Abstract
OBJECTIVE
To assess the impact of informed consent on el–derly patients' colorectal cancer (CRC) screening preferences.
DESIGN
Randomized, controlled trial.
SETTING
Four general internal medicine practices.
PATIENTS
We studied 399 elderly patients visiting their primary care provider for routine office visits.
INTERVENTIONS
Patients were randomized to receive either a scripted control message briefly describing CRC screening methods or one of two informational interventions simulating an informed consent presentation about CRC screening. One intervention described CRC mortality risk reduction in relative terms; the other, in absolute terms.
MEASUREMENTS AND MAIN RESULTS
The main outcome measure was intent to begin or continue fecal occult blood testing (FOBT), flexible sigmoidoscopy, or both. There was no difference in screening interest between the control group and the two information groups (p = .8). The majority (63%) of patients intended to begin or continue CRC screening. Informed patients were able to gauge more accurately the positive predictive value of screening (p = .0009). Control patients rated the efficacy of screening higher than did patients receiving relative risk reduction information, who rated it higher than did patients receiving absolute risk reduction information (p = .0002).
CONCLUSIONS
Elderly patients appeared to understand CRC screening information and use it to gauge the efficacy of screening, but provision of information had no impact on their preferences for screening. In view of the large proportion who preferred not to be screened, we conclude that elderly patients should be involved in the screening decision. However, factors other than provision of information must determine their CRC screening preferences.
Keywords: colorectal cancer screening, elderly, informed consent
Among cancers affecting both men and women, colorectal cancer (CRC) is the second most frequently diagnosed and the second leading cause of cancer death in the United States,1 and it is first among those for which accepted screening tests are available. In the past 5 years, evidence from both randomized trials and case-control studies has confirmed that screening for asymptomatic CRC with either fecal occult blood testing (FOBT) or flexible sigmoidoscopy can significantly reduce the likelihood of dying from CRC.2–5 This evidence has led all relevant organizations to recommend some form of CRC screening. Although most authorities recommend beginning screening at 50 years of age, there is no consensus regarding at which age screening should be discontinued. This uncertainty stems from several factors. On the one hand, CRC incidence increases with age; hence, the elderly may be particularly appropriate targets for screening. On the other hand, the value of screening older patients may be offset by competing morbidity and mortality risks. Although studies examining the impact of CRC screening have included elderly patients, they have not determined whether screening maintains its efficacy in this age group. Moreover, it is conceivable that older patients may not prioritize cancer screening as highly as younger patients, in that preservation of function and alleviation of chronic illnesses may be more prominent health care concerns.
Given the uncertain benefit of CRC screening in the elderly, together with the inconvenience, costs, and risks of screening and its sequelae, it is appropriate to involve elderly patients in the decision whether to continue screening into later years. Accordingly, we examined in a randomized trial whether providing of information about CRC screening to patients altered patient preferences for screening. In addition, it is unclear whether physicians are capable of providing cancer screening information in such a way as to ensure that their older patients understand the relevant issues to help them make screening decisions. This study was designed to determine whether elderly patients were capable of comprehending screening information, and whether different formats of presenting risk information influenced patient preferences for screening.
METHODS
Elderly patients (≥65 years) visiting their primary care doctors for routine office visits when a research assistant was available, between July 1996 and November 1997, were eligible for inclusion. Exclusion criteria included personal history of colon cancer, not speaking English, severe hearing impairment, and significant cognitive impairment; patients were excluded if they were unable to identify the current president and the current month. Four primary care practices were included in the study: one university-based, one suburban, one rural office practice, and one rural community health center.
After consenting to participate, patients were randomized to receive one of three colorectal cancer screening informational scripts, read aloud by a trained research assistant. The control script briefly described FOBT and flexible sigmoidoscopy. The relative risk reduction (RRR) information script provided a 3-minute discussion of FOBT, flexible sigmoidoscopy, and their test characteristics, the evidence supporting mortality reduction in general populations described in terms of relative risk reduction (graphic provided), and the uncertain benefit of screening older persons. The absolute risk reduction (ARR) information script was identical to the RRR information script, except that CRC mortality reduction was described in terms of absolute risk reduction. The informational scripts were reviewed and revised by a panel of primary care physicians and a gastroenterologist for accuracy and content validity. The interventions and survey were piloted with 43 elderly patients before development of the final scripts and instrument. The three informational scripts and accompanying graphics are presented in Appendix.
