Abstract
OBJECTIVE
To examine the cost-effectiveness of moving from usual care to more organized management strategies for patients on chronic warfarin therapy.
DESIGN
Using information available in the scientific literature, supplemented with data from a large health system and, when necessary, expert opinion, we constructed a 5-year Markov model to evaluate the health and economic outcomes associated with each of three different anticoagulation management approaches: usual care, anticoagulation clinic testing with a capillary monitor, and patient self-testing with a capillary monitor.
PATIENTS
Three hypothetical cohorts of patients beginning long-term warfarin therapy were used to generate model results.
MAIN RESULTS
Model results indicated that moving from usual care to anticoagulation clinic testing would result in a total of 1.7 thromboembolic events and 2.0 hemorrhagic events avoided per 100 patients over 5 years. Another 4.0 thromboembolic events and 0.8 hemorrhagic events would be avoided by moving to patient self-testing. When direct medical care costs and those incurred by patients and their caregivers in receiving care were considered, patient self-testing was the most cost-effective alternative, resulting in an overall cost saving.
CONCLUSIONS
Results illustrate the potential health and economic benefits of organized care management approaches and capillary monitors in the management of patients receiving warfarin therapy.
Keywords: anticoagulation management, cost-effectiveness analysis, decision analytic model, warfarin
Warfarin is the mainstay of long-term anticoagulation therapy in the United States. Although warfarin therapy reduces disability and fatal thromboembolic events, it can also cause disabling and fatal hemorrhagic events.1 Therefore, to maximize the efficacy of warfarin, frequent monitoring of the international normalized ratio (INR) of the prothrombin time is required.
The majority of patients receiving chronic warfarin therapy are managed in traditional office settings, but some are managed in formal anticoagulation clinics.2 Such clinics have been shown to improve the time spent within therapeutic range, as well as to reduce thromboembolic events and hemorrhagic complications.3,4 With the 1997 Food and Drug Administration approval of capillary monitors for patient use, it became possible not only to have INR values available immediately, but also for patients to determine their INR without traveling to a clinic or laboratory. The few studies that have evaluated the effectiveness of home testing have found that patients testing at home check their INRs more often and are within the therapeutic range more often than those tested in an anticoagulation clinic.5–7 Others have found that patient self-testing decreases adverse events,8 and is preferred by patients.9
Despite evidence that the tighter therapeutic control allowed by the introduction of organized anticoagulation clinics and capillary monitors may prevent complications, the introduction of such clinics and devices has been relatively slow, and the cost-effectiveness of the different testing and monitoring alternatives remains unknown. We therefore sought to evaluate the cost-effectiveness of three management alternatives: usual care, anticoagulation clinic testing, and patient self-testing.
METHODS
The Decision Model
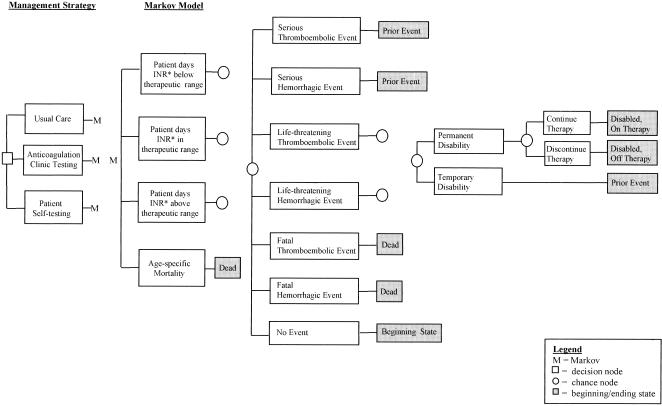
An overview of the underlying decision tree is presented in Figure 1. For modeling purposes, we assumed “usual care” to consist of a venipuncture blood draw for an INR, delayed test results, and no organized anticoagulation clinic. “Anticoagulation clinic testing” consisted of in-clinic capillary monitors for INRs, immediate test results, and an organized anticoagulation clinic. (Organized anticoagulation clinics generally consist of dedicated nursing or pharmacy staff who use explicit protocols and processes to monitor and adjust dosage among patients on warfarin therapy and to seek physician consult). “Patient self-testing” consisted of at-home capillary monitors for INRs with immediate test results managed by patients telephoning these results to an organized anticoagulation clinic.
FIGURE 1.
Model overview. *indicates international normalized ratio of the prothrombin time.
