Abstract
The V3 loop and the bridging sheet domain of human immunodeficiency virus type 1 (HIV-1) subtype B envelope glycoprotein gp120 have been implicated in CCR5 coreceptor utilization. In this study, mutant envelope glycoproteins of a subtype C isolate containing substitutions in the V3 or C4 region were generated to determine which are required for efficient CCR5-dependent cell fusion and viral entry. We found that the V3 crown and C4 residues are relatively dispensable for cell-cell fusion, although some residues may be involved in the regulation of early postentry steps in viral replication. In contrast, seven highly conserved residues located in the V3 stem are critical for CCR5 utilization, which can explain the apparent paradox that the functional convergence in CCR5 usage by genetically divergent HIV-1 strains involves a variable region. The finding that C4 residues do not have a critical role may appear to contradict the current model that bridging sheet residues are involved in the gp120-CCR5 interaction. However, a plausible interpretation is that these C4 residues may have a distinct role in the binding and fusion steps of the gp120-CCR5 interaction.
Human immunodeficiency virus type 1 (HIV-1) enters target cells through fusion of the virus envelope with the target cell membrane, which involves sequential interactions between the external envelope glycoprotein gp120, the primary receptor CD4, and a seven-transmembrane chemokine coreceptor (24, 30). The β-chemokine receptor CCR5 can act as a coreceptor for most primary non-syncytium-inducing (NSI) viruses (1, 5) that are transmitted between individuals and that predominate early in the course of natural infection (14). Syncytium-inducing viruses later emerge in infected patients that can utilize the α-chemokine receptor CXCR4 (22).
On the basis of studies of HIV-1 subtype B, efficient CCR5 binding is dependent on the presence of the V3 loop of gp120 (30) and the V3 loop sequence influences the specific chemokine receptors used by different HIV-1 strains (29). More recently, two functionally distinct regions of the V3 loop, designated the stem and the crown, were shown to be required for soluble subtype B gp120 binding to CCR5, whereas the V3 crown alone determines the coreceptor specificity of the virus (3).
Conserved gp120 structures have also been implicated in CCR5 binding. It is believed that the base of the V3 loop is in close proximity to the bridging sheet region, which is a four-stranded, antiparallel β sheet that includes the V1/V2 stem and two strands derived from the C4 region (11). This region is thought to undergo conformational changes as a result of CD4 binding, exposing CD4-induced epitopes that facilitate the interaction between some highly conserved gp120 residues and the chemokine coreceptor (11, 20). Five residues within the C4 region (I420, K421, Q422, P438, and G441) were reported to be the most critical for CCR5 binding to a subtype B gp120 protein (19). The highly conserved nature of these residues is thought to provide a molecular basis for the observed functional convergence in CCR5 utilization by genetically divergent HIV-1 strains.
The current model of HIV-1 coreceptor usage is one in which the V3 loop of gp120 dictates coreceptor choice and the conserved bridging sheet residues contribute to binding contacts with CCR5. However, previous studies were done with HIV-1 subtype B, and therefore it is not known whether the V3 and bridging sheet residues of other HIV-1 subtypes determine such viral characteristics. Given the high prevalence of HIV-1 subtype C in the global epidemic and its predominance in the most heavily affected region of southern Africa, we chose to determine whether corresponding regions in the gp120 protein from an HIV-1 subtype C isolate are also important for CCR5 utilization.
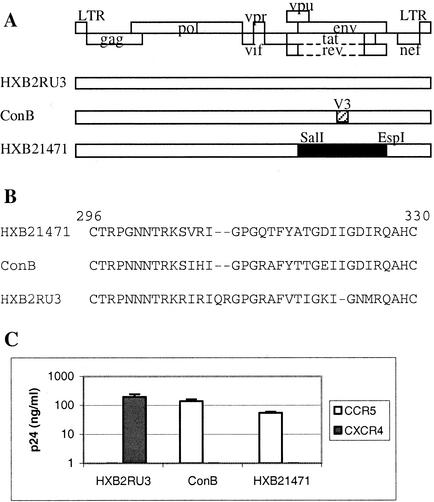
CCR5 utilization by a chimeric HIV-1 clone containing the subtype C envelope.
