Abstract
OBJECTIVE
To estimate the cost-effectiveness of low-molecular-weight heparin (LMWH) in the treatment of proximal lower extremity deep venous thrombosis.
DESIGN
Cost-effectiveness analysis that includes the treatment of the index case and simulated 3-month follow-up.
SETTING
Acute care facility.
PATIENTS AND PARTICIPANTS
Hypothetical cohorts of 1,000 patients who present with proximal deep venous thrombosis.
INTERVENTIONS
Intravenous unfractionated heparin (UH), LMWH (40% at home, 60% in hospital), or selective UH/LMWH (UH for hospitalized patients and LMWH for patients treated at home).
MEASUREMENTS AND MAIN RESULTS
The outcomes were recurrent thrombosis, mortality, direct medical costs, and marginal cost-effectiveness ratios from the payer's perspective. At the base-case and under most assumptions in the sensitivity analysis, the LMWH and the selective UH/LMWH strategies dominate the UH strategy i.e., they result in fewer cases of recurrent thrombosis and fewer deaths, and they save resources. The savings occur primarily by decreasing the length of stay. The LMWH strategy resulted in lower costs as compared with the UH strategy when the proportion of patients treated at home was more than 14%. Treating 1,000 patients with the LMWH strategy as compared with the UH/LMWH strategy would result in 10 fewer cases of recurrent thrombosis, 1.2 fewer deaths, at an additional cost of $96,822; the cost-effectiveness ratio was $9,667 and $80,685 per recurrent thrombosis or death prevented, respectively.
CONCLUSIONS
Treatment with LMWH leads to savings and better outcomes as compared with UH in patients with lower extremity deep venous thrombosis. The selective UH/LMWH strategy is an alternative option.
Keywords: thromboembolism; thrombophlebitis; heparin, low molecular weight; heparin; cost-benefit analysis
Approximately 2 million new cases of venous thromboembolism occur each year in the United States.1 Among patients adequately treated, thromboembolism may recur in up to 30% at 8 years, and the postphlebitic syndrome may develop in up to 28% at 5 years.2 Hospitalization and treatment with high-dose unfractionated heparin (UH) is the standard treatment for patients with deep venous thrombosis and pulmonary embolism.1,3–7
Low-molecular-weight heparin (LMWH) is as effective as UH in the treatment of venous thromboembolism.8–14 In some studies, patients treated with LMWH had fewer episodes of recurrent thromboembolism, major bleeding, and heparin-induced thrombocytopenia.1,8–10,15
The LMWHs have recently been approved to treat venous thrombosis in the United States. In some European countries up to 24% of patients are treated with LMWH.2 Though LMWH is 5 to 10 times more expensive than UH, it has three principal advantages: LMWH is administered subcutaneously, monitoring its anticoagulation effect is not necessary in most patients,16 and it gives the potential for treating patients at home.11,12
The cost of treating all patients with LMWH poses a unique problem to hospitals reimbursed on an episode-of-care basis. Such hospitals have strong financial disincentives for the routine use of LMWH. However, a hospital or health care system at risk for the long-term costs of caring for a defined population in a capitated system would have an incentive to treat patients at home with LMWH. In the latter example, the concern is that managed care companies may impose pressure on physicians to treat patients at home who indeed require hospitalization. In both cases, patients who cannot be treated at home might receive UH as it minimizes costs. Institutions with fixed and separate budgets for pharmacy, hospital care, and home care may have difficulties deciding which heparin to use. In this example, the pharmacy has no interest in future costs and would suggest the least-expensive alternative. In any of these examples, the selective use of LMWH for patients eligible for home treatment may emerge as a good intermediate solution.
Economic evaluation within clinical trials has shown the benefits of the use of LMWH in the treatment of venous thrombosis.17–18 However, the care of patients participating in clinical trials may be so atypical that such results cannot be extrapolated to other settings.19–20 We use cost-effectiveness analysis (CEA)21–24 to understand the clinical benefit (or harm) and the cost (or savings) of different strategies in the treatment of deep venous thrombosis under usual clinical practice. In this work, we address the following questions: Is LMWH cost-effective in the treatment of deep venous thrombosis compared with UH? And if so, how does the selective use of UH for hospitalized patients or LMWH for patients treated at home compare with LMWH for all?