As illustrated in Figure 1, for each monitoring alternative, the time patients spend below, in, and above therapeutic range is estimated and used to derive the associated risk of first and subsequent thromboembolic and hemorrhagic events. Once an event occurs, individuals are at risk of temporary and permanent disabilities. Among individuals who become permanently disabled, there is a risk of discontinuing anticoagulation therapy and thus being at an increased risk of subsequent thromboembolic events.
The decision tree was operationalized as a Markov model in Excel. In the model, hypothetical cohorts of patients are followed for 5 years following warfarin therapy initiation. To parallel the clinical data on thromboembolic and hemorrhagic events available, the model was built to be reflective of patients initiating therapy at age 57 years.10,11 With each 1-year cycle of the model, patients move among five defined health states (no prior event; prior event, nondisabled, continuing therapy; prior event, permanently disabled, continuing therapy; prior event, permanently disabled, discontinued therapy; and dead). Transitions among these states were defined using event probabilities drawn from the scientific literature, supplemented with information available within the authors' institution and, when voids remained, expert opinion.
Specifically, four key clinical assumptions were needed: (1) estimates of the time spent below, in, and above therapeutic range for each of the three management alternatives; (2) the risk of incurring an adverse event associated with these times; (3) the risk of disability following an adverse event; and (4) among those permanently disabled, the risk of discontinuing anticoagulation therapy. These clinical assumptions and the corresponding transition probabilities are described below.
For the baseline model, the estimated times spent below and above therapeutic range for usual care reflected those reported by Chiquette et al.,3 Hasenkam et al.,7 Gottlieb and Salem-Schatz,12 and our own clinical experiences. Using the average of these values, we assumed patients receiving usual care spend 33% of their time (range, 15%–50%) below and 17% of their time (range, 10%–25%) above therapeutic range. For anticoagulation clinic testing, we assumed that patients spend 26% percent of their time (range, 8%–40%) below and 9% of their time (range, 1%–17%) above therapeutic range to reflect the averages of those reported by a number of large anticoagulation clinical trials,13–21 as well as data from four observational studies.3,6,22,23 For patient self-testing, patients were assumed to spend 6% of their time (range, 5%–15%) below and 5% of their time (range, 1%–7%) above therapeutic range, reflecting the average of those reported by White et al.,23 Anderson et al.,9 Ansell et al.,6 and Hasenkam et al.7
Associated with these estimates of time spent below, in, and above therapeutic range are first and subsequent thromboembolic and hemorrhagic event rates (Table 1). Transition probabilities for event rates were derived from a longitudinal study of patients seen in five anticoagulation clinics.5,10,11 Published data from this study were supplemented with unpublished data provided by Dr. Stephan Fihn and colleagues. The average age of this population was 57 years and 81% were male. Thirty percent had a primary indication of deep vein thrombosis or pulmonary embolism, while 26% and 14% were on warfarin for valvular disease and atrial fibrillation, respectively. Finally, 21% had experienced a cerebral or systemic embolism, 7% had other circulatory conditions, and the remaining 3% were on warfarin for other conditions.10
Table 1.
Event Rate per 100 Patient Years by Time Spent Below, In, and Above Therapeutic Range
| Below | In | Above | ||||
|---|---|---|---|---|---|---|
| Events | Baseline | (Range) | Baseline | (Range) | Baseline | (Range) |
| Thromboembolic first events | ||||||
| Fatal | 0.05 | (0–0.30) | 0.01 | (0–0.05) | 0.00 | (0–0.05) |
| Life-threatening | 1.58 | (1.0–6.0) | 0.47 | (0–1.0) | 0.11 | (0–1.0) |
| Serious | 14.68 | (8.0–16.0) | 2.52 | (1.0–6.0) | 2.29 | (1.0–6.0) |
| Thromboembolic subsequent events | ||||||
| Fatal | 0.01 | (0–0.3) | 0.00 | (0–0.05) | 0.01 | (0–0.05) |
| Life-threatening | 0.32 | (0–5.0) | 0.04 | (0–1.0) | 0.44 | (0–1.0) |
| Serious | 2.45 | (0–6.0) | 0.88 | (0–6.0) | 1.60 | (1.0–6.0) |
| Hemorrhagic first events | ||||||
| Fatal | 0.05 | (0–0.3) | 0.04 | (0–0.3) | 0.20 | (0–0.6) |
| Life-threatening | 0.61 | (0–1.0) | 0.49 | (0–1.0) | 2.66 | (0.5–4.0) |
| Serious | 5.87 | (3.0–8.0) | 5.12 | (3.0–8.0) | 12.83 | (10.0–20.0) |
| Hemorrhagic subsequent events | ||||||
| Fatal | 0.02 | (0–0.3) | 0.02 | (0–0.3) | 0.07 | (0–0.6) |
| Life-threatening | 0.30 | (0–1.0) | 0.25 | (0–1.0) | 0.96 | (0.5–4.0) |
| Serious | 1.30 | (0–8.0) | 1.85 | (0–8.0) | 5.73 | (3.0–20.0) |
Although the study by Fihn et al. was done before INR reporting was commonplace: however, the classification system for prothrombin time therapeutic range used in their study afforded the ability to translate into INR-defined therapeutic ranges.10 To do so, we assumed an international sensitivity index (ISI) of 2.3.10 As proposed by Fihn et al., events were classified into three categories requiring medical care: serious, life-threatening, or fatal.10,11 Serious events include bleeding that requires endoscopy, recurrent deep vein thrombosis, and transient ischemic attack. Life-threatening events are those that require urgent treatment or cause irreversible sequelae; examples include myocardial infarction, stroke, and bleeding leading to surgery.
