Abstract
OBJECTIVE
To examine patient and physician preferences in regard to 5 colorectal cancer screening alternatives endorsed by a 1997 expert panel, determine the impact of patient and physician values regarding certain test features on screening preference, and assess physicians' perceptions of patients' values.
DESIGN
Cross-sectional survey.
SETTING
A general internal medicine practice at an academic medical center in 1998.
PARTICIPANTS
Patients (N = 217; 76% response rate) and physicians (N = 39; 87% response rate) at the study setting.
MEASUREMENTS AND MAIN RESULTS
Patients preferred fecal occult blood testing (43%) or colonoscopy (40%). In patients for whom accuracy was the most important test feature, colonoscopy (62%) was the preferred screening method. Patients for whom invasive test features were more important preferred fecal occult blood testing (76%; P < .001). Patients and physicians were similar in their values regarding the various test features. However, there was a significant difference between physicians' perceptions of which test features were important to patients compared with the patients' actual responses (P < .001). The largest discrepancy was for accuracy (patient actual 54% vs physician opinion 15%) and discomfort (patient actual 15% vs physician opinion 64%).
CONCLUSIONS
Patients have distinct preferences for colorectal cancer screening tests that are associated with the importance placed on certain test features. Physicians incorrectly perceive those factors that are important to patients. Physicians should incorporate patient values in regard to certain test features when discussing colorectal cancer screening with their patients and when eliciting their screening preferences.
Keywords: colorectal cancer screening, patient preferences, patient attitudes, physician attitudes, screening guidelines
Colorectal cancer is a significant cause of cancer morbidity and mortality in the United States. In 2000, it is estimated that over 56,000 deaths from the disease and approximately 130,000 new cases were diagnosed.1 There have been numerous studies showing the benefit of early detection through screening in reducing mortality from colorectal cancer.2–8 In addition, screening reduces the incidence of this disease through the identification and removal of premalignant adenomatous polyps.9–12 A number of screening strategies have been proposed that appear to perform similarly in reducing mortality from colorectal cancer.13–15 Thus, current data suggest that there is not an optimal test for colorectal cancer screening.
In 1997, a multidisciplinary expert panel convened by the Agency for Health Care Policy and Research (now the Agency for Healthcare Research and Quality) published guidelines after evaluating the different screening strategies for colorectal cancer.15 Utilizing evidence-based decision and cost-effectiveness analyses, they concluded that the following 5 different screening strategies were all acceptable and had approximately equal cost-effectiveness for “average-risk” persons beginning at age 50 years: 1) fecal occult blood testing annually; 2) flexible sigmoidoscopy once every 5 years; 3) combination of fecal occult blood testing annually with flexible sigmoidoscopy every 5 years; 4) colonoscopy once every 10 years; or 5) double-contrast barium enema once every 5 to 10 years.15 Unfortunately, the panel could not determine the single best method for colorectal cancer screening. Instead, it recommended that patients participate with their physicians in selecting one of the above screening methods.
It has been suggested that involving patients in the medical decision-making process will lead to more compliant behavior with health care recommendations.16,17 This is particularly relevant for colorectal cancer screening. Despite the availability of effective screening methods for mortality reduction, utilization of colorectal cancer screening methods has been suboptimal.18,19 It is felt that the effective use of communication and educational materials will enhance patient acceptance and compliance with colorectal cancer screening.15 The discussion of patient values and preferences is a critical step in engaging patients to participate in medical decision making.16,20 Presently, 2 published studies have examined patient preferences for colorectal cancer screening methods.21,22 However, neither included each of the 5 acceptable screening methods recommended by the expert panel. Moreover, neither examined physicians' personal preferences or their perceptions of patient values. Physicians' attitudes are important because of their influential impact on the patient-physician encounter.16
This study examines whether patients have particular preferences regarding the 5 alternative colorectal cancer screening methods as recommended by the expert panel and assesses the impact of values regarding certain test features on these preferences. In addition, physicians' personal preferences and perceptions of patient values are explored.
METHODS
Study Design
The study was performed using a cross-sectional survey design over a 4-month period in 1998 at Boston Medical Center in Boston, Massachusetts. The study protocol was reviewed and approved by the medical center's institutional review board.