The main outcome measures were interest in beginning or continuing CRC screening (FOBT and flexible sigmoidoscopy) and intent to begin or continue CRC screening, ascertained after delivery of the informational or control message. Interest was measured on a 5-point Likert scale, and intent was dichotomous (yes/no). Secondary outcome measures included patients' estimate of FOBT positive predictive value (to measure comprehension), and patients' perception of CRC mortality reduction by screening (to measure perceived efficacy of screening). To estimate FOBT positive predictive value, patients were asked, “What is the chance that an abnormal stool card test will actually turn out to be cancer?” and given three choices: almost all, about half, or very few (correct). To measure perceived efficacy of screening, patients were asked to gauge the degree to which screening would reduce the risk of dying from cancer: a great deal, somewhat, or very little (no correct response). Baseline covariates obtained on all patients included sociodemographic data, family history of colon cancer, personal history of noncolon cancer, CRC screening history, history of colonic polyps (by self-report), and self-perceived health status.
Baseline characteristics of the randomization groups were compared using one-way analysis of variance (ANOVA) for continuous variables and χ 2 for categorical variables. Mean interest in CRC screening in the control group and two intervention groups was compared using one-way ANOVA. Intent to screen was compared using χ 2. Trends were examined using the Mantel-Haenszel test for linear association. All testing was two-sided, and p < .05 was considered significant. A sample size of 318 subjects (106 in each of the three experimental groups) was calculated to be sufficient to detect a 20% difference in screening interest (1 point change on a 5-point Likert scale) with an α error of .02 and a power of .90, assuming SD = 2 on the Likert scale. An α of .02 was chosen to account for multiple comparisons between the control group and two intervention groups.
RESULTS
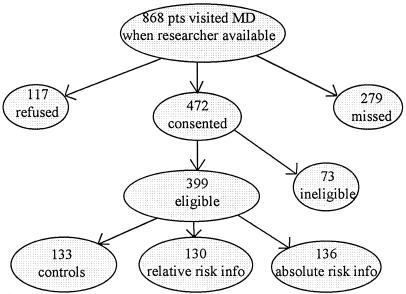
Patient enrollment into the randomized trial is outlined in Figure 1. Of the 868 elderly patients who visited their physicians when a research assistant was on-site, 279 were not interviewed because there was insufficient time before their office visit or the interviewer was with other subjects, and 117 refused to participate. Of the 472 who consented to participate, 73 were ineligible, leaving 399 who were enrolled and randomized to receive the control information (n = 133), the RRR information (n = 133), or the ARR information (n = 136). Enrolled patients were slightly younger than those who were missed, ineligible, or refused (73.9 years vs 75.4 years, p = .001), and were somewhat more likely to be white (75.4% vs 69.5%, p = .05). There was no difference in type of medical insurance. Baseline characteristics of patients in the three randomization groups are presented in Table 1. There were no statistically significant differences in patient characteristics between the three groups.
FIGURE 1.
Patient enrollment.
Table 1.
Patient Characteristics*
| Characteristic | Control Group n = 133) | Relative Risk Reduction Information Group (n = 133) | Absolute Risk Reduction Information Group (n = 136) |
| Mean age (SD), y | 75 (6) | 74 (6) | 74 (6) |
| Female, % | 62 | 63 | 65 |
| Nonwhite, % | 23 | 24 | 28 |
| High school grads, % | 47 | 55 | 55 |
| Income < $15,000, % | 62 | 61 | 57 |
| Very good to excellent health, %† | 34 | 32 | 32 |
| Family history of colon cancer, % | 11 | 10 | 10 |
| Colonic polyp history, % | 14 | 15 | 16 |
| Previously screened, % | 77 | 75 | 77 |
There were no statistically significant differences in patient characteristics between groups
Based on self-reported health status
There was no difference in screening interest or intent to begin or continue CRC screening between the three information groups. Mean (SD) interest in FOBT on the 5-point Likert scale (1 = low interest, 5 = high interest) in the control, RRR information, and ARR information groups was 3.3 (1.5), 3.4 (1.5), and 3.3 (1.5), respectively. Mean (SD) interest in flexible sigmoidoscopy in the three groups was 2.5 (1.5), 2.6 (1.5), and 2.7 (1.4). The proportion of elderly patients probably or definitely interested in screening is displayed in the top half of Table 2. Although slightly more than half of the patients showed interest in beginning or continuing FOBT, only about one third of patients indicated interest in flexible sigmoidoscopy; there were no significant differences based on the amount or type of screening information received.