We assumed that 60% of all life-threatening thromboembolic events24–28 and 10% of all life-threatening hemorrhagic events5 resulted in permanent disability. Among some patients incurring a disabling event, the risk associated with continued warfarin use most likely outweighs the potential benefits. Our model therefore allowed for a proportion of those who were permanently disabled to discontinue warfarin therapy. To our knowledge, no published data are available on the percentage of individuals who discontinue therapy after becoming permanently disabled. As a baseline, we assumed that 50% would do so after a permanently disabling event. Among individuals who discontinued therapy, the risks of a subsequent thromboembolic event (17%) and hemorrhagic event (1%) were derived from those reported for members of the control group of a large randomized anticoagulation trial for secondary prevention.15
Using data from several studies that have assigned utilities to the adverse events associated with anticoagulation therapy,29–33 we also estimated the quality-adjusted life years (QALYs) expected with each management alternative. For patients who suffered a temporarily disabling event, a utility of 0.75 was assigned for the 30 days following the event. For individuals who suffered a permanently disabling event, a utility of 0.50 was assigned from the time of the event until death or the end of the 5-year modeling period, whichever came first. Because the validity and reliability of these reported QALYs is untested, we elected to vary these estimates over a wide range in the sensitivity analyses.
For each of these assumptions, we conducted sensitivity analyses to determine the robustness of model results to underlying assumptions. One-way sensitivity analysis was conducted for assumptions of particular interest, and multiple-way sensitivity analysis was conducted using Crystal Ball,34 a program that enables Monte Carlo analysis. The more uncertain the data on which we based an assumption, the wider the range we tested around the assumption in the sensitivity analyses.
All data were compiled and all analyses and interpretations were conducted by the authors, independent of Roche Diagnostic (formerly Boehringer Mannheim) staff, the funding source for the study. The two exceptions to this were data on the base model device cost and estimated time required to train patients in its use, both of which were provided by Roche Diagnostic staff.
Cost Data
The direct medical care costs of anticoagulation monitoring and of thromboembolic and hemorrhagic events are used to estimate the model. These costs were drawn from the scientific literature, supplemented with information available within the authors' institution and, when needed, expert opinion. To approximate the reference case as recommended by the Panel on Cost-Effectiveness in Health and Medicine,35 we included the costs associated with anticoagulation testing, monitoring, and adverse events (including nursing home costs), as well as the costs incurred by patients and their caregivers in receiving care. Model results are presented from two perspectives: a medical provider perspective, which includes all direct medical care costs, including nursing home costs; and a societal perspective, which includes the costs incurred by medical providers, and by patients and their caregivers in receipt of care. All costs and health outcomes were discounted at a base rate of 3%,35 and all monetary amounts reflect 1997 dollars.
As shown in Table 2, annual anticoagulation management costs were derived using an estimated testing frequency of 14 times per year for usual care,12 23 times per year for anticoagulation clinic testing,6 and 52 times per year for patient self-testing.8 The frequencies for usual care and anticoagulation clinic testing are consistent with experiences within our own institution; all reflect the average testing frequency for patient populations that include those who spend time both within and outside the therapeutic range. Included in the direct medical care costs of anticoagulation management were the costs of equipment, supplies, and staff time (i.e., nurse and physician time). Although we assumed the average physician time per consult to be consistent across the three management alternatives (i.e., 2 minutes),36 we assumed the frequency with which the physician is consulted to be reduced once an anticoagulation clinic had been established (i.e., 90% vs. 10%).36 Nursing time per test was assumed to be 13 minutes for usual care, 15 minutes for anticoagulation clinic testing, and 8 minutes for patient self-testing. Valuation of these resources was achieved by using prevailing wage and price data from our institution and therefore reflects the acquisition cost to a provider organization. Also reflective of the acquisition cost to a provider organization was the price used in the base model for the capillary monitor—$1,385.