METHODS
Decision Model
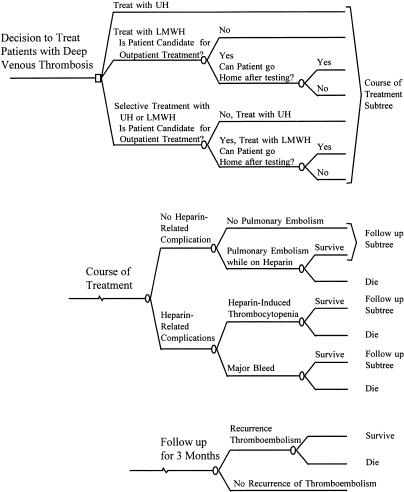
We constructed a decision model in which a cohort of 1,000 hypothetical patients with proximal deep venous thrombosis of the lower extremity could receive intravenous UH, or LMWH, or intravenous UH for patients who require hospitalization and LMWH for patients treated at home (selective UH/LMWH)(Fig. 1). We specified costs and benefits from the health care system (payer) perspective. We compared costs, recurrence of thromboembolism, death, and marginal cost-effectiveness ratios among the strategies.23 Marginal cost-effectiveness ratio is expressed as the net cost incurred (or saved) per each additional (or prevented) clinical outcome. This cost-effectiveness ratio is obtained by the formula: (Cost-Effectiveness Ratio) = (Net Cost)/(Net Outcome), where (Net Cost) = (Cost Treatment A) − (Cost Treatment B), and (Net Outcome) = (Outcome B) − (Outcome A). A treatment is “dominant” if it costs less and has better outcomes, which is the most desirable situation.
FIGURE 1.
Decision tree. UH indicates unfractionated heparin; LMWH, low-molecular-weight heparin.
During treatment with heparin, patients may develop pulmonary embolism, major bleeding, or heparin-induced thrombocytopenia for which they require a vena cava interruption procedure. Major bleed is defined as a decrease in hemoglobin of at least 2 g/dL, bleed located in the cranium, retroperitoneum, or prosthetic joint, or a bleed resulting in transfusion of at least 2 U of blood.3 We assume that some patients die from such complications. We assume that patients receive anticoagulants for 3 months (time period used on most trials). During this period, patients have a probability of developing a recurrent venous thrombosis and dying from it (primarily from pulmonary embolism). Estimates of values for the base case and the range for sensitivity analysis are shown in Table 1. In the base-case analysis, we assume that 40% of patients in the LMWH or selective UH/LMWH strategies are treated at home. In clinical trials, 35% to 70% of patients were eligible for home treatment.11–13 We assume that 30% of such patients are never hospitalized (36%–48% in research settings) and the rest stay in the hospital for 2.5 days (2.2 ± 3.8 days in research settings).11,12 We assume 6 days of treatment with UH or LMWH; in practice, patients received heparin for 5.5 to 6.5 days.11–13
Table 1.
Input Variables
| Input Variable* | Base-Case Estimate | Range for Sensitivity Analysis | Range in Published Studies | References |
|---|---|---|---|---|
| Recurrent thromboembolism within 3 mo | ||||
| Unfractionated heparin, % | 6.00 | 4–8 | 2.9–9.6 | (1,4,8–12,25,26) |
| LMWH, % | 4.40 | 3.2–6.1 | 0–6.9 | (4,8–12,25) |
| Relative risk LMWH/UH | 0.73 | 0.54–1.3 | (25,27) | |
| Pulmonary embolism during heparin treatment | ||||
| Unfractionated heparin, % | 0.60 | 0.5–1 | 0.4–1.9 | (11,12,28 |
| LMWH, % | 0.60 | 0.4–1.2 | — | Estimated |
| Relative risk LMWH/UH | 1.0 | 0.8–1.2 | — | Estimated |
| Heparin-related complications | ||||
| Major bleed† | ||||
| Unfractionated heparin, % | 4.00 | 3–8 | 0–15 | (1,3,4,8–12,15,29) |
| LMWH, % | 1.60 | 0.6–7.2 | 0–15 | (8–12,15) |
| Relative risk LMWH/UH | 0.40 | 0.2–0.9 | (8,27) | |
| Heparin-induced thrombocytopenia | ||||
| Unfractionated heparin, % | 1 | 0–2 | 1–3 | (1,4,15) |
| LMWH, % | 0 | 0–2 | < 1 | (1,10,15,30) |
| Relative risk LMWH/UH | 0 | 0–1 | — | Estimated |
| Mortality, % | ||||
| Pulmonary embolism | 5 | 1–20 | 1.5–21 | (1,28,31–36) |
| Heparin-induced thrombocytopenia | 5 | 0–50 | < 50 | (37–40) |
| Bleed | 0.10 | 0–5 | — | (15) |
| After recurrent thromboembolism | 8.80 | 5–14 | — | (28) |
| Duration of therapy, d | 6 | 5–10 | 5.5–6.5 | (11–13) |