In order to examine the role of the V3 loop and of conserved C4 residues of HIV-1 subtype C gp120 in CCR5 utilization, we constructed an HIV-1 infectious molecular clone containing a subtype C envelope gene derived from the CCR5-using (R5) primary isolate from Botswana, 00BW1471 (GenBank accession no. AF443091). Amino acid alignment of the V3 and C4 regions showed that the isolate 00BW1471 and consensus subtype C sequences are identical, with the exception of two residues in the V3 loop (at position 300, a Gly for an Asn, and at position 307, a Val for an Ile; Fig. 1B) (26). The env gene of isolate 00BW1471 was used to replace that of a well-characterized CXCR4-using (X4) HIV-1 subtype B molecular clone, HXB2RU3 (Fig. 1A). The ability of this chimeric construct, designated HXB21471, to use CCR5 as an entry coreceptor was subsequently demonstrated in infection assays with human glioma cell lines U87-CD4-CCR5 and U87-CD4-CXCR4, which stably express CD4/CCR5 and CD4/CXCR4, respectively. Briefly, cell-free supernatants were collected 72 h after transfection of 293 cells with proviral DNA by using the Superfect transfection reagent (Qiagen, Hilden, Germany). Equivalent amounts of viruses, as standardized by p24, were used to infect the target cells, and the p24 levels in the infected culture supernatants were determined 7 days postinfection by enzyme-linked immunosorbent assay (ELISA; NEN Life Science Products, Boston, Mass.). As shown in Fig. 1C, HXB21471 replicated in the CCR5-positive cells but not in the CXCR4-positive cells. ConB, a previously described subtype B molecular clone that uses CCR5 as its entry coreceptor (25), was included as a positive control. The sequence of ConB differs from that of HXB2RU3 only in the V3 loop, which represents the consensus sequence of NSI subtype B viruses (Fig. 1B). As expected, ConB replicated to a high level in CCR5-positive cells but not in CXCR4-positive cells (Fig. 1C). Conversely, HXB2RU3 replicated only in cells expressing CXCR4. This experiment demonstrated that the HIV-1 subtype C envelope protein contains important determinants of coreceptor utilization and confirmed previous observations that the env gene of an R5 virus is sufficient to confer on an otherwise X4 virus the ability to use CCR5 as a coreceptor (29). The HXB21471 chimeric virus was subsequently used as the parental clone to generate mutant subtype C envelope glycoproteins.
FIG. 1.
(A) Schematic drawing of the proviral clones used in this study. The open rectangle represents sequences from subtype B molecular clone HXB2RU3. The hatched rectangle in ConB represents the consensus V3 sequence of HIV-1 subtype B NSI viruses. The filled rectangle in HXB21471 represents the env sequence (positions 5789 to 8904) derived from subtype C primary isolate 00BW1471. LTR, long terminal repeat. (B) V3 sequences of the proviral clones from positions 296 to 330 on the basis of the numbering of 00BW1471. (C) Coreceptor utilization by the proviral clones as measured by p24 levels in virus-infected cultures at day 7. The results represent the means and standard deviations of data from three independent experiments.
Construction of HXB21471 envelope mutants.
To determine the extent of involvement of the V3 loop and of conserved C4 residues in CCR5 utilization, alanine-scanning mutagenesis was conducted by overlap extension PCR (10) and all mutations were verified by DNA sequencing. Among the 33 V3 residues of chimeric clone HXB21471, 31 amino acids, from Thr-297 to His-329, were individually replaced with an alanine residue. Alanine was chosen as a substituent because its small nonpolar methylene side chain is less likely to impose severe constraints on gp120 and contributes little to protein-protein interactions. The two naturally occurring alanines at positions 317 and 328 were changed instead into a glycine. Among the C4 residues, alanine substitutions were made at positions 420, 421, 422, 438, and 441.