Patients were recruited from a university-based general internal medicine practice at Boston Medical Center. Eligible patients were between the ages of 40 and 75 years, under the care of a primary care faculty member, and able to understand English. Forty years of age was selected as the lower limit to include a population of patients (age 40–49 years) who most likely would not yet have undergone any colorectal cancer screening. Secondary analyses were planned to compare colorectal cancer screening test preferences in patients who had previously been screened with those who had never undergone colorectal cancer screening. Because we assumed that there would be a higher proportion of patients who had undergone in their lifetime at least 1 of the colorectal cancer screening tests under consideration (fecal occult blood testing, sigmoidoscopy, colonoscopy, or barium enema), we wanted to increase the sample size of patients who had never been screened. With current recommendations stating that colorectal cancer screening for average-risk individuals should begin at age 50 years, we concluded that the younger age group (age 40–49 years) would likely not have yet undergone colorectal cancer screening but were close enough in age that this topic would have some importance to them.
Recruitment was performed using a convenience sample of new and return patient visits to the study site. A research assistant identified eligible patients by age criteria after reviewing the appointment lists at the beginning of a clinic session. The research assistant would approach eligible patients consecutively as they were checking out after their physician visit. Patients were given an introduction to the study and then asked to participate. Written consent was obtained from those patients who agreed to participate. The research assistant then administered the survey instrument to the patient in a structured interviewer format by reading the information from the instrument. The patient followed along visually.
Physicians were recruited from the Section of General Internal Medicine at Boston Medical Center and included practicing primary care faculty members as well as general internal medicine fellows. All of the 45 primary care physicians from this group were considered eligible. A survey was distributed to them in their office mailboxes, and each physician was advised that participation in the study was voluntary and responses confidential. Surveys were self-administered and returned to one of the study's principal investigators (BSL).
Instruments
Patient Decision Aid
The project utilized a decision aid that was developed with the intent to educate patients about colorectal cancer screening (See Appendix, available at http://www.blackwellscience.com/jgi). After the educational portion, this instrument elicited patient preferences regarding the 5 alternative screening methods recommended by the expert panel and patient values regarding certain test features. The design and administration of the decision aid used the Informed Decision-making Model as a framework. This model identifies critical elements in the patient-physician interaction that result in patient participation in decision making.23
One of the key components of the Informed Decision-making Model is the explanation of the pros (benefits) and cons (risks) of the alternatives presented to the patient.23 Our decision aid addressed this issue by describing select test features of each of the screening methods (Table 1) Great care was taken to develop this portion of the decision aid in an unbiased manner because of its impact on patients' decision making. The selection and description of the particular test features in the decision aid involved multiple steps. Previous work in which colorectal cancer screening tests were discussed with patients was reviewed.22,24,25 Qualitative research methods in the form of a focus group and telephoned patient interviews determined which test features were important to patients. Twenty-six patients, participating in a pilot study, gave feedback on a preliminary version of the decision aid. In addition, input from health care professionals occurred through pre-testing of the decision aid with them as well as peer-reviewed feedback at research conferences.
Table 1.
Screening Test Features Described in the Instrument
| Frequency: How often the test is recommended to be performed |
| Discomfort: Potential unpleasant effects from the test |
| Complications: Potential adverse events from the test |
| Inconvenience: Things a patient needs to do in preparing for the test |
| Time: How long it takes to perform the test |
| Accuracy: How effective the test is in ultimately detecting a cancer or polyp if present |
| Further testing: If screening test is positive, what diagnostic procedure is needed |
The format of the decision aid included: 1) a verbal statement by the research assistant discussing the epidemiology of colorectal cancer, the effectiveness of screening, the lack of consensus as to the best screening method, the expert panel and its recommendations, and the decision aid's objective in eliciting patient preferences for the alternative screening methods; 2) a listing of the recommended alternative screening methods; 3) a written description of each of the screening methods along with a visual aid (e.g., photograph); 4) information on features of each of the screening methods (see Table 1); 5) a question after the discussion of each feature to assess the patient's level of understanding; and 6) questions asking patients to rank order their preferences regarding the screening methods and the importance of the various test features in determining the preference.
Physician Survey
As in the patient survey, physicians were asked to rank order their preferences in regard to the various colorectal cancer screening methods and the importance of the test features that influenced their preference. In addition, physicians rank ordered the test features they thought patients would find most important in choosing a screening method and selected the screening method they would recommend to their patients.
Statistical Analysis
Descriptive statistics were used to characterize the study population, the screening preferences, and the important test features used to formulate these preferences. X2and Fisher's exact tests were used to test for significant differences between physicians' responses compared to those given by patients. These tests of significance were also used to compare how physicians thought patients would respond in ranking the importance of certain test features in influencing screening preferences with how the patients actually responded. All statistical analyses were performed using SAS software, version 6.12 (SAS Institute, Cary, NC).