| Duration of LMWH therapy in the hospital, d | 2.50 | 1–10 | — | (11) |
| Day of occurrence of heparin-induced complication | 4 | 3–7 | — | Estimated |
| Relative risk of recurrent thrombosis after a complication | 1.50 | 1–10 | < 37 | Estimated,30) |
| Eligibility for outpatient treatment, LMWH strategy, % | 40 | 0–70 | 35–70 | (11–13) |
| Patients never hospitalized, LMWH strategy, % | 30 | 0–50 | 36–48 | (11,12) |
| Cost of treatment,† | ||||
| Deep venous thrombosis | ||||
| Unfractionated heparin | 2,863 | 1,500–5,000 | 1,573–7,736 | (17,41–48) |
| LMWH, 6 d in the hospital | 3,144 | 2,500–5,000 | 1,030–3,102 | (17,41,42,49) |
| LMWH, 6 d at home | 1,540 | 1,000–2,000 | — | See Appendix |
| Pulmonary embolism | 6,760 | 5,000–15,000 | 6,072–12,004 | (43,46–48,50) |
| Major bleed | 3,660 | 2,000–10,000 | 1,812–11,214 | (17,43,46–48) |
| Heparin-induced thrombocytopenia | 3,520 | 2,000–10,000 | — | See Appendix |
LMWH indicates low molecular-weight heparin; UH, intravenous unfractionated heparin.
Associated with a decrease in hemoglobin of ≥2g/dL, retroperitoneal, intracranial, prosthetic joint, or if it resulted in transfusion of ≥2 U of blood.
All costs are expressed in 1996 U.S. dollars. See Appendix for detailed explanation of derivation of costs and range for sensitivity analysis. Data from literature were converted to 1996 U.S. dollars by applying the exchange rate for foreign currency (Federal Reserve Bank of St. Louis: http://www.stls.frb.org/fred/data/exchange.html) and using the Consumer Price Index for medical care (Bureau of Labor Statistics: http://stats.bls.gov/blshome.html).
Sensitivity Analysis
We varied all probabilities and cost estimates in the sensitivity analysis. Sensitivity analysis tests the robustness of conclusions to variations in base-case estimates.51 In one-way sensitivity analyses, the estimates were each modified, one at a time, while maintaining the other values at the base-case value. We also performed two-way sensitivity analysis and considered best-case and worst-case scenarios.
Probabilities
Estimates for recurrent thromboembolism, pulmonary embolism, death, major bleeding, and heparin-induced thrombocytopenia were obtained from meta-analyses8–10,25,27 and other reports (Table 1). We used the estimate from the Cochrane collaboration for the relative risk of recurrent thromboembolism at 3 months (relative risk [RR] 0.73) and extended the range in the sensitivity analyses (range, 0.54 to 1.3). More effective estimates have been reported by some (RR 0.40; 95% confidence interval [CI] 0.3–0.8),8 but not others (RR 0.85; 95% CI 0.63–1.14).27 We assume that the various LMWHs are similar in effectiveness.
Costs
We include direct medical costs to treat the initial episode of deep venous thrombosis, major bleed, heparin-induced thrombocytopenia, pulmonary embolism, and recurrent thromboembolism (see Table 1 and Appendix). To address the issue of cost shifting to patients, we assume that the direct cost of care at home is paid by the health system. Cost data were derived from cost accounting at our institution for each of the services rendered (charge data were not used for our estimates). Cost to Medicare was used as a proxy for physicians' fees for professional services. All monetary values are in 1996 U.S. dollars. We excluded the fixed costs of hospital care, focusing only on the relevant variable costs associated with the choice of drugs and dosage. Our cost estimates to treat an episode of deep venous thrombosis, major bleed, and pulmonary embolism are similar to those in the published literature. We used Data 3.0.17 to calculate cost-effectiveness data (TreeAge Software, Inc, Williamston, Mass).