Table 2.
Annual Anticoagulation Management Costs Per Patient
| Number of Tests per Year | |||||
|---|---|---|---|---|---|
| Management Strategy | Baseline | (Range) | Costs to Managed Care Organization | Costs to Patients and Their Caregivers | Total Management Costs |
| Usual care | 14 | (9–23) | $157* | $239sup§ | $396 |
| Anticoagulation clinic testing | 23 | (11–28) | $233† | $520∥ | $753 |
| Patient self-testing | 52 | (29–73) | $660‡ | $200¶ | $860 |
Baseline estimates assume 2 minutes physician (MD) time for 90% of tests valued at $72/h, 13 minutes of nursing (RN) time per test valued at $23.40/h, equipment and supply cost of $4 per test
Baseline estimates assume 2 minutes MD time for 10% of tests valued at $72/h, 15 minutes of RN time per test valued a t $23.40/h, $4 reagent cartridge per test and $1,385 per capillary monitor per 200 patients served, allocated over 5 years of use
Baseline estimates assume 2 minutes of MD time for 10% of tests valued at $72/h, 8 minutes of RN time per test valued at $23.40/h, $4 reagent cartridge per test, and $1,385 per capillary monitor allocated over 5 y of use
Baseline estimates assume 17 minutes of patient (PT) and caregiver (CG) time per test valued at $14.10/h with CG accompanying 30% of PTs, 26 mi per test valued at $0.30/mi (CG assumed to travel with PT) and 52 travel minutes per test valued at $14.10/h (no mileage or travel time for 7 tests assumed to coincide with routine office visits)
Baseline estimates assume 20 minutes of PT and CG time per test valued at $14.10/h with CG accompanying 30% of PTs, 26 mi per test valued at $0.30/mi (CG assumed to travel with PT) and 52 travel minutes per test valued at $14.10/h (no mileage or travel time for 7 tests assumed to coincide with routine office visits)
Baseline estimates assume 15 minutes of PT and CG time per test valued at $14.10/h with CG assisting 9% of PTs with self-testing
Included in the costs of anticoagulation management faced by patients and their caregivers were time and travel costs (Table 2). Time costs, which include travel, waiting, training, and testing times, were estimated to be 17 minutes for usual care, 20 minutes for anticoagulation clinic testing, and 15 minutes for self-testing.36 They were valued using prevailing national wage rates for individuals aged 55 to 64 years ($14.10 per hour).37 From experiences in our anticoagulation clinic, we assumed 30% of patients were accompanied by a family member for clinic-based testing. Nine percent were assumed to receive help with home testing.38 So as to estimate only the marginal costs associated with INR testing, no travel costs were associated with any testing assumed to occur during routine visits. (In the base model, we assumed seven such visits per year.)
Table 3 presents the average cost for adverse events used in the model. Included in these were office visit, emergency department, and hospital admission costs. The underlying resource use (e.g., length of stay) from which these estimates were derived was from the stroke-related literature,39,40 as well as a sample of 50 patients receiving anticoagulation therapy from our institution. Valuation of these resources was achieved by using our institution's cost estimates and published data.41 For individuals who were institutionalized, the cost of nursing home care was also included.
Table 3.
Adverse Event Costs
| Average Cost per Event | ||
|---|---|---|
| Event Severity | Thromboembolic Events | Hemorrhagic Events |
| Fatal | $5,112 | $11,232 |
| Life-threatening | $19,280 | $20,980 |
| Serious | $10,684 | $3,044 |
RESULTS
Baseline Analysis
Table 4 presents the number of adverse events by severity expected with each of the three management strategies. As illustrated in Table 4, 3.7 events per 100 patients could be avoided by moving from usual care to anticoagulation clinic testing, and another 4.9 events per 100 patients could be avoided by moving from anticoagulation clinic testing to patient self-testing over a 5-year period.
Table 4.