Table 2.
Interest in and Intent to Undergo Colorectal Cancer Screening Among Elderly Patients*
| Interest/Intent | Controls, % | RRR Info Group, % | ARR Info Group, % | p Value |
|---|---|---|---|---|
| Interest in undergoing screening† | ||||
| Probably or definitely interested in FOBT | 51.9 | 54.4 | 51.5 | .9 |
| Probably or definitely interested in flex sig | 32.2 | 36.0 | 32.3 | .8 |
| Intent to undergo screening‡ | ||||
| Intent to begin or continue FOBT | 55.6 | 61.0 | 55.4 | .6 |
| Intent to begin or continue flex sig | 32.3 | 36.0 | 33.1 | .8 |
| Intent to begin or continue FOBT and/or flex sig | 59.4 | 66.9 | 63.1 | .4 |
*RRR indicates relative risk reduction; ARR, absolute risk reduction; FOBT, fecal occult blood testing; flex sig, flexible sigmoidoscopy.
Measured on 5-point Likert scale
Measured as yes/no
The lower half of Table 2 depicts the proportion of patients actually intending to begin or continue CRC screening. Overall, 63% of patients intended to begin or continue CRC screening (FOBT, flexible sigmoidoscopy, or both), but there was no difference in intent by information group.
Although the type of CRC screening information we provided did not affect preferences, patients who received information were able to gauge more accurately the positive predictive value of screening. In response to the question, “What is the chance that an abnormal stool card test will actually turn out to be cancer?” 53.8% of control patients responded correctly, compared with 71.1% of patients who received screening information (p for difference = .0007). There was no difference in correct response rates between the two information groups (RRR and ARR information groups).
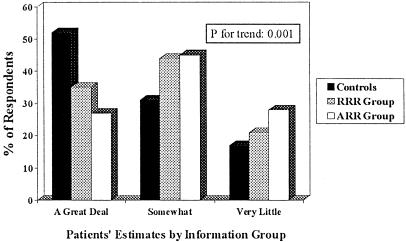
There were also significant differences between control and information groups in the perceived efficacy of screening in reducing CRC mortality. As depicted in Figure 2, control patients rated the efficacy of screening higher than did patients receiving RRR information, who rated it higher than did patients receiving ARR information (p for trend = .0002).
FIGURE 2.
Patients' estimates of colorectal cancer mortality reduction by screening. RRR indicates relative risk reduction; ARR, absolute risk reduction.
DISCUSSION
In this study, information about CRC screening and its potential effect on mortality, whether couched in terms of relative or absolute risk reduction, had no impact on screening interest or intent among elderly primary care patients. Overall, approximately two thirds of this elderly cohort intended to begin or continue some form of CRC screening.
It is exceedingly unlikely that this study actually failed to detect a clinically meaningful difference in screening interest between the information groups because of inadequate power. The sample size was sufficiently large to have had a 90% probability of detecting a 1-point difference in CRC screening interest between groups (on the 5-point Likert scales) with an α error of .02. The actual differences found between the control and intervention groups varied only between 0 and 0.2 points. Even if a true difference of under 1 point on the Likert scale went undetected, this would be of doubtful clinical significance.
Although the information provided had no effect on preferences, elderly patients appeared capable of understanding CRC screening information, given that informed patients demonstrated a more accurate estimation of FOBT predictive value. This finding is presumably explained by the fact that patients who received RRR and ARR informational interventions were provided with identical information about the positive predictive value of FOBT, whereas control patients were given no such information. Given the paucity of literature on comprehension of screening information among the elderly, it is reassuring to find that they are capable of retaining such information. Although one might argue that our patient population was “enriched” by excluding patients with significant cognitive impairment, such patients are often not considered appropriate candidates for screening because of their limited life expectancy or inability to tolerate the diagnostic and therapeutic interventions involved in early detection and treatment of colon cancer.
The amount and nature of information given also appear to have influenced how elderly patients gauged the efficacy of CRC screening in reducing CRC mortality. Control patients, who received only a brief description of FOBT and flexible sigmoidoscopy, rated efficacy of screening the highest, followed by the patients who received RRR information and those who received ARR information, respectively. This finding is in keeping with other studies, generally involving younger subjects, which have shown that the “framing effect”—the format in which one poses probabilistic information—influences patients' estimates of risks and benefits,6–11 as well as doctors' estimates,12,13 and, in most cases, screening and treatment preferences. However, none of these previous studies examined the impact of framing on an exclusively elderly patient population or the impact of RRR versus ARR information on cancer screening preferences.