Effects of mutations on cell-cell fusion.
The ability of HIV-1 gp120 to mediate cell-cell fusion is commonly used to characterize the efficiency of coreceptor utilization by an HIV-1 strain. To investigate the relative contribution of the introduced amino acid substitutions in HIV-1 subtype C gp120 on CCR5 usage, we quantitatively assessed the ability of the mutant envelope proteins to induce fusion with U87-CD4-CCR5 cells. Fusion was analyzed by a previously described reporter gene activation assay (17), with a few modifications. Our system employed 293 effector cells that express the HIV-1 gp120 protein and contain a vaccinia virus-encoded T7-driven lacZ gene and target cells that stably express CD4 and CCR5 and vaccinia virus-encoded T7 RNA polymerase. Envelope-mediated cell-cell fusion results in cytoplasmic mixing and was scored by the level of β-galactosidase (β-Gal) selectively produced in fused cells after 6 h (17). Cell fusion was calculated with the formula (mutant OD405 ÷ WT OD405) × 100%, where OD405 is optical density at 405 nm and WT is wild type. The ability of cells expressing the HXB21471 envelope to selectively fuse with target cells expressing CCR5 was determined by comparing levels of β-Gal by using the cell lines U87-CD4-CCR5 and U87-CD4-CXCR4. As expected, significant β-Gal activity was detected in cell lysates only when CCR5-positive target cells were used (data not shown). In contrast, only background levels of β-Gal were detected when CCR5-positive cells were mixed with cells expressing the HXB2RU3 envelope (data not shown), which was used as a negative control since this clone utilizes CXCR4 and not CCR5.
The β-Gal activity produced by the subtype C mutant clones was compared with that of HXB21471 (WT) to ascertain the effect of the introduced mutations on CCR5 utilization. As shown in Fig. 2A, alanine substitution in only one (I420A) out of the five highly conserved C4 residues (19) resulted in a significant reduction (more than 50%) in cell-cell fusion, on the basis of β-Gal production. β-Gal activity by the other C4 mutants was 67 to 94% of the WT level. In the V3 loop, the R298A and I322A substitutions severely disrupted envelope-mediated cell fusion, as judged by a >90% decrease in β-Gal activity compared to that of the WT. Five other substitutions (N301A, N302A, T303A, I325A, and R326A) were also found to significantly reduce cell-cell fusion by more than 50%. All mutants that failed to induce CCR5-dependent cell-cell fusion were also tested in similar fusion assays with U87-CD4-CXCR4 target cells, and none gained the ability to use CXCR4 as a coreceptor (data not shown).
FIG. 2.
(A) Effects of V3 and C4 mutations on CCR5-dependent cell-cell fusion as measured by a reporter gene activation assay. β-Gal activity is expressed as a percentage of that seen with the WT clone, HXB21471. Hatched bars indicate values of <50%. (B) Effects of V3 and C4 mutations on proviral DNA copy numbers, as measured by PCR. Values are normalized to the amount of β-globin DNA in each sample. The values shown are percentages of the WT value. Hatched bars indicate mutants that exhibited ≤10% proviral DNA. Grey bars indicate mutants that retained >75% of the WT fusogenic potential but had a 10-fold reduction in proviral DNA. (C) Effects of V3 and C4 mutations on gp120-sCD4 binding, as measured by ELISA. The hatched bar indicates a value of <50% of the WT value. The data shown represent the means and standard deviations of data from three independent experiments.
Effects of mutations on proviral DNA integration.