Number of Adverse Events per 100 Patients Over 5 Years
| Thromboembolic Events | Hemorrhagic Events | ||||||
|---|---|---|---|---|---|---|---|
| Management Strategy | Total | Fatal | Life-threatening | Serious | Fatal | Life-threatening | Serious |
| Usual care | 30.65 | 0.05 | 1.50 | 11.91 | 0.17 | 2.24 | 14.78 |
| Anticoagulation clinic testing | 26.95 | 0.04 | 1.27 | 10.48 | 0.14 | 1.85 | 13.17 |
| Patient self-testing | 22.10 | 0.03 | 0.82 | 6.92 | 0.12 | 1.62 | 12.60 |
Because the majority of these events are not fatal, the expected differences in life years among the three alternatives are relatively small (i.e., <0.2). However, as many life-threatening and serious events result in disability, moving from usual care to anticoagulation clinic testing would result in an increase of 0.5 QALY per 100 patients, and moving from anticoagulation clinic testing to patient self-testing would result in an additional 0.8 QALY per 100 patients over the 5-year period.
Table 5 presents the discounted 5-year costs associated with each of the three management alternatives. Although the increased frequency of testing that occurs with anticoagulation clinic testing (23 vs 14) drives up the costs of testing compared with those incurred in usual care, these cost increases are more than offset with the savings due to avoided adverse events and their sequelae, making anticoagulation clinic testing a financially appealing alternative to usual care in terms of associated medical care costs. On the other hand, moving from anticoagulation clinic testing to patient self-testing is expected to result in an overall increase in medical care costs, as the savings due to reduced adverse events are not completely offset by the increased costs of testing.
Table 5.
Five-Year Costs per 100 Patients by Management Strategy*
| Management Strategy | Medical Care Costs | Patient and Caregiver Costs | All Costs |
|---|---|---|---|
| Usual care | $419,514 | $110,223 | $529,737 |
| Anticoagulation clinic testing | $405,560 | $240,110 | $645,671 |
| Patient self-testing | $526,014 | $96,713 | $622,727 |
All costs are reported in 1997 dollars
In addition to the direct medical care costs, we considered the costs incurred by patients and their caregivers in receiving care. Because of the increased frequency of testing (and the associated time and travel costs), an increase of almost $130,000 per 100 patients is realized in the costs incurred by patients and their caregivers when moving from usual care to anticoagulation clinic testing. On the other hand, because of the reduction in time and travel costs associated with patient self-testing, even with a substantial increase in the frequency of testing, an overall cost saving of over $140,000 per 100 patients would be realized when moving from anticoagulation clinic testing to patient self-testing.
The net result is that the discounted incremental cost-effectiveness ratios differ substantially depending on whether or not the costs incurred by patients and their caregivers are included. As illustrated in Table 6, when these costs are considered (“All Costs”), moving from usual care to anticoagulation clinic testing results in a cost-effectiveness ratio of $31,327 per avoided event (or $232,226 per QALY), and moving from anticoagulation clinic testing to patient self-testing results in an expected cost saving. However, when patient and caregiver costs are ignored, moving from usual care to anticoagulation clinic testing results in a cost saving, while moving from anticoagulation clinic testing to patient self-testing results in a cost-effectiveness ratio of $24,818 per avoided event (or $153,504 per QALY).
Table 6.
Cost-Effectiveness Ratios
| Ratio | Medical Care Costs | All Costs |
|---|---|---|
| Cost per Event | ||
| Usual care to anticoagulation clinic testing | Cost saving | $31,327 |
| Anticoagulation clinic testing to patient self-testing | $24,818 | Cost saving |
| Cost per QALY | ||
| Usual care to anticoagulation clinic testing | Cost saving | $232,226 |
| Anticoagulation clinic testing to patient self-testing | $153,504 | Cost saving |
Sensitivity Analyses
We conducted one-way sensitivity analyses for model assumptions including time spent outside therapeutic range, disability rates, the resource use associated with adverse events, the proportion of the disabled population that discontinued therapy, the resources required for anticoagulation management, the wage rate used to value patient and caregiver time, and the discount rate. In general, model findings of overall cost increases or savings were most sensitive to assumptions regarding time spent below and above therapeutic range. For example, a relatively small change in the time spent below and above therapeutic range (i.e., 4%–6%) led to a cost increase instead of a savings in medical care costs when moving from usual care to anticoagulation clinic testing. In addition, the model findings of cost increases or savings are sensitive to annual testing frequency. For example, if frequency of testing for patient self-testing increases to 57 times a year (base 52), then moving from anticoagulation testing to patient self-testing would no longer be cost saving when all costs are included, although the cost would still be a modest $608 per event avoided or $3,758 per QALY. Converseley, overall model results were not sensitive to the discount rate used (range, 0%–10%), or assumptions regarding the percentage of patients discontinuing therapy after a permanent disability (range, 20%–80%), the wage rate used to value patient and caregiver time (range, $11–$17 per hour), and the annual number of tests that would occur during routine visits (range, 4–14).