RESULTS
Of the 285 patients approached, 219 agreed to participate (76% response rate). Two patients were not able to complete the survey portion of the instrument that elicited preferences regarding the various colorectal cancer screening tests. This resulted in 217 patients for whom preferences were determined. An additional 18 patients did not complete other portions of the survey due to time constraints. Surveys were returned from 39 of the 45 eligible physicians (87% response rate). Table 2 describes the characteristics of the responders and nonresponders (for both patient and physician samples) in this study. There were no statistically significant differences between responders and nonresponders in the patient sample according to gender or age. A significantly greater proportion of nonwhites in the patient sample declined participation in the study. Tests of significance were not performed to detect differences between responders and nonresponders in the physician sample because of the low number of nonresponders (n = 6) in this group. The largest contribution to the total patient study population by any one physician was 18%. Of the 54 patients age 40 to 49 years, 20 (37%) had never undergone a colorectal cancer screening test.
Table 2.
Study Population Characteristics
| Patients | |||
|---|---|---|---|
| Responders (n = 217) | Nonresponders (n = 66) | P Value | |
| Female, % | 56 | 62 | >.05 |
| <60 y, % | 64 | 59 | >.05 |
| Nonwhite, % | 39 | 56 | .001 |
| >High school | |||
| Degree, % | 52 | NA | |
| Previous colorectal screening (ever), % | |||
| None | 18 | NA | |
| Fecal occult blood testing | 70 | NA | |
| Flexible sigmoidoscopy | 36 | NA | |
| Colonoscopy | 28 | NA | |
| Barium enema | 24 | NA | |
| Physicians | ||
|---|---|---|
| Responders (n = 39) | Nonresponders (n = 6) | |
| Female, % | 51 | 50 |
| <60 y, % | 97 | 100 |
| Nonwhite, % | 41 | 0 |
| Appointment, % | ||
| Faculty attending | 79 | 83 |
| GIM fellows | 21 | 17 |
NA, not available (data not collected). Test of significance were not performed for differences between responders and nonresponders in the physician group because of the low number of nonresponders.
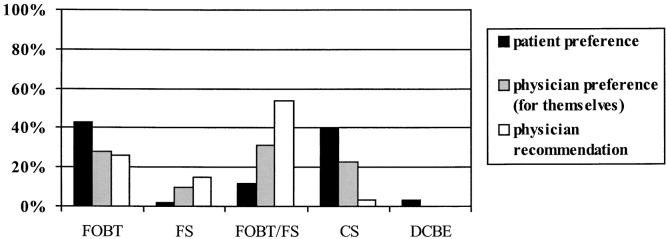
The preferred screening method for the study population is displayed in Figure 1. The majority of patients preferred fecal occult blood testing annually (43%) or colonoscopy once every 10 years (40%). There was much less interest in the combination method of fecal occult blood testing annually and flexible sigmoidoscopy once every 5 years (12%), double-contrast barium enema once every 5 to 10 years (3%), or flexible sigmoidoscopy once every 5 years (2%). When physicians were asked, “Which colorectal cancer screening method would you prefer for yourself?” they were divided among fecal occult blood testing (28%), combination method (31%), or colonoscopy (23%). This difference in preferences between patients and physicians is statistically significant (P = .001).
FIGURE 1.
Patient and physician screening method preferences and physician recommendations. FOBT, fecal occult blood test; FS, flexible sigmoidoscopy; FOBT/FS, combination method; CS, colonscopy; DCBE, double-contrast barium enema. Patients preferred FOBT or CS, whereas physicians preferred FOBT, FOBT/FS, or CS (P = .001). Physicians recommend FOBT/FS most often, which is statistically different from patient preferences (P < .001) and physicians' preferences for themselves (P < .001).
A majority of the physicians recommended the combination method (54%) as the colorectal cancer screening strategy of choice for their patients (Fig. 1). Fecal occult blood testing (23%) and flexible sigmoidoscopy (15%) were recommended by a smaller number of the physicians; colonoscopy (3%) and barium enema (0%) were rarely recommended. There were statistically significant differences between the screening recommendations made by physicians to their patients and the patients' preferences (P < .001) and the physicians' own preferences (P < .001).