To confirm the effects of the introduced mutations in HIV-1 subtype C gp120 on CCR5 usage, we tested the abilities of the mutant viruses to enter cells stably expressing CD4 and CCR5. Viral entry was analyzed by detecting proviral DNA integration. Briefly, U87-CD4-CCR5 cells were infected with equivalent amounts of viruses, as standardized by p24, and genomic DNA was extracted from the cells 24 h postinfection with the Qiagen Genomic DNeasy Kit. PCR amplification of HIV-1 gag from the genomic DNA was performed by using a LightCycler (Roche, Mannheim, Germany) with the primers 5′AGTGGGGGGACATCAAGCAGCCATGCAAAT-3′ and 5′-TACTAGTAGTTCCTGCTATGTCACTTCC3′. A second set of primers that amplify a region of the human β-globin gene was used to normalize for cell numbers (21). As expected, only the U87-CD4-CCR5 cells, and not the related cell line expressing CXCR4, supported entry of the WT HXB21471 virus (data not shown). As shown in Fig. 2B, mutations in gp120 that disrupted cell-cell fusion by more than 50% was found to block CCR5-dependent viral entry. Mutants R298, N302A, T303A, I322A, I325A, R326A, and I420A showed a dramatic 90% reduction in proviral DNA copy number, and N301A decreased proviral DNA by 75%. The significant reduction in proviral DNA levels confirmed that CCR5 utilization by these mutant envelope clones was markedly less efficient than that by the WT clone. However, we also identified two mutants, I309A and G441A, that effectively mediated cell-cell fusion by more than 75% of the WT level but the relative proviral DNA copy number was reduced 10-fold (Fig. 2B). The discrepancy between levels of cell-cell fusion and levels of proviral DNA suggested that these mutant viruses may use CCR5 to enter cells but replication is blocked at a step prior to complete proviral DNA integration.
Effects of mutations on CD4 binding.
Since binding of HIV-1 gp120 to CD4 is often a prerequisite for CCR5 binding, we wanted to determine if the observed effects on CCR5 utilization by the introduced mutations are indirectly caused by reduced CD4 binding. The ability of gp120 to bind CD4 was measured by ELISA as previously described (15), with a few modifications. Cell lysates were harvested 72 h after transfection of 293 cells with proviral DNA. A saturating amount of gp120 in cell lysates was incubated in wells coated with 2 μg of D7324 per ml, a sheep antibody (Ab) to the conserved C-terminal 15 amino acids of HIV-1 gp120 (National Institutes of Health AIDS Research and Reference Reagent Program). Equal amounts of soluble CD4 (sCD4) were then added to the wells, and bound sCD4 was detected by using T4-4, a rabbit polyclonal Ab against CD4 (National Institutes of Health AIDS Research and Reference Reagent Program). The concentration of sCD4 used displayed about half-maximal binding to captured gp120. Bound T4-4 Ab was detected by using alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin and the AMPAK system (Dako, Cambridgeshire, United Kingdom). sCD4 binding was calculated with the formula (mutant OD490 ÷ WT OD490) × 100%. All except one mutant retained the ability to bind sCD4 efficiently (75 to 135% of the WT level; Fig. 2C). The only exception is the mutation I420A, which reduced the sCD4 binding capacity by more than twofold (41% of the WT level). A similar mutation in a subtype B gp120 protein was also shown to decrease sCD4 binding by about 50% (19). The effect on sCD4 binding of the I420A mutation could thus account for the observed reduction in CCR5 utilization by this clone. However, these results indicated that the V3 mutations that disrupted CCR5 utilization are not due to reduced CD4 binding. This is in agreement with previous reports that V3 residues are not involved in gp120-CD4 interactions (31).
Effects of mutations on expression, processing, and virion incorporation.