A multi-way sensitivity analysis, in which all assumptions within the model were allowed to vary within their specified ranges, confirmed overall results. Moving from usual care to anticoagulation clinic testing is likely to result in a reduction in the number of events (88% certainty), an increase in life years (79% certainty), and a decrease in disabled life years (80% certainty). Similarly, moving from anticoagulation clinic testing to patient self-testing will also result in a reduction in the number of events (91% certainty), an increase in life years (85% certainty), and a decrease in disabled life years (95% certainty). In addition, when only medical care costs are considered, results indicate that moving from usual care to anticoagulation clinic testing is likely to be cost saving (80% certainty), and when patient and caregiver costs incurred are included, moving from anticoagulation clinic testing to patient self-testing may result in a cost saving (48% certainty).
DISCUSSION
Consistent evidence exists that the more time a patient spends within his or her therapeutic range, the fewer events he or she has. The practical issues of how to manage patients most appropriately to keep them within therapeutic range, however, remain unclear. The establishment of anticoagulation clinics seems to be the current best answer, and a randomized trial evaluating their effectiveness is currently underway.2 Incorporating newer technologies, like capillary monitors, into the management process may further improve patient outcomes. This model addresses both the health and economic implications of more organized management of patients receiving chronic warfarin therapy: anticoagulation clinic testing and patient self-testing.
Model results illustrate the potential health benefits of such a move: over a 5-year period, moving from usual care to anticoagulation clinic testing would reduce the number of adverse events per 100 patients by 3.7, and moving from anticoagulation clinic testing to patient self-testing would result in a reduction of another 4.9 events.
The model also illustrates the importance of considering fully the economic impact to each of the different entities involved. From a medical care provider perspective, this model demonstrates that an anticoagulation clinic testing approach would provide a cost-effective, in fact a cost-saving, alternative to usual care. However, the burden that such an approach would place on patients and their caregivers cannot be ignored. The increased frequency of testing associated with anticoagulation clinic testing relative to usual care substantially increases the time- and travel-related costs to patients and their caregivers. Once these costs are included, patient self-testing becomes the most cost-effective alternative to usual care.
Two potential limitations of the model warrant reiteration. First, the adverse event rates used were based on a population that included relatively few patients with atrial fibrillation. Ideally, we would have based estimates of event rates on data derived from a population more reflective of those currently receiving warfarin therapy, that is, one with a larger percentage of patients with atrial fibrillation. However, we know of no database that includes thromboembolic and hemorrhagic event rates by either management alternative or time within therapeutic range for such a population. Second, although we are conducting a randomized clinical trial comparing time spent within and outside therapeutic range by different management alternatives, to date no such trial data exist. Therefore, we were forced to rely on observational data for modeling assumptions regarding time spent below, in, and above therapeutic range. Although model findings of cost savings are sensitive to these assumptions, the relative ranking of the alternatives generally is not. That is, almost without exception, anticoagulation clinic testing is the most cost-effective alternative to usual care when costs to patients and their caregivers are ignored, and once these costs are included, patient self-testing becomes the most clinically effective and cost-effective alternative.
Acknowledgments
The authors thank Stephan Fihn, Jorja Henikoff, Mary McDonell, and the members of the Warfarin Optimized Outpatient Follow-Up Study Group for their help and willingness to share data from their study.
Financial support for this work was received from Boehringer Mannheim Corp.