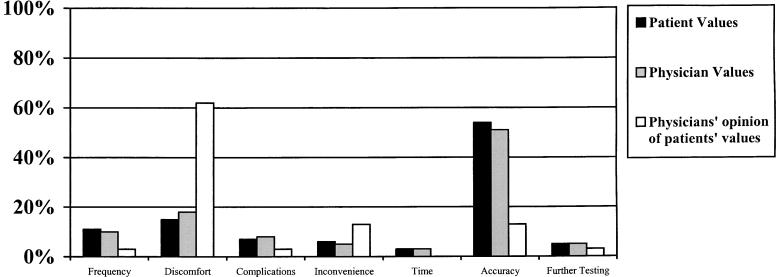
Figure 2 illustrates the test feature most valued when selecting a screening method. A high percentage of patients (54%) and physicians (51%) considered accuracy the most important test feature. Nearly equal percentages of patients and physicians responded that the most important test feature was discomfort (patients 15%, physicians 18%), frequency (patients 11%, physicians 10%), complications (patients 7%, physicians 8%), inconvenience (patients 6%, physicians 5%), further work-up (patients 5%, physicians 5%), or time to do the test (patients 3%, physicians 3 %). The differences between patients and physicians were not statistically significant. Figure 2 also illustrates the differences in the percentage of physicians perceiving what the most important test feature for patients would be compared with the percentage of patients who actually valued that particular test feature the most (P < .001). The largest discrepancies were in the percentage that considered the most important test feature to be accuracy (patient actual 54% vs physician opinion 15%) and the percentage that considered discomfort to be the most important (patient actual 15% vs physician opinion 64%).
FIGURE 2.
Most important test feature in selecting a screening method. Percentages reflect the number of respondents who answered that the particular test feature was most important in selecting a colorectal cancer screening test. Patients and physicians are similar in valuing certain test features. Physicians' opinion of what patients value is statistically different from what patients say is important to them (P < .001).
The influence of patient demographics on patient preferences and most valued test feature was also assessed. There were statistically significant differences (P = .01) between genders in the selection of a screening method, with the largest differences seen in the preference for the combination method (males 17%, females 7%). No significant differences were seen when assessing preferences across age, race, or educational level. For the most important test features considered, there was a statistically significant difference (P = .047) across education level. The largest difference in percentages was found for those who valued accuracy the most (greater than a high school degree 60%, high school degree or less 48%). No differences were seen across gender, age, or race according to the most important test feature identified.
There were 184 patients (82%) who had undergone at least 1 of the colorectal cancer screening tests during their lifetime. In this group, the screening methods most preferred were fecal occult blood testing (42%) and colonoscopy (41%). There was less interest for the combination method (12%), double-contrast barium enema (3%), and flexible sigmoidoscopy (2%). There were no statistically significant differences with regard to screening method preference when comparing this group of patients with those who had never undergone colorectal cancer screening. In the group of patients never previously screened (n = 33), the most preferred screening methods were fecal occult blood testing (48%) and colonoscopy (36%). Because of the relatively small number of patients who had no previous testing, there may not have been adequate power to detect significant differences. In addition, analyses did not reveal statistically significant differences in screening method preference and most valued test feature between those patients who had ever had a lower gastrointestinal (LGI) endoscopic procedure (flexible sigmoidoscopy or colonoscopy, n = 111) versus those who had not (n = 88). There were 18 participants who did not respond to the question of previous experience with colorectal tests because of time constraints. For those who had LGI endoscopy in the past, fecal occult blood testing was preferred by 41% and colonoscopy by 40%. Among those who had never had LGI endoscopy, 44% preferred fecal occult blood testing and 41% preferred colonoscopy. Accuracy was the most valued test feature in the greatest number of patients for both groups (previous LGI endoscopy 59%, no LGI endoscopy 50%) followed by discomfort (previous LGI endoscopy 9%, no LGI endoscopy 18%). Similar nonsignificant findings were seen when comparing those patients who had previous invasive testing (LGI endoscopy or barium enema, n = 123) versus those who had not (n = 76).
