, methods, and time periods. In New Jersey, there have been a number of initiatives by state agencies and the health care community to increase access to health care services among HIV-positive women, with strong efforts beginning in the mid-1990s to reduce perinatal transmission by encouraging use of antiretrovirals during pregnancy. New Jersey requires mandatory testing and counseling of all pregnant women, which the DHSS implements by frequent contact with health care facilities (including emergency departments) and providing education to clinicians.56 These efforts may have had a spillover effect on HIV care for women in general and helped to reduce gender disparities in care.
Strengths of this study include its large statewide sample, representing beneficiaries receiving medical care from a broad range of providers, as well as the fact that it does not rely on self-report to determine utilization, using instead data on filled prescriptions. Reliance on administrative data also means that individualized consent was not required and the entire population could be studied. However, the administrative data strategy also has significant limitations. It is possible that some individuals continue to fill prescriptions without actually taking the drugs, leading to a possible underestimate of dropout from treatment. In addition, claims data, even in combination with Registry data, provide only limited covariate information to control for disease stage such as CD4+ counts, viral load, health status, and other patient characteristics.
The evaluation of persistence, particularly at the end of life, could be complicated in some cases by virologic failure and consequent regimen switching (this is one of the reasons we focused on nondecedent individuals in our main analysis). However, we believe that in most cases of appropriate regimen switching, it is likely that a new regimen would have been started with little time lapse. Although there has been recent interest in the concept of structured interruptions or “drug holidays,” this was not a commonly recommended form of care during our study period. Guidelines for structured interruptions or drug holidays were published almost 3 years after the end of our study period, in February 2001. The link between adherence and clinical outcomes among HIV-infected patients has been well documented.27,36,57,58 For example, in a study using filled prescriptions, patients who missed filling their PI prescription for 1 month were significantly less likely to achieve undetectable viral loads.58 Similarly, patients reporting higher adherence have been shown to have greater HIV suppression.27
Adhering to complex combination antiviral regimens for HIV disease is a considerable challenge, particularly for low-income individuals likely to be struggling with economic and social issues, such as the Medicaid beneficiaries in our study population. There is a need for additional research on whether case management strategies improve adherence over time, as our results suggest, and on the relative strengths of alternative care management strategies in helping patients in the difficult task of long-term adherence. Another important issue concerns the intensity of medical management and frequency of follow-up. Is greater frequency of medical visits associated with greater adherence? Our study results are consistent with this assumption. Controlling for other covariates, our study found a positive association between number of visits and initiation and persistence of PI/NNRTI use. One additional visit per year was associated with a 4% increase (CI, 3% to 5%) in the odds of initiation and, among those who initiated treatment, with a 1% increase in proportion of follow-up time on therapy. However, further research on this issue is needed because the frequency of physician visits is likely both to be affected by and to affect health status, and may function in part as an indicator of unmeasured differences in health status. Frequency is also likely both to be affected by and to affect initiation and persistence in PI/NNRTI use.
While limitations of administrative data suggest the need for caution in interpreting the findings, our results are of particular concern with respect to racial and ethnic disparities in receipt of care. Our findings extend previous research documenting racial and ethnic differences in the initiation of antiretroviral therapies by raising concerns about disparities in persistence of use of these therapies. In the era of highly active but demanding antiretroviral therapy regimens, these results suggest the need for further examination of the barriers to persistent use of these therapies. In particular, they suggest the need for increased efforts to implement and evaluate new care strategies and supportive services that may be helpful in helping all subgroups of patients remain on their regimens on a consistent and long-term basis.
Acknowledgments
The authors wish to acknowledge the programming assistance provided by Ms. Yang Lou and research assistance provided by Ms. Michelle McGrath. The authors also gratefully acknowledge the assistance of the New Jersey Department of Human Services and the New Jersey Department of Health and Senior Services in cooperating with the research team and providing data used in this study, with special thanks to Dr. Gloria Rodriguez, Samuel Costa (now of the Centers for Disease Control), Dr. Sander Kelman and Mr. Donald Hartz.