Assumptions
We assume that recurrent thromboembolism occurs only once within 3 months. We do not include costs or outcomes associated with minor bleed, emergency department visits, and warfarin monitoring because we assume them to be equal among the strategies. We assume that the probability of death after a recurrent thromboembolism was the same between patients who did and patients who did not sustain a complication during the hospital stay (pulmonary embolism, bleed, and heparin-induced thrombocytopenia). Data support the assumption that mortality after a recurrent thromboembolism is higher in patients who present with pulmonary embolism,28,31 as compared with deep venous thrombosis of the legs. We assume that mortality was similar among treatment options. Some meta-analyses have shown survival advantage among LMWH-treated patients,8,27 and others have not.10,25 By not including these variables, we may underestimate the benefits of the LMWH. We assume that the cost of recurrent thrombosis is equal to the cost of treatment of the initial episode (additional costs incurred owing to pulmonary embolism modeled in the sensitivity analysis). We do not discount costs or outcomes as the outcomes are expected to occur within 3 months. Postphlebitic syndrome was not included as an outcome as it occurs beyond the time frame of our analysis and there are no long-term data to examine it.
Funding
An intra-institutional grant funded this project. No funds were provided from any of the companies that manufacture any of the heparins, and the authors do not hold stock in any such companies. None of the authors have received at any time fees for consulting or the like. Study design, analyses, and manuscript preparation were performed entirely by the authors.
RESULTS
Base Case
The base-case clinical outcomes and costs per 1,000 patients treated are shown in Table 2. The LMWH strategy dominates the UH strategy; i.e., 16.7 fewer cases of recurrent thromboembolism and 2 fewer deaths occur, saving the health care system $310,765.
Table 2.
Base-Case Costs and Outcomes of Unfractionated Heparin (UH), Low-Molecular-Weight Heparin (LMWH), and Selective UH/LMWH in the Treatment of Deep Venous Thrombosis
| Strategy | |||
|---|---|---|---|
| Measure of Effectiveness | UH for all | LMWH for all | Selective UH/LMWH* |
| Estimates per 1,000 patients treated, $ | |||
| Cost, total | 3,203,329 | 2,892,564 | 2,795,742 |
| During index case | 3,040,187 | 2,775,095 | 2,662,779 |
| During follow-up (3 mo) | 163,142 | 117,469 | 132,963 |
| Recurrent thrombosis, n | 61.43 | 44.74 | 54.76 |
| Mortality, n | 6.2 | 4.2 | 5.4 |
| During index case | 0.8 | 0.3 | 0.6 |
| During follow-up (3 mo) | 5.4 | 3.9 | 4.8 |
| Cost-effectiveness as compared to UH for all† | |||
| Recurrent thrombosis | — | Dominant | Dominant |
| Mortality | — | Dominant | Dominant |
UH if hospitalized; LMWH if home treatment.
A strategy is dominant if it costs less and has better outcomes.
The selective UH/LMWH strategy also dominates the UH strategy; i.e., 6.7 fewer cases of recurrent thromboembolism and 0.8 fewer deaths occur, saving the health care system $407,587.
The LMWH strategy does not dominate the selective UH/LMWH strategy. It results in 10 fewer cases of recurrent thromboembolism and 1.2 fewer deaths, but at an additional cost to the health care system of $96,822. The cost-effectiveness ratios of the LMWH strategy as compared with the selective UH/LMWH strategy are $9,667 and $80,685 per recurrent thromboembolism or death prevented, respectively. Most of the savings occurred during the treatment of the index case by decreasing the length of stay.
Sensitivity Analysis
Under the assumption of equal effectiveness for recurrent thromboembolism, the LMWH strategy and the selective UH/LMWH strategies were $269,492 and $395,262 less expensive than the UH strategy (per 1,000 patients treated), respectively.