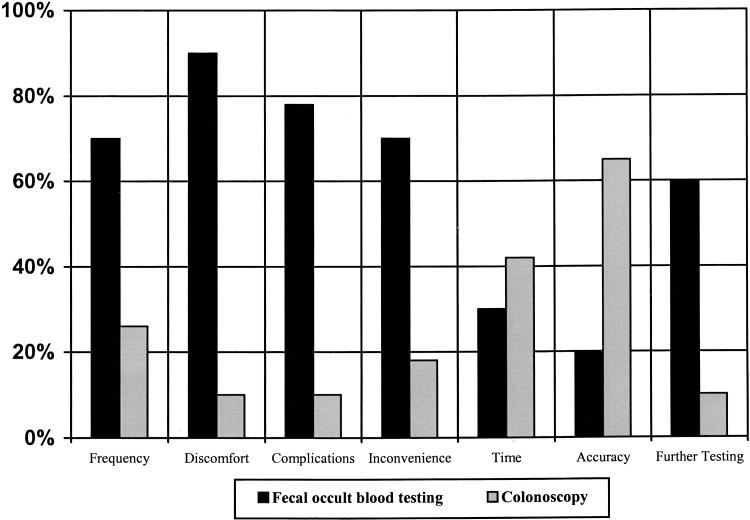
The preferred screening method chosen by patients depended in part upon the test feature most valued (Fig. 3). Patients who viewed frequency, discomfort, complications, inconvenience, or further work-up as the most important test feature selected fecal occult blood testing more often. For those who valued accuracy the most, colonoscopy was the preferred screening method.
FIGURE 3.
Patients' most valued test feature and the screening method selected. Each column is a reflection of only those patients selecting a particular test feature as most important. For example, of the patients who value “discomfort” the most, 90% preferred fecal occult blood testing and 10% preferred colonoscopy. Not all columns add to 100% because only preferences for fecal occult blood testing and colonoscopy were included in this figure. The other screening methods were excluded from the data presentation because they were preferred in a minority of instances and also to simplify the presentation of the data. The preferred screening method chosen (fecal occult blood testing or colonoscopy) depends upon the test feature most valued by patients (P < .001).
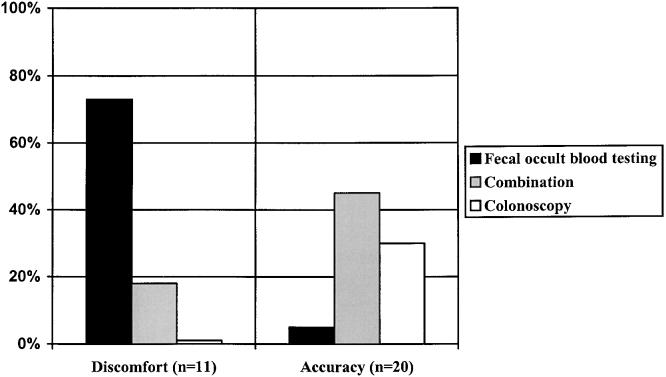
The test features most valued by physicians also were associated with their personal screening method preference (Fig. 4). Those who valued accuracy the most preferred combination method (45%) or colonoscopy (30%), whereas physicians who valued discomfort the most preferred fecal occult blood testing (73%). The number of physicians who valued frequency, complications, inconvenience, time, or further work-up was too small for meaningful analysis.
FIGURE 4.
Physicians' most important test feature in selecting a screening method. The preferred screening method chosen (fecal occult blood testing, combination method, or colonoscopy) depends upon the test feature most valued by physicians (P < .001). For example, the majority of physicians who valued discomfort selected fecal occult blood testing whereas those who valued accuracy selected the combination method or colonoscopy.
Finally, patients were asked, “Which of the screening tests would you have done if recommended by your doctor?” Some of the respondents (n = 18) were not able to complete this question because of time constraints. Overall, if recommended by their physician, 94% would do fecal occult blood testing, 74% would have a flexible sigmoidoscopy, 74% would undergo the combination of fecal occult blood testing and flexible sigmoidoscopy, 81% would have a colonoscopy, and 71% would complete a double-contrast barium enema. For those preferring fecal occult blood testing who responded to the above question (n = 84 with 9 nonrespondents), 96% responded that they would do the fecal occult blood testing if recommended by their physician. Likewise, 93% of patients who preferred colonoscopy who responded to the above question (n = 79 with 6 nonrespondents) stated they would have a colonoscopy done if recommended. All of the patients who preferred flexible sigmoidoscopy (n = 5) or the combination method (n = 23) said they would complete the respective testing if their physician recommended it. In the group of patients who preferred double-contrast barium enema (n = 6), 50% would undergo this test if recommended. Interestingly, 87% of those who preferred colonoscopy would undergo fecal occult blood testing if recommended by their physician, whereas only 68% of those who preferred fecal occult blood testing would undergo colonoscopy (P = .004).