This research was supported by National Institutes of Health grants R01-MH58984, R01-DA11362, R01-DA11855, and P01-MH43450 (NIA supplement).
The findings and opinions reported here are those of the authors and do not necessarily represent the views of any other individuals or organizations.
REFERENCES
- 1.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–4. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 2.Palella J, Frank J, Delaney KM, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter CCJ, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1997: updated recommendations of the International AIDS Society, USA panel. JAMA. 1997;277:1962–9. [PubMed] [Google Scholar]
- 4.Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 protease inhibitors: a review for clinicians. JAMA. 1997;277:145–53. [PubMed] [Google Scholar]
- 5.Fauci AS. AIDS in 1996: much accomplished, much to do. JAMA. 1996;276:155–6. [PubMed] [Google Scholar]
- 6.Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Morb Mortal Wkly Rep. 1998;47:43–82. [Google Scholar]
- 7.Bonfanti P, Capetti A, Rizzardini G. HIV disease treatment in the era of HAART. Biomed Pharmacother. 1999;53:93–105. doi: 10.1016/s0753-3322(99)80066-3. [DOI] [PubMed] [Google Scholar]
- 8.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–7. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 9.Erice A, Mayers DL, Strike DG, et al. Brief report: primary infection with zidovudine-resistant human immunodeficiency virus type 1. N Engl J Med. 1993;328:1163–5. doi: 10.1056/NEJM199304223281605. [DOI] [PubMed] [Google Scholar]
- 10.Hecht FM, Grant RM, Petropoulos CJ, et al. Sexual transmission of an HIV-1 variant resistant to multiple reverse-transcriptase and protease inhibitors. N Engl J Med. 1998;339:307–11. doi: 10.1056/NEJM199807303390504. [DOI] [PubMed] [Google Scholar]
- 11.Boden D, Hurley A, Zhang L, et al. HIV-1 drug resistance in newly infected individuals. JAMA. 1999;282:1135–41. doi: 10.1001/jama.282.12.1135. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–15. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 13.Bing EG, Kilbourne AM, Brooks RA, Lazarus EF, Senak M. Protease inhibitor use among a community sample of people with HIV disease. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:474–80. doi: 10.1097/00042560-199904150-00010. [DOI] [PubMed] [Google Scholar]
- 14.Sorvillo F, Kerndt P, Odem S, et al. Use of protease inhibitors among persons with AIDS in Los Angeles County. AIDS Care. 1999;11:147–55. doi: 10.1080/09540129948045. [DOI] [PubMed] [Google Scholar]
- 15.Sackoff JE, McFarland JW, Shin SS. Trends in prescriptions for highly active antiretroviral therapy in four New York City HIV clinics. J AIDS. 2000;23:178–83. doi: 10.1097/00126334-200002010-00010. [DOI] [PubMed] [Google Scholar]
- 16.Mehta S, Moore RD, Graham NMH. Potential factors affecting adherence with HIV therapy. AIDS. 1997;11:1665–70. doi: 10.1097/00002030-199714000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Bangsberg D, Tulsky JP, Hecht FM, Moss AR. Protease inhibitors in the homeless. JAMA. 1997;278:63–5. [PubMed] [Google Scholar]
- 18.Bayer R, Stryker J. Ethical challenges posed by clinical progress in AIDS. Am J Public Health. 1997;87:1599–602. doi: 10.2105/ajph.87.10.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Roon EN, Verzijl JM, Juttmann JR, Lenderink AW, Blans MJ, Egberts AC. Incidence of discontinuation of highly active antiretroviral combination therapy (HAART) and its determinants. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:290–4. doi: 10.1097/00042560-199903010-00012. [DOI] [PubMed] [Google Scholar]
- 20.Malow RM, McPherson S, Kilmas N, et al. Adherence to complex combination antiretroviral therapies by HIV-positive drug abusers. Alcohol Drug Abuse. 1998;49:1021–4. doi: 10.1176/ps.49.8.1021. [DOI] [PubMed] [Google Scholar]