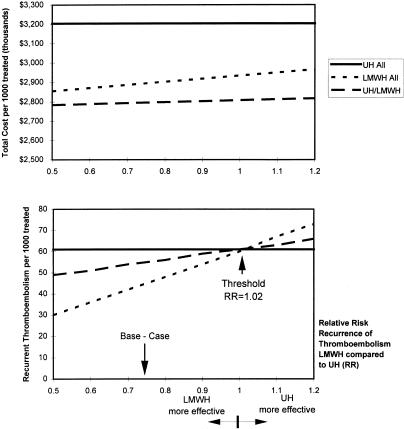
Recurrent thromboembolism and mortality were insensitive to any assumptions in one-way sensitivity analyses. The LMWH strategy always resulted in fewer cases of thromboembolism and deaths than the UH strategy or the selective UH/LMWH strategy. Threshold analysis showed that the LMWH strategy led to fewer cases of recurrent thromboembolism or deaths at relative risks of recurrent thromboembolism of less than 1.02Fig. 2) or less than 1.11, respectively. The total cost of treatment was insensitive to the relative risk of recurrent thromboembolism. The cost was higher for the UH strategy, followed by the LMWH strategy, and then by the selective UH/LMWH strategy Figure 2).
FIGURE 2.
Costs and outcomes of treatment with unfractionated heparin for all (UH), low-molecular-weight heparin for all (LMWH), and selective (UH/LMWH) according of relative effectiveness.
In one-way sensitivity analysis, the LMWH strategy always resulted in lower costs than the UH strategy, unless the proportion of eligible patients for home treatment was less than 14% (base case, 40%), or patients who received LMWH remained hospitalized for more than 6.5 days (base case, 2.5 days). In one-way sensitivity analysis, the LMWH strategy always resulted in higher costs than the selective UH/LMWH strategy, unless the cost of treatment with UH was more than $3,018 (base case, $2,376).
The costs of treatment and clinical outcomes per 1,000 patients treated in two-way sensitivity analysis are shown in Table 3. The LMWH strategy was dominant over the UH strategy when the relative risk of recurrent thromboembolism was less than 1 and the proportion of patients eligible for home therapy was as low as 14%. The LMWH strategy resulted in higher costs and fewer cases of recurrent thromboembolism or death than the selective UH/LMWH strategy.
Table 3.
Two-Way Sensitivity Analysis. Unfractionated Heparin (UH), Low-Molecular-Weight Heparin (LMWH), and Selective UH/LMWH
| Estimates per 1,000 Patients Treated | Treated at Home (%) | Relative Risk of Recurrent Thromboembolism at 3 Months, LMWH vs UH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1 | 1.1 | 1.2 | ||
| Cost of treatment (thousands), $ | |||||||||
| UH | 0 | 3,203 | 3,203 | 3,203 | 3,203 | 3,203 | 3,203 | 3,203 | 3,203 |
| LMWH | 70 | 2,505 | 2,519 | 2,533 | 2,547 | 2,561 | 2,575 | 2,588 | 2,602 |
| 40* | 2,854 | 2,870 | 2,886 | 2,902 | 2,918 | 2,934 | 2,950 | 2,966 | |
| 20 | 3,087 | 3,105 | 3,122 | 3,139 | 3,156 | 3,173 | 3,191 | 3,208 | |
| 10 | 3,204 | 3,222 | 3,240 | 3,257 | 3,275 | 3,293 | 3,311 | 3,329 | |
| Selective UH/LMWH | 70 | 2,470 | 2,478 | 2,487 | 2,495 | 2,503 | 2,512 | 2,520 | 2,528 |
| 40* | 2,784 | 2,789 | 2,794 | 2,799 | 2,803 | 2,808 | 2,813 | 2,818 | |
| 20 | 2,994 | 2,996 | 2,999 | 3,001 | 3,003 | 3,006 | 3,008 | 3,010 | |
| 10 | 3,099 | 3,100 | 3,101 | 3,102 | 3,103 | 3,105 | 3,106 | 3,107 | |
| Recurrent Thromboembolism, n | |||||||||
| UH | 0 | 61 | 61 | 61 | 61 | 61 | 61 | 61 | 61 |
| LMWH | 0–70 | 30 | 36 | 42 | 48 | 54 | 60 | 67 | 73 |
| Selective UH/LMWH | 40* | 49 | 51 | 54 | 56 | 59 | 61 | 63 | 66 |
| Mortality, n | |||||||||
| UH | 0 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| LMWH | 0–70 | 3 | 4 | 4 | 5 | 5 | 6 | 6 | 7 |
| Selective UH/LMWH | 40* | 5 | 5 | 5 | 6 | 6 | 6 | 6 | 6 |
Base-case assumptions.