DISCUSSION
This study has shown that patients have distinct preferences regarding a colorectal cancer screening test when offered the 5 acceptable methods described by the expert panel.15 We found that the majority of patients preferred either fecal occult blood testing (43%) or colonoscopy (40%). Less preferred were the combination method of fecal occult blood testing and flexible sigmoidoscopy (12%), barium enema (3%), and flexible sigmoidoscopy (2%).
In other studies in which patient preferences regarding colorectal cancer screening tests were determined, patients also exhibited preferences for certain screening methods. Leard et al. determined preferences from patients at 3 internal medicine clinics where fecal occult blood testing, flexible sigmoidoscopy, colonoscopy, and barium enema were discussed,22 but not the combination method of fecal occult blood testing and flexible sigmoidoscopy. Participants preferred fecal occult blood testing (31%) and colonoscopy (38%) with much less interest in flexible sigmoidoscopy (13%) and barium enema (14%). Pignone et al. also elicited patient preferences in which participants were asked to select either fecal occult blood testing, flexible sigmoidoscopy, or the combination method.21 Neither colonoscopy nor barium enema were discussed in that study. They found that the majority of patients preferred fecal occult blood testing (37%) or the combination method (47%).
The present study, like those of Leard and Pignone, suggests that patient preferences are oriented toward either the most accurate screening method offered (colonoscopy or combination method) or the least invasive modality (fecal occult blood testing). An explanation for this phenomenon may be that patient preferences are influenced by values regarding particular test features. This study shows an association between the test feature determined most important by the patient and the screening method they select. Patients who are negatively influenced by invasive test features (discomfort, inconvenience, complications, and time exposed to the negative effects of the test) are more likely to select fecal occult blood testing, whereas patients who value accuracy the most are more inclined to select colonoscopy.
An important finding in this study is that physicians incorrectly perceive those factors that are valued by patients in forming their preferences. While physicians and patients had similar values in regard to the various screening test features, physicians overestimated the impact of patient discomfort on screening preferences. A minority of physicians (18%) and patients (15%) in this study felt that discomfort was the most important feature influencing their selection of a colorectal cancer screening test for themselves, whereas 64% of physicians thought that discomfort would be the most important consideration for patients in forming a preference for a specific screening test. Previous studies have shown that the perception of patient discomfort negatively influences a physician's decision to recommend certain tests such as screening sigmoidoscopy.26,27 Thus, these incorrect perceptions may cause physicians to recommend screening tests that are different from those preferred by their patients. Because of this, a suggested clinical approach would be to have health care providers highlight pertinent test features and determine patient values regarding these test features when discussing colorectal cancer screening with their patients. By identifying a particular patient's values in regard to these test features, health care providers can better assist the patient in selecting a screening method for colorectal cancer. This insight is important when engaging patients to participate in medical decision making,20,28,29 as the expert panel has recommended in regard to selecting a colorectal cancer screening method.15
Another significant finding is the high level of patient interest for colonoscopy as the preferred colorectal cancer screening modality. The results of this study suggest that a minority of physicans recommend screening colonoscopy to their patients. This may be due to the high out-of-pocket costs to patients. Generally, this procedure has not been covered by third-party payors when performed in average-risk individuals for screening purposes. As of July 1, 2001, Medicare has begun to pay for a screening colonoscopy once every 10 years in average-risk individuals.30 In time, it may appear appropriate for other third-party payors to consider reimbursement for screening colonoscopy. Previous studies, such as the recent cost-effectiveness analysis by Sonnenberg et al., have shown this procedure to be a cost-effective screening modality.31 Frazier et al., however, found that screening colonoscopy every 10 years was dominated (less effective and more costly) by other alternatives such as fecal occult blood testing (rehydrated) or fecal occult blood testing (unrehydrated) plus sigmoidoscopy every 5 years.32 This study did find that a one-time screen with colonoscopy at age 55 years can achieve a 30% to 50% reduction in colorectal cancer mortality. Thus it is arguable whether screening colonoscopy is cost-effective. Given the high level of patient preference, the clinical benefit, and the potential cost-effectiveness of screening colonoscopy, this procedure may become a widely accepted and utilized screening method in the future by many physicians and patients.