- 21.Kalichman SC, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. J Gen Intern Med. 1999;14:267–73. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–9. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 23.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19:124–33. Mar. [PubMed] [Google Scholar]
- 24.Bassetti S, Battegay M, Furrer H, et al. Why is highly active antiretroviral therapy (HAART) not prescribed or discontinued? J AIDS. 1999;21:114–9. [PubMed] [Google Scholar]
- 25.Eldred LJ, Wu AW, Chaisson RE, Moore RD. Adherence to antiretroviral and pneumocystis prophylaxis in HIV disease. J AIDS. 1998;18:117–225. doi: 10.1097/00042560-199806010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Kastrissios H, Suarez JR, Hammer S, Katzenstein D, Blaschke TF. The extent of non-adherence in a large AIDS clinical trial using plasma dideoxynucleoside concentrations as a marker. AIDS. 1998;12:2305–11. doi: 10.1097/00002030-199817000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Haubrich RH, Little SJ, Currier JS, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. AIDS. 1999;13:1099–107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- 28.Rigsby MO, Rosen MI, Beauvais JE, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15:841–7. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laine C, Newschaffer CJ, Zhang D, Cosler L, Hauck WW, Turner BJ. Adherence to antiretroviral therapy by pregnant women infected with human immunodeficiency virus: a pharmacy claims-based analysis. Obstet Gynecol. 2000;95:167–73. doi: 10.1016/s0029-7844(99)00523-2. [DOI] [PubMed] [Google Scholar]
- 30.Crystal S, Sambamoorthi U, Merzel C. The diffusion of innovation in AIDS treatment: zidovudine use in two New Jersey cohorts. Health Serv Res. 1995;30:593–614. [PMC free article] [PubMed] [Google Scholar]
- 31.Broers B, Morabia A, Hirschel B. A cohort study of drug users' compliance with zidovudine treatment. Arch Intern Med. 1994;154:1121–7. [PubMed] [Google Scholar]
- 32.Center for Medicaid and State Operations. Medicaid and acquired immune deficiency syndrome (AIDS) and human immunodeficiency virus (HIV) infection. April 2000 http://www.hcfa.gov/medicaid/obs11.htm. [Google Scholar]
- 33.Berk ML, Schur CL. Access to care: how much difference does Medicaid make? Health Aff (Millwood) 1998;17:169–80. doi: 10.1377/hlthaff.17.3.169. [DOI] [PubMed] [Google Scholar]
- 34.Henry K. The case for more cautious, patient-focused antiretroviral therapy. Ann Intern Med. 2000;132:306–11. doi: 10.7326/0003-4819-132-4-200002150-00009. [DOI] [PubMed] [Google Scholar]
- 35.Regenstein M, Anthony SE. Medicaid managed care for persons with disabilities. Economic and Social Research Institute; August 1998. http://newfederalism.urban.org/html/occa11.html#medi. [Google Scholar]
- 36.Turner BJ, Newschaffer CJ, Zhang D, et al. Antiretroviral use and pharmacy-based measurement of adherence in postpartum HIV-infected women. Med Care. 2000;38:911–25. doi: 10.1097/00005650-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Fasciano NJ, Cherlow AL, Turner BJ, Thornton CV. Profile of Medicare beneficiaries with AIDS: application of an AIDS casefinding algorithm. Health Care Financ Rev. 1998;19:19–38. [PubMed] [Google Scholar]
- 38.Sambamoorthi U, Collins S, Crystal S. Characteristics of dually-eligible Medicaid beneficiaries. Health Soc Policy. doi: 10.1300/J045v14n01_02. in press. [DOI] [PubMed] [Google Scholar]
- 39.Merzel C, Crystal S, Sambamoorthi U, Karus D, Kurland C. New Jersey's Medicaid waiver for acquired immunodeficiency syndrome. Health Care Financ Rev. 1992;13:27–44. [PMC free article] [PubMed] [Google Scholar]