DISCUSSION
Our analysis suggests that at the base case or at any given range of assumptions, treating patients with proximal deep venous thrombosis with LMWH will result in fewer cases of recurrent thromboembolism or deaths within 3 months as compared with the selective UH/LMWH strategy or the UH strategy. The LMWH and the selective UH/LMWH strategies dominated the UH strategy; i.e., they resulted in fewer adverse clinical outcomes and saved resources of the health care system. The decrease in costs originated primarily by a decrease in the length of stay.
Economic evaluation and clinical trials provide the best source of data regarding the efficacy of an intervention in research settings.19 Such economic analyses are able to measure directly the costs of interventions and outcomes. However, the care of patients participating in clinical trials may be so atypical that such results cannot be extrapolated to other settings.19 Differences in operational efficiency of systems and institutions participating in clinical trials may differ significantly from usual practice.20 Also, it may be difficult to decide whether other interventions, tests, or services are carried out because of the research protocol or the usual treatment.20 Confirming results from such economic evaluations with CEA may provide convergent validity on the use of LMWH.
The economic evaluation of treating patients with deep venous thrombosis with LMWH as compared with UH has been published for two clinical trials,17,18 one cost minimization study,41 and two cost-effectiveness analyses.52,53 Hull et al. showed that the LMWH strategy (tinzaparin, RR of recurrent thrombosis of 41%) dominates the UH strategy; i.e., 41 fewer cases of recurrent thromboembolism and 49 fewer deaths occur per 1,000 patients treated, saving the health care system $482,00017 (all patients hospitalized, data calculated from the report and converted to 1996 U.S. dollars by using the Consumer Price Index for medical care). The savings would have increased to $1,096,370 if 37% of patients had been treated outside the hospital setting. The second report showed a 64% reduction in cost (95% CI 56%–72%), primarily due to a 40% reduction in length of stay.18 The latter clinical trial evaluated nadroparin and found a nonsignificant 20% risk reduction of recurrent thrombosis.12 The cost-minimization study performed in Germany estimated savings of $1,687,820 per 1,000 patients treated with LMWH as compared with UH.41 The authors assumed similar efficacy and side effects, hospital stay of 2.7 days for all patients who receive LMWH and 8.1 days for patients receiving UH, and 15% of patients requiring a skilled nursing visit, and two visits to the physician. Gould et al. found that LMWH is highly cost-effective for the management of deep venous thrombosis.52 The LMWH strategy dominated the UH strategy; cost savings were realized at the same time as lower mortality. The authors used the societal perspective and the patient's lifetime as the analytic horizon. They assumed 30% of patients receiving LMWH at home and 25% discharged from the hospital after 3 days. The second cost-effectiveness analysis using the third-party payer and an analytic horizon of 3 months also showed that the LMWH strategy dominates the UH strategy.53
Our CEA extends the results of prior reports17,18,41,52,53 in several ways. First, by modeling the benefits and risks of LMWH using a different approach, we reach similar conclusions that the use of LMWH results in better outcomes at a lower cost; however, our estimates of savings are more conservative. Second, not every patient is a candidate for treatment at home with LMWH. The selective use of UH for hospitalized patients or LMWH for patients treated at home is an attractive option for institutions with limited pharmacy budgets, or until more data become available supporting the improved outcomes in patients who receive any of the LMWH. We chose to model three strategies and acknowledge that the selective UH/LMWH is a combined approach of the UH and LMWH strategies. The cost-effectiveness ratio of LMWH versus selective UH/LMWH reflects the differences between inpatient LMWH and inpatient UH (costs and health effects cancel out in the 40% of those in each cohort treated as outpatients with LMWH). And third, this CEA may allow consumers, policymakers, and administrators to model and understand the trade-offs between strategies. This is important when the relative effectiveness of interventions, percentage of patients eligible for treatment at home, operational efficiencies of institutions, and reimbursement structure vary under usual clinical practice. Our analysis is consistent with others.52,53
The potential improvement in clinical outcomes and cost savings are illustrated in the sensitivity analysis. Some studies have reported that the effectiveness and risks of using LMWH are similar to those of UH10–12; even under these equal effectiveness assumptions, the LMWH dominates the UH strategy. Savings should occur even when as few as 14% of patients who receive LMWH are treated at home (base case, 40%). Our findings do not change across a wide range of values used for relative risk of recurrent thromboembolism, major bleeding, heparin-induced thrombocytopenia, hospitalization rates, length of hospitalization, duration of therapy, and LMWH cost, among others.