The differences we found between information groups in screening test knowledge and perceived efficacy did not translate into differences in screening interest; however, these findings demonstrate that elderly subjects are capable of understanding and manipulating relatively complex probabilistic information. This capacity has been termed “numeracy” by Schwartz et al., who examined patients' understanding of the benefits of screening mammography in terms of their facility with probabilistic thinking.14 They found that understanding was closely linked with numeracy, and that many of the patients in their study had poor numeracy skills. Although the present study did not examine numeracy in depth, the results suggest that this exclusively elderly cohort was able to utilize numeric information.
Others have examined patient preferences for CRC screening and arrived at divergent conclusions, although none focused on elderly populations. Leard et al. found that 96% of the primary care patients they surveyed desired some form of CRC screening, a much greater interest than was found in the present study, although the patient population was younger, more educated, more racially homogenous (white), and more likely to have been screened previously.15 Their scripted presentation of screening options was also lengthier (10 minutes) and included barium enema and colonoscopy, in addition to FOBT and flexible sigmoidoscopy. Pignone et al. recently found that patients' interest in CRC screening increased significantly after they received CRC screening information.16 Again, the study involved general adult patients rather than elderly patients and was a before-and-after study rather than a randomized clinical trial. Moreover, an objective of the study was to increase screening rates rather than assess the impact of an informational intervention (M. P. Pignone, personal communication). Despite these methodologic differences, it may be the case that elderly patients, while capable of understanding screening information, are less influenced by it than are younger populations.
There are several limitations to the present study. First, the majority of patients were poor and many were not well educated, limiting the generalizablity of the findings, although we demonstrated that they were clearly able to comprehend at least some of the information provided. Second, FOBT and flexible sigmoidoscopy screening history were based on patient self-report. Third, screening interest and intent are surrogate markers for actual screening behavior; clearly, physician preferences and practices will strongly influence ultimate colorectal screening decisions, and the impact of direct physician counseling may differ significantly from that of the scripted informational interventions described herein. Fourth, we chose to describe only FOBT and flexible sigmoidoscopy in our information scripts, rather than include barium enema and colonoscopy. We limited the options because at the time of the study FOBT and flexible sigmoidoscopy were (and still are) the two screens with the best evidence to support their use. Moreover, these two tests are the “common denominator” of all major organizations' recommendations. We also acknowledge that the CRC mortality risk reduction figure of 33% that we selected for the informational script is derived from studies of FOBT, not sigmoidoscopy, for which the risk reduction is in the 40% to 55% range. We chose this figure because it derives from the most rigorous evidence available—randomized controlled trials. Finally, it is always difficult in an informed consent discussion to strike a true balance between the benefits and burdens of a proposed intervention; it is certainly possible that the content and wording of the information influenced the results of the study in ways we did not intend.
Even after clinical trials help to illuminate the effectiveness of CRC screening in the elderly, it will remain incumbent on health care providers to involve their elderly patients in the decision whether to screen. Others have shown that owing to competing mortality risks the marginal benefit of detecting and treating any single disease declines with age.17 In the specific case of CRC, it has been demonstrated that the presence, number, and severity of comorbid illnesses increase with age, and that these comorbidities adversely affect CRC-specific survival18; hence, detecting and intervening on CRC is less likely to benefit older individuals. In addition, elderly patients may have health care priorities other than screening and early detection, such as preservation of function, which should be elicited before embarking on CRC screening. Balancing these caveats, our ability to prognosticate the value of screening for the individual elderly patient will always be nothing more than an actuarial estimate. Many patients who are aging successfully will live to benefit from screening, will benefit from the reassurance of a negative test result, and will place screening and early detection as high priorities. Given these considerations, the issues surrounding CRC screening in the elderly seem destined to remain as murky as those related to prostate cancer screening and mammography in younger women for the foreseeable future.19 This conclusion accentuates the importance of engaging elderly patients in the CRC screening decision.20,21
In summary, we found that providing elderly patients with a balanced discussion of the benefits, burdens, and uncertainties of CRC screening affected their perception of screening effectiveness, but had no impact on their preferences for such screening. This negative result does not diminish the importance of involving elderly patients in CRC screening decisions, but does suggest that factors other than information must be more important in determining screening interest.