REFERENCES
- 1.Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993;95:315–28. doi: 10.1016/0002-9343(93)90285-w. [DOI] [PubMed] [Google Scholar]
- 2.Matchar DB, Samsa GP, Cohen SJ. Should we just let the anticoagulation service do it? The conundrum of anticoagulation for atrial fibrillation. J Gen Intern Med. 1996;11:768–70. doi: 10.1007/BF02598998. [DOI] [PubMed] [Google Scholar]
- 3.Chiquetter E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with routine medical care. Circulation. 1995;92:I–686. Abstract. [Google Scholar]
- 4.Cortelazzo S, Finazzi G, Viero P, Galli M, Remuzzi A, Parenzan L, et al. Thrombotic and hemorrhagic complications in patients with mechanical heart valve prosthesis attending an anticoagulation clinic. Thromb Haemost. 1993;69:316–20. [PubMed] [Google Scholar]
- 5.White RH, McKittrick T, Takakuwa J, et al. Management and prognosis of life-threatening bleeding during warfarin therapy. Arch Intern Med. 1996;156:1197–1201. [PubMed] [Google Scholar]
- 6.Ansell JE, Patel N, Ostrovsky D, Nozzolillo E, Peterson AM, Fish L. Long-term patient self-management of oral anticoagulation. Arch Intern Med. 1995;155:2185–9. [PubMed] [Google Scholar]
- 7.Hasenkam JM, Knudsen L, Kimose HH, et al. Practicability of patient self-testing or oral anticoagulant therapy by the international normalized ratio (INR) using a portable whole blood monitor. A pilot investigation. Thromb Res. 1997;85:77–82. doi: 10.1016/s0049-3848(96)00224-1. [DOI] [PubMed] [Google Scholar]
- 8.Bernardo A, Halhuber C. Long-term experience with patient self-management of oral anticoagulation. Ann Hematol. 1996;72:A62. Abstract. [Google Scholar]
- 9.Anderson DR, Harrison L, Hirsh J. Evaluation of a portable prothrombin time monitor for home use by patients who require long-term oral anticoagulant therapy. Arch Intern Med. 1993;153:1441–7. [PubMed] [Google Scholar]
- 10.Fihn SD, McDonell MB, Martin DC, et al. Risk factors for complications of chronic anticoagulation: a multicenter study. Ann Intern Med. 1993;118:511–20. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Fihn SD, Callahan CM, Martin DC, et al. The risk for and severity of bleeding complications in elderly patients treated with warfarin. Ann Intern Med. 1996;124:970–9. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb LK, Salem-Schatz S. Anticoagulation in atrial fibrillation. Does efficacy in clinical trials translate into effectiveness in practice? Arch Intern Med. 1994;154:1945–53. doi: 10.1001/archinte.154.17.1945. [DOI] [PubMed] [Google Scholar]
- 13.The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators The effect of low dose warfarin on the risk of stroke in patients with non-rheumatic atrial fibrillation. N Engl J Med. 1990;323:1505–11. doi: 10.1056/NEJM199011293232201. [DOI] [PubMed] [Google Scholar]
- 14.Connolly SJ, Laupacis A, Gent M, Roberts RS, Cairns JA, Joyner C. Canadian atrial fibrillation anticoagulation study. J Am Coll Cardiol. 1991;18:349–55. doi: 10.1016/0735-1097(91)90585-w. [DOI] [PubMed] [Google Scholar]
- 15.European Atrial Fibrillation Trial Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischemic attack or minor stroke. Lancet. 1993;342:1255–62. [PubMed] [Google Scholar]
- 16.The European Atrial Fibrillation Trial Study Group. Optimal oral anticoagulant therapy in patients with non-rheumatic atrial fibrillation and recent cerebral ischemia. N Engl J Med. 1995;333:5–10. doi: 10.1056/NEJM199507063330102. [DOI] [PubMed] [Google Scholar]
- 17.Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with non-rheumatic atrial fibrillation. Veterans Affairs stroke prevention in nonrheumatic atrial fibrillation investigators. N Engl J Med. 1992;327:1406–12. doi: 10.1056/NEJM199211123272002. [DOI] [PubMed] [Google Scholar]
- 18.Petersen P, Godtfredsen J, Boysen G, Anderson ED, Anderson B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989:175–8. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- 19.Stroke Prevention in Atrial Fibrillation Investigators. Stroke prevention in atrial fibrillation study. Circulation. 1991;84:527–39. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- 20.Stroke Prevention in Atrial Fibrillation Investigators. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: stroke prevention in atrial fibrillation II study. Lancet. 1994;343:687–91. [PubMed] [Google Scholar]