To verify that the alanine substitutions did not cause drastic changes in expression, processing, or virion incorporation, Western blot analyses of cell and viral lysates were performed as previously described (28), by using reference subtype C pooled HIV-1-positive serum as the detection Ab. Figure 3 shows the Western blots of selected V3 mutants, namely, those that had a significant effect on either cell-cell fusion or viral entry, and of all of the C4 mutants. In the cell lysates (Fig. 3A and C), all mutant envelope proteins were expressed efficiently and the ratio of gp160 to gp120 was also similar to the WT ratio, suggesting no defect in processing. Furthermore, the only difference in the electrophoretic mobility of the envelope proteins was seen for mutations at positions 301 and 303, which destroy the known N-linked glycosylation site (NXT). As shown in Fig. 3B and D, similar amounts of gp120 were also detected in viral lysates. The ratio of gp120 to p24 in the virions was analyzed by densitometry, and there was no evidence that poor incorporation of gp120 accounted for the reduction in CCR5 utilization.
FIG. 3.
Western blot analysis of envelope proteins in cellular (A and C) and viral (B and D) lysates. Pooled sera from individuals infected with HIV-1 subtype C were used to detect the expression of envelope proteins. Panels A and B are Western blots of V3 mutations that significantly affected CCR5-dependent cell fusion or viral entry. Panels C and D are Western blots of all of the C4 mutants.
Previous studies have demonstrated that the gp120 V3 loop is a major determinant of coreceptor usage. In the context of HIV-1 subtype B, most of the residues that influence coreceptor choice have been mapped to positions flanking the crest of the V3 loop (3, 23, 27). The V3 stem (functionally defined by Cormier et al. to include amino acids 296 to 305 and 321 to 330), along with several C4 residues, is responsible for gp120 binding to the N-terminal domain of CCR5, whereas additional residues in the V3 crown (residues 306 to 320) are required for binding to cell surface CCR5 (3, 4). In this study, we showed by alanine-scanning mutagenesis that the most critical residues of an HIV-1 subtype C gp120 for CCR5 interaction consist primarily of those located in the V3 stem. Individual residues in the V3 crown and in the C4 region are relatively dispensable for envelope-mediated cell fusion, although some of these residues may be involved in the regulation of early postfusion entry steps of this virus.
The seven critical V3 residues (R298, N301, N302, T303, I322, I325, and R326) of this HIV-1 subtype C gp120 protein form a subset of those previously identified by using subtype B (4). These residues are all located in the V3 stem and are highly (>90%) conserved among R5 isolates of both subtypes B and C (18). Changing these residues significantly perturbed subtype C gp120 interactions with CCR5, resulting in a >50% reduction in cell-cell fusion and a >90% reduction in proviral DNA integration. Correspondingly, similar mutations of these residues in subtype B have been shown to decrease gp120 binding to CCR5 by more than 50% (4). Mutation of Arg-298 has also been shown to prevent a subtype B HIV-1 strain from establishing productive infection in CCR5-positive cells (28, 29). Furthermore, residues N301, N302, and T303 in the N-terminal segment of the V3 loop form a highly conserved N-linked glycosylation site, and loss of the N-glycan at this position has been reported to influence coreceptor usage by subtype B viruses (13, 16). We also note that the critical V3 residues include two arginines and two isoleucines. The importance of basic and hydrophobic residues in the V3 loop is consistent with the interpretation that contacts between gp120 and CCR5 may involve electrostatic and hydrophobic interactions, since acidic and aromatic residues in the extracellular regions of CCR5 are critical for coreceptor function (7, 8). Taken together, our results lend support to the idea that a limited set of highly conserved residues in the V3 stem constitutes the main determinants of CCR5 interaction for genetically divergent viruses.