Acknowledgments
Dr. Wolf is the recipient of an American Cancer Society Cancer Control Career Development Award for Primary Care Physicians. The authors thank Andrea Chang, Steven N. Schechterman, and John I. Morgan for their critical involvement in data collection, and Dr. John T. Philbrick for his thoughtful review of the manuscript.
APPENDIX
Colorectal Cancer Screening Informational Scripts
Control Script
Now I'm going to be asking you some questions about screening for colon cancer. Screening means testing for colon cancer in people who have no signs or symptoms of cancer. The two screening tests I'll be talking about are the stool card test and the sigmoidoscopy test. The stool card test involves taking home three small cards from your doctor's office, smearing a small stool sample on each card from three separate bowel movements, and returning the cards to your doctor. Sigmoidoscopy involves passing a narrow flexible tube through the rectum to examine the colon. Colonoscopy is similar to sigmoidoscopy, except the tube is longer.
Absolute and Relative Risk Reduction Informational Scripts1
I'd like to tell you a bit more about colon cancer or cancer of the bowel and the tests we have to look for it. Older individuals have a small chance (about 6 in 100) of developing colon cancer at some point during their lifetime, and about a 2–3 in 100 chance of dying from it. [Colon cancer is the third leading cause of cancer and the third leading cause of cancer death in both men and women.]
The purpose of colon cancer screening is to look for early cancer or growths that could turn into cancer in people who have no symptoms of colon cancer so that it could be caught early. There are basically two types of colon cancer screening, stool cards and sigmoidoscopy. Let me describe these choices briefly.
The stool cards test for tiny amounts of blood in your stool that you can't see with the naked eye, as this may be the first sign of cancer. This involves taking home three cards once a year, on which you smear a small amount of bowel movement. You then send them back to your doctor. The test isn't perfect—it only finds about 1 of every 4 cancers. For example, if four people had colon cancer, the card test might only detect cancer in one of them. It could miss the other three. The test also causes a lot of “false alarms”; only about 1 in 10 of abnormal stool card results actually turns out to be due to a cancer. Nine times out of 10 there is no cancer.
The other screening test, sigmoidoscopy, involves passing a flexible tube, or scope, with a camera at the end of it part way up the colon to look directly for cancer and growths. The advantage of this test is that it allows the doctor to look directly into the colon. The main disadvantages are that it is uncomfortable and requires you to take two enemas beforehand to clean out the bowel. Because it looks only part way up the colon, it finds only about half of all cancers and growths. If you were to have either an abnormal stool card test or an abnormal sigmoidoscopy, your doctor would probably advise you to have a longer scope, called a colonoscopy, passed through the rectum into the entire colon to search for cancer. This procedure involves taking a powerful laxative beforehand, but allows the doctor to see the entire colon. If early cancer is found, surgery to remove the affected part of the colon is generally recommended. Screening sigmoidoscopy is not covered by many insurance programs, including Medicare, and usually runs about $150. The stool cards are also not covered by insurance, but cost only about $6.
Another option is to not be tested at all, unless signs of colon cancer develop, though at that point, cancer is much less likely to be curable.
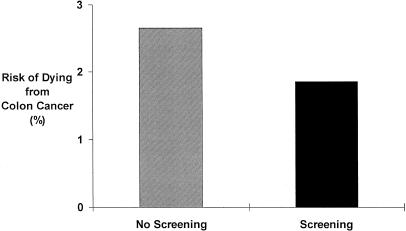
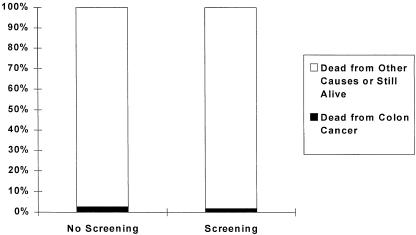
Studies have shown that stool cards done yearly or sigmoidoscopy done every 3 to 10 years can significantly lower the risk of dying from colon cancer. This figure (Appendix Figure 1 for relative risk information script; Appendix Figure 2 for absolute risk information script) shows you how screening with either stool cards or sigmoidoscopy affects your chances of dying from colon cancer over the next 10 years. The bar on the left shows the chances without screening and the bar on the right shows the chances with screening. [As you can see, the chances of dying from colon cancer drop by about 30% or 30 in 100 if you are screened.] The black area at the bottom shows the chance of dying from colon cancer, and the gray area shows the chance of dying from other causes or being still alive 10 years from now. As you may be able to see, the black area does get a little smaller with screening, indicating that your risk of dying drops from a little over 2 in a hundred to a little under 2 in 100.