An interesting and important issue that was not addressed in this study is whether physician ordering of a colorectal cancer screening test according to an individual patient's preference would impact the patient's compliance in completing the test. While it appeared that the majority of the study population had intentions to complete their preferred colorectal cancer screening test if recommended by their physician, we did not prospectively follow these patients to assess which colorectal cancer screening tests were ordered nor did we evaluate patient completion of colorectal screening after the decision aid had been administered. Pignone et al. showed that the use of a decision aid (i.e., video-based) improved the rate of colorectal cancer screening completion.33 However, it was not reported in this study whether ordering a test according to the patient's preference improves the screening rate. Further work assessing the impact of informed decision making and patient preferences with regard to colorectal cancer screening compliance may prove to be clinically useful.
Our study has several important potential limitations. First, patients are probably influenced by the information presented to them. Attempts were made to comprehensively and accurately discuss in a nonbiased manner those test features that were potentially important to patients. We sought both patient and health care professional input into selecting the test features that are presented in the instrument. In addition, a review of the literature provided specific information that was used in the descriptions of the test features.
Another potential limitation was that costs of the various screening methods were excluded from the survey instruments. We acknowledge that preferences would probably be influenced by out-of-pocket costs to the patient. However, the diversity of health insurance coverage in this study population, with varying degrees of coverage for each of the colorectal cancer screening tests, made costs a complex construct to measure. Therefore, it was decided that costs would be excluded from the instrument for this study. Instead, the focus of the study was to assess preferences assuming full coverage for the screening tests. The research assistants did not specifically instruct all of the patients to select their preferences without regard to costs. Instead, only when the patients asked were they advised by the research assistants to disregard costs. Future work should explore issues regarding costs and patients' willingness to pay for specific colorectal cancer screening tests.
Related to this is the impact of costs on physician recommendation for certain colorectal cancer screening tests. In the physician survey, the effect of potential out-of-pocket costs to the patient as well as the levels of physician reimbursement associated with the various screening tests was not evaluated. It is acknowledged that this may have an impact on the specific types of colorectal cancer screening tests that physicians recommend to their patients. For instance, physicians may not be ordering screening colonoscopies because of the high out-of-pocket patient costs associated with this test. This may account for some of the difference between the relatively high level of patient preference and low level of physician recommendation for colonoscopy seen in this study. As third-party payers, such as Medicare, begin coverage for screening colonoscopy, it will be worth noting physician practice patterns with regard to colorectal cancer screening test ordering and assessing patient completion with modalities such as colonoscopy.
A third limitation is the generalizability of the results from this study. A single, university-based, general internal medicine practice was used to conduct the study. Further studies assessing patient and physician attitudes toward colorectal cancer screening tests should be performed to assess whether similar results can be replicated in other clinical settings.
Moreover, there was a racial difference between responders and nonresponders in the patient sample. It is not known whether the subsample of nonwhite nonresponders would have altered the results significantly. Of note, the majority of nonwhite nonresponders were African American (92%). While beyond the scope of this study, the difficulty of recruiting African-American participants into research studies is an important issue that has been acknowledged in the literature and needs to be addressed. Barriers such as lack of knowledge about research, lack of understanding and trust of informed consent procedures, and distrust of researchers have been identified.34 Further work addressing these barriers may lead to enhanced participation of African Americans in research studies.
Finally, the patient population was obtained using a convenience sample of persons who had come in for a physician appointment and therefore may not be representative of the population as a whole. In particular, persons who do not regularly see their physicians may have attitudes different from those exhibited by this study's patient population. Administration of the survey in a population-based study in the future would address this issue.
In conclusion, this study has shown that individual patients place different values on the various test features of colorectal cancer screening tests. The test feature considered most important by the patient is associated with patient preferences for certain colorectal cancer screening tests. In general, physicians misperceive the test features most valued by patients that influence screening method preference. Furthermore, patient demographic factors are not useful in predicting screening method preference. Because predicting an individual patient's preference is difficult, physicians should discuss with their patients, on a case-to-case basis, the available colorectal cancer screening method options, including the various features associated with each test. Patient values regarding these test features should then be elicited and patient preferences for a screening test determined. Through this process, the patient and physician can make a more informed decision with regard to colorectal cancer screening.
Acknowledgments
The authors would like to gratefully acknowledge the contributions and advice of the late Dr. Mark Moskowitz to this project.
The authors would like to thank Jeanne Speckman, MSc, for her assistance in data management and statistical support of the project.
BSL's efforts on this study were supported by the Physician Training Award in Preventive Medicine, American Cancer Society, Award 97-3.