We did not observe dramatic effects on CCR5-dependent cell fusion and viral entry by substitution of residues located in the V3 crown and in the C4 region of subtype C gp120, although similar mutations in subtype B have resulted in 2- to 10-fold reductions in gp120 binding to cell surface CCR5 (4, 19). We found that only one mutation (I420A) in C4 decreased cell-cell fusion by more than 50%, which, upon subsequent analysis, could be accounted for by the reduced ability to bind the CD4 receptor. Thus, the relative importance of the V3 crown and C4 residues may vary depending on the context of the envelope protein in which it is found. Alternatively, the differences may reflect the fact that the assays used in the two experiments measure different steps of the gp120-CCR5 interaction. Discrepancies between detectable CCR5 binding and cell fusion have been reported previously (2) and may be attributed to the following mechanisms. First, the two assays have different sensitivities. Detectable gp120-CCR5 binding generally requires more receptors per cell and higher gp120-CCR5 affinity (2). Thus, certain mutations may allow gp120 to retain low-affinity interactions with CCR5 that are sufficient for cell fusion but are below the detection limit of the binding assay. Second, CCR5 interaction is likely influenced by the form of envelope protein used (6). Binding assays use soluble monomeric gp120, whereas fusion assays use envelope proteins expressed on the cell surface, which represent the oligomeric form found on the virion surface. Third, the structures of CCR5 that support gp120 binding and fusion have been mapped to overlapping but distinct regions. The N-terminal domain of CCR5 is more important for gp120 binding, while the extracellular loops are more critical for fusion and virus infection (12). Some mutations may thus significantly affect gp120 binding but only modestly affect fusion activity. To definitively show that the roles of the V3 crown and C4 residues are different in subtypes B and C, comparisons can be made with a similar mutational analysis of a subtype B R5 isolate. Performance of gp120-CCR5 binding assays with our subtype C mutant clones would also serve this purpose, although binding assays are physiologically relevant only if the native oligomeric form of gp120 is used, and current technology does not allow the purification of such a molecule. Further mutational analysis can also be done by using different subtype C gp120 molecules to determine whether our conclusions will be applicable to other isolates.
A recent report shows that the chimeric virus LJL, an LAI X4 virus containing the V3 stem from the R5 virus JR-FL, can enter cells expressing CXCR4 but not cells expressing CCR5. However, the LLJ virus containing the V3 crown from JR-FL in the LAI backbone is able to enter CCR5-positive cells, albeit with 10- to 100-fold lower efficiency (3). The interpretation was that, in the context of a virion, the V3 crown alone is necessary and sufficient to determine coreceptor usage. This finding is not necessarily inconsistent with our findings. Although we show that individual residues in the V3 crown are not as critical as those in the V3 stem for CCR5 interaction, our finding does not argue against the importance of the V3 crown itself. Our mutations were done in the context of a conformationally correct V3 crown, which likely influences the V3 loop structure. To reconcile these seemingly incompatible findings, we propose that the inability of the LJL virus, which contains the JR-FL V3 stem, to enter CCR5-positive cells is due to the fact that V3 stem residues can mediate CCR5 interaction only if the V3 loop is in the proper conformation. The V3 crown sequence of LAI (and some other X4 viruses) contains an insertion of two amino acids adjacent to the GPG crest, which may alter the V3 loop to the extent that it becomes incompatible with the strict conformational requirements needed for specific interactions with CCR5. On the other hand, the ability of the LLJ virus to enter CCR5-positive cells despite containing the V3 stem from an X4 virus can be explained by the fact that the N-terminal segment of the V3 stem is conserved between the two strains and one out of the three critical residues in the C-terminal segment is present. The presence of five (out of the seven) critical residues required for CCR5 interaction may allow viral entry of this chimeric construct with low efficiency since the crown region of JR-FL preserves the correct R5 V3 loop conformation. Such an interpretation supports the notion that while the V3 crown may direct coreceptor specificity, V3 stem residues are the ones critical for CCR5 interaction.
Early steps in the virus replication cycle after binding and fusion include virus uptake, uncoating, reverse transcription, and proviral DNA integration. In this study, we identified a few mutants that retained >75% of the WT fusogenic potential but were severely inhibited in proviral DNA integration. It is thus possible that certain V3 crown and C4 residues may influence some postfusion events following viral entry. In agreement with this, it has recently been reported that viruses of the M-tropic R5 JRFL strain harboring silent mutations in the V3 region failed to replicate efficiently in macrophages because of a postentry defect, mainly at the step of reverse transcription (9). However, the effect of V3 nucleotide sequences on postentry steps of HIV-1 replication may be specific for macrophages since the silent mutants were still able to productively infect a CCR5-expressing cell line (9). Furthermore, in our study, we observed that the effects of amino acid substitutions in gp120 were generally more dramatic in the infection assays than in the cell-cell fusion assays. Our results may thus purely reflect the fact that a virus infectivity assay can amplify a modest effect at the stage of virus entry, whereas a cell-cell fusion assay will yield a relatively lower score for such an effect. Further investigation is needed to distinguish between these two possibilities.