Some aspects in the interpretation of this study are worth emphasizing. First, we assumed that all LMWHs are equally effective. We varied the effectiveness of the LMWH in the sensitivity analysis to include such uncertainty. Second, patients with suspected pulmonary embolism or recurrent thrombosis were not modeled to receive LMWH. Results of recent trials of LMWH in patients with deep venous thrombosis and pulmonary embolism are promising.13,14,54 Third, we assumed that mortality was similar among treatment options, and we may have underestimated the effectiveness of the LMWH. A recent meta-analysis showed a lower mortality among LMWH-treated patients (RR 0.71; 95% CI 0.53–0.94).27 However, no firm data exist on this survival advantage among patients treated at home. Finally, in research settings, 35% to 70% of patients were eligible for outpatient treatment and 36% to 48% of patients were never hospitalized,11–13 whereas we assumed 40% and 30%, respectively. Our assumptions would not apply to health care systems that are not prepared to care for these patients at home. Clinically unstable patients or patients at risk of developing pulmonary embolism, thrombocytopenia, or bleeding should still be hospitalized. The savings by decreasing the length of stay may seem obvious; however, cost shifting to ambulatory facilities and patients needs to be addressed before a widespread policy of treating patients at home is implemented.
In conclusion, LMWH appears to be the treatment of choice for patients with deep venous thrombosis in whom pulmonary embolism is not suspected. However, for patients who require hospitalization, the use of intravenous UH is an alternative option.
Acknowledgments
We thank Ms. Amy Jackson for preparing the manuscript, the Clinical Information and Support Office of the University Health Systems of Eastern North Carolina for providing cost data, and Drs. Diana Antonacci, James Byrd, and Wilhelmine Wiese for critically reviewing the paper.
Appendix
Estimation of Costs of Treatment Shown in Table 1
| 1. Cost to treat deep venous thrombosis, cost using unfractionated heparin = $2,863 per patient. Consisting of medication cost, $92 (cost and administration fees to pharmacy, 37,500 IU/d); hospital room, $2,297 (6 days of hospitalization); professional fee, $256 (initial and subsequent visits); and testing, $219 (activated partial thromboplastin time and cell blood count tests). This cost estimate is consistent with that reported in the literature, $1,573 to $7,736.17,41–45,49 Data from literature were converted to 1996 U.S. dollars by applying the exchange rate for foreign currency (Federal Reserve Bank of St. Louis, available at: http://www.stls.frb.org/fred/data/exchange.html) and using the Consumer Price Index for medical care (Bureau of Labor Statistics, available at: http://stats.bls.gov/blshome. html). The variability of estimates reported in the literature is due to the perspective and the inclusion of cost estimates for oral anticoagulants. |
| 2. Cost to treat deep venous thrombosis. Cost of using LMWH was estimated for treatment at the hospital and at home. Six days in the hospital = $3,144 per patient. Consisting of medication cost, $501 (cost to pharmacy including administration fees, 140 mg of enoxaparin or 16,800 IU of dalteparin, calculated for a 70-kg person at a dose of 1 mg/kg/12 h of enoxaparin, or 120 IU/kg/12 h of dalteparin); hospital room, $2,297; professional fee, $256 (initial and subsequent visits); and testing, $91 (cell blood count tests). Six days at home= $1,540 per patient. Consisting of medication cost, $501; hospital room, $0; professional fee, $148 (initial visit and teaching of how to use subcutaneous medication); testing, $91 (cell blood count tests); and home care, $801 (two skilled nursing visits, home care plan oversight by physician, one outpatient visit, and health aid visits). Based on these estimates, a daily rate for each was calculated and the costs were added. For example, at the base-case scenario the cost is $2,208. It was calculated as follows: ($3,144/6) × 2.5 + ($1,540/6) × 3.5 (duration of therapy of 2.5 in the hospital and 3.5 days at home for a total duration of therapy of 6 days). We did not include wages forgone. Our cost estimate is consistent with ones reported in the literature, $1,030 to $3,102.17,41,42,49 |