REFERENCES
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. Ca Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. (published erratum appears in N Engl J Med 1993;329:672) N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 3.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondegaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 5.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-ocult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 6.Selby JV, Friedman GD, Quesenberry CP, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 7.Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst. 1992;84:1572–5. doi: 10.1093/jnci/84.20.1572. [DOI] [PubMed] [Google Scholar]
- 8.Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst. 1993;85:1311–8. doi: 10.1093/jnci/85.16.1311. [DOI] [PubMed] [Google Scholar]
- 9.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 10.Atkin WS, Cuzick J, Northover JM, Whynes DK. Prevention of colorectal cancer by once-only sigmoidoscopy. Lancet. 1993;341:736–40. doi: 10.1016/0140-6736(93)90499-7. [DOI] [PubMed] [Google Scholar]
- 11.Muller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. Ann Intern Med. 1995;123:904–10. doi: 10.7326/0003-4819-123-12-199512150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd Ed. Alexandria, Va: International Medical Publishing; 1996. [Google Scholar]
- 14.Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers and update 2001: testing for early lung cancer detection. Ca Cancer J Clin. 2001;51:38–75. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 16.Brody DS. The patient's role in clinical decision-making. Ann Intern Med. 1980;93:718–22. doi: 10.7326/0003-4819-93-5-718. [DOI] [PubMed] [Google Scholar]
- 17.Eraker SA, Kirscht JP, Becker MH. Understanding and improving patient compliance. Ann Intern Med. 1984;100:258–68. doi: 10.7326/0003-4819-100-2-258. [DOI] [PubMed] [Google Scholar]
- 18.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–22. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Trends in screening for colorectal cancer—United States, 1997 and 1999. Morb Mortal Wkly Rep. 2001;50:162–6. [PubMed] [Google Scholar]
- 20.Quill TE, Brody H. Physician recommendations and patient autonomy: finding a balance between physician power and patient choice. Ann Intern Med. 1996;125:763–9. doi: 10.7326/0003-4819-125-9-199611010-00010. [DOI] [PubMed] [Google Scholar]
- 21.Pignone M, Bucholtz D, Harris R. Patient preferences for colon cancer screening. J Gen Intern Med. 1999;14:432–7. doi: 10.1046/j.1525-1497.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leard LE, Savides TJ, Ganiats TG. Patient preferences for colorectal cancer screening. J Fam Pract. 1997;45:211–8. [PubMed] [Google Scholar]
- 23.Braddock CH, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions: informed decision making in the outpatient setting. J Gen Intern Med. 1997;12:339–45. doi: 10.1046/j.1525-1497.1997.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pignone MP, Bucholtz D, Harris R. Patient interest and preferences for colon cancer screening. J Gen Intern Med. 1998;13(Suppl 1):96. doi: 10.1046/j.1525-1497.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolan JG. Are patients capable of using the analytic hierarchy process and willing to use it to help make clinical decisions? Med Decis Making. 1995;15:76–80. doi: 10.1177/0272989X9501500111. [DOI] [PubMed] [Google Scholar]
- 26.Schoen RE, Weissfeld JL, Kuller LH. Sigmoidoscopy use among primary care physicians. Prev Med. 1995;24:249–54. doi: 10.1006/pmed.1995.1041. [DOI] [PubMed] [Google Scholar]
- 27.American Cancer Society. 1989 survey of physicians' attitudes and practices in early cancer detection. Ca Cancer J Clin. 1990;40:77–101. doi: 10.3322/canjclin.40.2.77. [DOI] [PubMed] [Google Scholar]
- 28.Eraker SA, Polister P. How decisions are reached: physician and patient. Ann Intern Med. 1982;97:262–8. doi: 10.7326/0003-4819-97-2-262. [DOI] [PubMed] [Google Scholar]
- 29.Redelmeier DA, Rozin P, Kahneman D. Understanding patients' decisions: cognitive and emotional perspectives. JAMA. 1993;270:72–6. [PubMed] [Google Scholar]
- 30. http.http://www.medicare.gov/publications/pubs/pdf/prevent.pdf.
- 31.Sonnenberg A, Delco F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133:573–84. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 32.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–61. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 33.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening—a randomized, controlled trial. Ann Intern Med. 2000;133:761–9. doi: 10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 34.Freimuth VS, Quinn SC, Thomas SB, Cole G, Zook E, Duncan T. African Americans' views on research and the Tuskegee Syphilis Study. Soc Sci Med. 2001;52:797–808. doi: 10.1016/s0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]