Acknowledgments
We thank T. Dudek for helpful discussions, B. Aiamkitsumrit and M. F. McLane for technical assistance, and C. VanWinkle and S. Teeravechyan for editorial assistance.
P.S. was supported by grant TW00004 from the Fogarty International Center.
REFERENCES
- 1.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 2.Baik, S. S., R. W. Doms, and B. J. Doranz. 1999. HIV and SIV gp120 binding does not predict coreceptor function. Virology 259:267-273. [DOI] [PubMed] [Google Scholar]
- 3.Cormier, E. G., and T. Dragic. 2002. The crown and stem of the V3 loop play distinct roles in human immunodeficiency virus type 1 envelope glycoprotein interactions with the CCR5 coreceptor. J. Virol. 76:8953-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cormier, E. G., D. N. Tran, L. Yukhayeva, W. C. Olson, and T. Dragic. 2001. Mapping the determinants of the CCR5 amino-terminal sulfopeptide interaction with soluble human immunodeficiency virus type 1 gp120-CD4 complexes. J. Virol. 75:5541-5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 6.Doranz, B. J., S. S. Baik, and R. W. Doms. 1999. Use of a gp120 binding assay to dissect the requirements and kinetics of human immunodeficiency virus fusion events. J. Virol. 73:10346-10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doranz, B. J., Z. H. Lu, J. Rucker, T. Y. Zhang, M. Sharron, Y. H. Cen, Z. X. Wang, H. H. Guo, J. G. Du, M. A. Accavitti, R. W. Doms, and S. C. Peiper. 1997. Two distinct CCR5 domains can mediate coreceptor usage by human immunodeficiency virus type 1. J. Virol. 71:6305-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farzan, M., N. Vasilieva, C. E. Schnitzler, S. Chung, J. Robinson, N. P. Gerard, C. Gerard, H. Choe, and J. Sodroski. 2000. A tyrosine-sulfated peptide based on the N terminus of CCR5 interacts with a CD4-enhanced epitope of the HIV-1 gp120 envelope glycoprotein and inhibits HIV-1 entry. J. Biol. Chem. 275:33516-33521. [DOI] [PubMed] [Google Scholar]
- 9.Harada, T., Y. Tsunetsugu-Yokota, Y. Koyanagi, T. Sata, T. Kurata, and A. Kojima. 2002. Role of nucleotide sequences in the V3 region in efficient replication of CCR5-utilizing human immunodeficiency virus type 1 in macrophages. Virology 299:192-203. [DOI] [PubMed] [Google Scholar]
- 10.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Genetics 77:51-59. [DOI] [PubMed] [Google Scholar]
- 11.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, B., M. Sharron, C. Blanpain, B. J. Doranz, J. Vakili, P. Setoh, E. Berg, G. Liu, H. R. Guy, S. R. Durell, M. Parmentier, C. N. Chang, K. Price, M. Tsang, and R. W. Doms. 1999. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 274:9617-9626. [DOI] [PubMed] [Google Scholar]
- 13.Li, Y., M. A. Rey-Cuille, and S. L. Hu. 2001. N-linked glycosylation in the V3 region of HIV type 1 surface antigen modulates coreceptor usage in viral infection. AIDS Res. Hum. Retrovir. 17:1473-1479. [DOI] [PubMed] [Google Scholar]
- 14.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 15.Moore, J. P. 1990. Simple methods for monitoring HIV-1 and HIV-2 gp120 binding to soluble CD4 by enzyme-linked immunosorbent assay: HIV-2 has a 25-fold lower affinity than HIV-1 for soluble CD4. AIDS 4:297-305. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama, E. E., T. Shioda, M. Tatsumi, X. Xin, D. Yu, S. Ohgimoto, A. Kato, Y. Sakai, Y. Ohnishi, and Y. Nagai. 1998. Importance of the N-glycan in the V3 loop of HIV-1 envelope protein for CXCR-4- but not CCR-5-dependent fusion. FEBS Lett. 426:367-372. [DOI] [PubMed] [Google Scholar]