- 40.Diggle P, Liang KY, Zeger S. Analysis of Longitudinal Data. Oxford and New York: Oxford University Press; 1994. [Google Scholar]
- 41.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons, Inc.; 1980. [Google Scholar]
- 42.Aday LA, Anderson RM. The national profile of access to medical care: where do we stand? Am J Public Health. 1984;74:1331–9. doi: 10.2105/ajph.74.12.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access in health care. JAMA. 1995;274:305–11. [PubMed] [Google Scholar]
- 44.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the department of veterans affairs. JAMA. 1994;271:1175–80. [PubMed] [Google Scholar]
- 45.Whittle J, Conigliaro J, Good CB, Joswiak M. Racial differences in the use of invasive cardiovascular procedures in the Department of Veterans Affairs medical system. N Engl J Med. 1993;329:621–7. doi: 10.1056/NEJM199308263290907. [DOI] [PubMed] [Google Scholar]
- 46.Carlisle DM, Leake BD, Shapiro MF. Racial and ethnic disparities in the use of cardiovascular procedures: associations with types of health insurance. Am J Public Health. 1997;87:263–7. doi: 10.2105/ajph.87.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gornick ME, Eggers PW, Reilly TW, et al. Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med. 1996;335:791–9. doi: 10.1056/NEJM199609123351106. [DOI] [PubMed] [Google Scholar]
- 48.Satariano ER, Swanson GM, Moll PP. Nonclinical factors associated with surgery received for treatment of early stage breast cancer. Am J Public Health. 1992;82:195–8. doi: 10.2105/ajph.82.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diehr P, Yergan J, Chu J, et al. The influence of race on the use of surgical procedures for treatment of peripheral vascular disease of the lower extremities. Arch Surg. 1995;130:381–6. doi: 10.1001/archsurg.1995.01430040043006. [DOI] [PubMed] [Google Scholar]
- 50.Center for Disease Control. Fighting HIV/AIDS in African American communities: a growing crisis. October 4 2000 http://www.cdc.gov/hiv/pubs/brochure/African-American.pdf. [Google Scholar]
- 51.Fairfield KM, Libman H, Davis RB, Eisenberg DM, Phillips RS. Delays in protease inhibitor use in clinical practice. J Gen Intern Med. 1999;14:395–401. doi: 10.1046/j.1525-1497.1999.08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitahata MM, Koepseli TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. N Engl J Med. 1996;334:701–6. doi: 10.1056/NEJM199603143341106. [DOI] [PubMed] [Google Scholar]
- 53.Wenger N, Gifford A, Liu H, et al. Patient characteristics and attitudes associated with antiretroviral adherence. 6th Conference on Retroviruses and Opportunistic Infections, Chicago, January 1999 Abstract 98. [Google Scholar]
- 54.Centers for Disease Control. AIDS among people aged 50 or older years—U.S., 1991–1996. JAMA. 1998;279:576. [Google Scholar]
- 55.Anderson K, Mitchell JM. Expenditures on services for persons with acquired immunodeficiency syndrome under a medicaid home- and community-based waiver program: are selection effects important? Med Care. 1997;35:425–39. doi: 10.1097/00005650-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Department of Health and Senior Services. Division of AIDS Prevention and Control begins broad initiative to decrease perinatal transmission. AIDSline. 1999;11:1–2. [Google Scholar]
- 57.Hakki M, Cinti SK. Gaps in pharmacy logs predict poor virologic outcome in HIV-infected patients on highly active antiretroviral therapy. Presented at the annual meeting Michigan Infectious Disease Society, Lansing, Mich, March 2000. [Google Scholar]
- 58.Singh N, Berman SM, Swindells S, et al. Adherence of human immunodeficiency virus-infected patients to antiretroviral therapy. Clin Infect Dis. 1999;29:824–30. doi: 10.1086/520443. [DOI] [PubMed] [Google Scholar]