- 21.Palaretti G, Leal N, Cocchen S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT) Lancet. 1996;348:423–8. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer FJM, Rosendaal FR, Vandenbroucke JP, Briet E. Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch Intern Med. 1993;153:1557–62. doi: 10.1001/archinte.153.13.1557. [DOI] [PubMed] [Google Scholar]
- 23.White RH, McCurdy SA, von Mardensdorff H, Woodruff De, Jr., Leftgoff L. Home prothrombin time monitoring after the initiation of warfarin therapy. Ann Intern Med. 1989;111:730–7. doi: 10.7326/0003-4819-111-9-730. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson PR, Wolfe CDA, Warburton FG, et al. A long-term follow-up of stroke patients. Stroke. 1997;28:507–12. doi: 10.1161/01.str.28.3.507. [DOI] [PubMed] [Google Scholar]
- 25.Bonita R, Solomon N, Broad JB. Prevalence of stroke and stroke-related disability. Estimates from the Auckland Stroke Studies. Stroke. 1997;28:1898–1902. doi: 10.1161/01.str.28.10.1898. [DOI] [PubMed] [Google Scholar]
- 26.Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Is the EuroQol a valid measure of health-related quality of life after stroke? Stroke. 1997;28:1876–82. doi: 10.1161/01.str.28.10.1876. [DOI] [PubMed] [Google Scholar]
- 27.Tennant A, Geddes JML, Fear J, Hillman M, Chamberlain MA. Outcome following stroke. Disabil Rehabil. 1997;19:278–84. doi: 10.3109/09638289709166539. [DOI] [PubMed] [Google Scholar]
- 28.Dighe MS, Aparasu RR, Rappaport HM. Factors predicting survival, changes in activity limitations, and disability in a geriatric post-stroke population. Gerontologist. 1997;37:483–9. doi: 10.1093/geront/37.4.483. [DOI] [PubMed] [Google Scholar]
- 29.Naglie G, Detsky AS. Treatment of chronic nonvalvular atrial fibrillation in the elderly: a decision analysis. Med Decis Making. 1992;12:239–49. doi: 10.1177/0272989X9201200401. [DOI] [PubMed] [Google Scholar]
- 30.Disch DL, Greenberg ML, Holzberger PT, Malenka DJ, Birkmeyer JD. Managing chronic atrial fibrillation: a Markov decision analysis comparing warfarin, quinidine, and low-dose amiodarone. Ann Intern Med. 1994;120:449–57. doi: 10.7326/0003-4819-120-6-199403150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Tsevat J, Eckman MH, McNutt RA, Pauker SG. Warfarin for dilated cardiomyopathy: a bloody tough pill to swallow? Med Decis Making. 1989;9:162–9. doi: 10.1177/0272989X8900900303. [DOI] [PubMed] [Google Scholar]
- 32.Seto TB, Taira DA, Tsevat J, Manning WJ. Cost-effectiveness of transesophageal echocardiographic-guided cardioversion: a decision analytic model for patients admitted to the hospital with atrial fibrillation. J Am Coll Cardiol. 1997;29:122–30. doi: 10.1016/s0735-1097(96)00448-2. [DOI] [PubMed] [Google Scholar]
- 33.Gage BF, Cardinalli AB, Albers GW, Owens DK. Cost-effectiveness of warfarin and aspirin for prophylaxis of stroke in patients with nonvalvular atrial fibrillation. JAMA. 1995;274:1839–45. [PubMed] [Google Scholar]
- 34.Sarent R, Wainwright E. Crystal Ball Version 4.0 User Manual. Broomfield, Colo: CG Press; 1996. [Google Scholar]
- 35.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press, Inc; 1996. [Google Scholar]
- 36.Ansell JE, Hamke AK, Holden A, Knapic N. Cost effectiveness of monitoring warfarin therapy using standard versus capillary prothrombin times. Am J Clin Pathol. 1989;91:587–9. doi: 10.1093/ajcp/91.5.587. [DOI] [PubMed] [Google Scholar]
- 37.US Bureau of the Census, Current Population Reports, Money Income in the United States: 1996 (With Separate Data on Valuation of Noncash Benefits) Washington, DC: US Government Printing Office; 1997. pp. 60–197. Table 7. [Google Scholar]
- 38.Murphy DJ, Williamson PS, Nease De., Jr Supportive family members of diabetic adults. Fam Pract Res J. 1994;14:323–31. [PubMed] [Google Scholar]
- 39.Mitchell JB, Ballard DJ, Whisnant JP, Ammering CJ, Samsa GP, Matchar DB. What role do neurologists play in determining the costs and outcomes of stroke patients? Stroke. 1996;27:1937–43. doi: 10.1161/01.str.27.11.1937. [DOI] [PubMed] [Google Scholar]
- 40.Holloway RG, Witter DM, Lawton KB, Lipscomb J, Samsa G. Inpatient costs of specific cerebrovascular events at five academic medical centers. Neurology. 1996;46:860. [PubMed] [Google Scholar]
- 41.Health Insurance Association of America . Source Book of Health Insurance Data, 1996. Washington, DC: 1997. pp. 854–60. [Google Scholar]