- 17.Nussbaum, O., C. C. Broder, and E. A. Berger. 1994. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J. Virol. 68:5411-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ping, L. H., J. A. Nelson, I. F. Hoffman, J. Schock, S. L. Lamers, M. Goodman, P. Vernazza, P. Kazembe, M. Maida, D. Zimba, M. M. Goodenow, J. J. Eron, S. A. Fiscus, M. S. Cohen, and R. Swanstrom. 1999. Characterization of V3 sequence heterogeneity in subtype C human immunodeficiency virus type 1 isolates from Malawi: underrepresentation of X4 variants. J. Virol. 73:6271-6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizzuto, C., and J. Sodroski. 2000. Fine definition of a conserved CCR5-binding region on the human immunodeficiency virus type 1 glycoprotein 120. AIDS Res. Hum. Retrovir. 16:741-749. [DOI] [PubMed] [Google Scholar]
- 20.Rizzuto, C. D., R. Wyatt, N. Hernandez-Ramos, Y. Sun, P. D. Kwong, W. A. Hendrickson, and J. Sodroski. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949-1953. [DOI] [PubMed] [Google Scholar]
- 21.Saiki, R. K., S. Scharf, F. Faloona, K. B. Mullis, G. T. Horn, H. A. Erlich, and N. Arnheim. 1985. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230:1350-1354. [DOI] [PubMed] [Google Scholar]
- 22.Schuitemaker, H., N. A. Kootstra, R. E. de Goede, F. de Wolf, F. Miedema, and M. Tersmette. 1991. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J. Virol. 65:356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speck, R. F., K. Wehrly, E. J. Platt, R. E. Atchison, I. F. Charo, D. Kabat, B. Chesebro, and M. A. Goldsmith. 1997. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J. Virol. 71:7136-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 25.Trujillo, J. R., W. K. Wang, T. H. Lee, and M. Essex. 1996. Identification of the envelope V3 loop as a determinant of a CD4-negative neuronal cell tropism for HIV-1. Virology 217:613-617. [DOI] [PubMed] [Google Scholar]
- 26.Vasil, S., R. Thakallapally, B. T. Korber, and B. T. Foley. 1998. Global variation in the HIV-1 V3 region, p. 118-129. In B. Korber, C. Kuiken, B. Foley, B. Hahn, F. McCutchan, J. Mellors, and J. Sodroski (ed.), Human retroviruses and AIDS 1998. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 27.Verrier, F., A. M. Borman, D. Brand, and M. Girard. 1999. Role of the HIV type 1 glycoprotein 120 V3 loop in determining coreceptor usage. AIDS Res. Hum. Retrovir. 15:731-743. [DOI] [PubMed] [Google Scholar]
- 28.Wang, W. K., T. Dudek, M. Essex, and T. H. Lee. 1999. Hypervariable region 3 residues of HIV type 1 gp120 involved in CCR5 coreceptor utilization: therapeutic and prophylactic implications. Proc. Natl. Acad. Sci. USA 96:4558-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, W.-K., T. Dudek, Y. J. Zhao, H. Brumblay, M. Essex, and T.-H. Lee. 1998. CCR5 co-receptor utilization involves a highly conserved arginine residue of human immunodeficiency virus type 1 gp120. Proc. Natl. Acad. Sci. USA 95:5740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 31.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 67:4557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]