APPENDIX FIGURE 1.
Impact of screening on risk of dying from colon cancer.
APPENDIX FIGURE 2.
Impact of screening on risk of dying from colon cancer.
One major point is that we don't know if screening for colon cancer in older people is really helpful—that is, whether it helps save lives. This is why your personal preferences for screening are so important.
The information I've provided is neither to talk you into nor out of having colon cancer screening, but is simply to help you better understand the issues. Now I'd like to ask you a few more questions about colon cancer screening.
Footnotes
Elements included in the relative risk reduction script, but not in the absolute risk reduction script, are indicated in brackets. Elements included in the absolute risk reduction script, but not in the relative risk reduction script, are italicized.
REFERENCES
- 1.Landis SH, Murray T, Bolden S. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 2.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 3.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–77. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 5.Selby JV, Friedman GD, Quesenberry CP, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 6.Malenka DJ, Baron JA, Johansen S, Wahrenberger JW, Ross JM. The framing effect of relative and absolute risk. J Gen Intern Med. 1993;8:543–8. doi: 10.1007/BF02599636. [DOI] [PubMed] [Google Scholar]
- 7.Banks SM, Salovey P, Greener S, et al. The effects of message framing on mammography utilization. Health Psychol. 1995;14:178–84. doi: 10.1037//0278-6133.14.2.178. [DOI] [PubMed] [Google Scholar]
- 8.O'connor AM. Effects of framing and level of probability on patients' preferences for cancer chemotherapy. J Clin Epidemiol. 1989;42:119–26. doi: 10.1016/0895-4356(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 9.Meyerowitz BE, Chaiken S. The effect of message framing on breast self-examination attitudes, intentions, and behavior. J Pers Soc Psychol. 1987;52:500–10. doi: 10.1037//0022-3514.52.3.500. [DOI] [PubMed] [Google Scholar]
- 10.Llewellyn-Thomas HA, McGreal MJ, Thiel EC. Cancer patients' decision making and trial-entry preferences: the effects of “framing” information about short-term toxicity and long-term survival. Med Decis Making. 1995;15:4–12. doi: 10.1177/0272989X9501500103. [DOI] [PubMed] [Google Scholar]
- 11.Hux JE, Naylor CD. Communicating the benefits of chronic preventive therapy: does the format of efficacy data determine patients' acceptance of treatment? Med Decis Making. 1995;15:152–7. doi: 10.1177/0272989X9501500208. [DOI] [PubMed] [Google Scholar]
- 12.Forrow L, Taylor WC, Arnold RM. Absolutely relative: how research results are summarized can affect treatment decisions. Am J Med. 1992;92:121–4. doi: 10.1016/0002-9343(92)90100-p. [DOI] [PubMed] [Google Scholar]
- 13.Naylor CD, Chen E, Strauss B. Measured enthusiasm: does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1993;117:916–21. doi: 10.7326/0003-4819-117-11-916. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127:966–72. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Leard LE, Savides TJ, Ganiats TG. Patient preferences for colorectal cancer screening. J Fam Pract. 1997;45:211–8. [PubMed] [Google Scholar]
- 16.Pignone MP, Bucholtz D, Harris R. Patient interest and preferences for colon cancer screening. J Gen Intern Med. 1998;13(suppl 1):96. doi: 10.1046/j.1525-1497.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch HG, Albertsen PC, Nease RF, Bubolz TA, Wasson JH. Estimating treatment benefits for the elderly: the effect of competing risks. Ann Intern Med. 1996;124:577–84. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 18.Yancik R, Wesley MN, Ries LAG, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients—a population-based study. Cancer. 1998;82:2123–34. [PubMed] [Google Scholar]
- 19.Ransohoff DF, Harris RP. Lessons from the mammography screening controversy: can we improve the debate? Ann Intern Med. 1997;127:1029–34. doi: 10.7326/0003-4819-127-11-199712010-00016. [DOI] [PubMed] [Google Scholar]
- 20.Sox HC. Screening for disease in older people. J Gen Intern Med. 1998;13:424–5. doi: 10.1046/j.1525-1497.1998.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassirer JP. Incorporating patients' preferences into medical decisions. N Engl J Med. 1994;330:1895–6. doi: 10.1056/NEJM199406303302611. [DOI] [PubMed] [Google Scholar]