| 3. Cost to treat pulmonary embolism= $6,760. This consists of medications, $138 (mean dose of 40,000 IU/day of unfractionated heparin, 9 days); hospital room, $3,445; professional fee, $382 (initial and subsequent visits); blood testing, $155 (activated partial thromboplastin time and cell blood count tests); ventilation-perfusion scanning, $958; and Greenfield filter insertion, $1,682. Our estimate is similar to ones reported in the literature, $6,072 to $12,004.43,46,47,55,56 |
| 4. Cost to treat major bleed= $3,660. Consisting of hospital room, $1,531 (4 days of hospitalization); professional fee, $190 (initial and subsequent visits); blood testing, $117 (activated partial thromboplastin time and cell blood count tests); Greenfield filter insertion, $1,682; and blood transfusion, $140 (cost to hospital for 1 U of blood). The estimates reported in the literature of $1,812 to $11,21417,46,48 include additional diagnostic testing (endoscopy and imaging) and treatment. Our range for sensitivity analysis includes those costs. |
| 5. Cost to treat heparin-induced thrombocytopenia= $3,520 per patient, which is equal to the cost to treat a major bleed, except for the blood transfusion. The costs of other interventions or medications are included in the sensitivity analyses. |
REFERENCES
- 1.Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism: a statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996;93:2212–45. doi: 10.1161/01.cir.93.12.2212. [DOI] [PubMed] [Google Scholar]
- 2.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a “standard care” nomogram: a randomized controlled trial. Ann Intern Med. 1993;119:874–81. doi: 10.7326/0003-4819-119-9-199311010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hirsh J, Warkentin TE, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1998;114:489S–510S. doi: 10.1378/chest.114.5_supplement.489s. [DOI] [PubMed] [Google Scholar]
- 5.Hyers TM. Venous thromboembolism. Am J Respir Crit Care Med. 1999;159:1–14. doi: 10.1164/ajrccm.159.1.9803109. [DOI] [PubMed] [Google Scholar]
- 6.Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. Chest. 1998;114:561S–578S. doi: 10.1378/chest.114.5_supplement.561s. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg JS. Management of venous thromboembolism. N Engl J Med. 1996;335:1816–28. doi: 10.1056/NEJM199612123352407. [DOI] [PubMed] [Google Scholar]
- 8.Siragusa S, Cosmi B, Piovella F, Hirsh J, Ginsberg JS. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: results of a meta-analysis. Am J Med. 1996;100:269–77. doi: 10.1016/S0002-9343(97)89484-3. [DOI] [PubMed] [Google Scholar]
- 9.Lensing AW, Prins MH, Davidson BL, Hirsh J. Treatment of deep venous thrombosis with low-molecular-weight heparins. A meta-analysis. Arch Intern Med. 1995;155:601–7. [PubMed] [Google Scholar]
- 10.Leizorovicz A, Simonneau G, Decousus H, Boissel JP. Comparison of efficacy and safety of low molecular weight heparins and unfractionated heparin in initial treatment of deep venous thrombosis: a meta-analysis. BMJ. 1994;309:299–304. doi: 10.1136/bmj.309.6950.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med. 1996;334:677–81. doi: 10.1056/NEJM199603143341101. [DOI] [PubMed] [Google Scholar]
- 12.Koopman MM, Prandoni P, Piovella F, et al. The Tasman Study Group. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared wtih subcutaneous low-molecular-weight heparin administered at home. N Engl J Med. 1996;334:682–7. doi: 10.1056/NEJM199603143341102. [DOI] [PubMed] [Google Scholar]
- 13.The Columbus Investigators. Low-molecular weight heparin in the treatment of patients with venous thromboembolism. N Engl J Med. 1997;337:657–62. doi: 10.1056/NEJM199709043371001. [DOI] [PubMed] [Google Scholar]
- 14.Simonneau G, Sors H, Charbonnier B, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. Tinzaparine ou Heparine Standard: Evaluations dans l'Embolie Pulmonaire. N Engl J Med. 1997;337:663–9. doi: 10.1056/NEJM199709043371002. [DOI] [PubMed] [Google Scholar]
- 15.Levine MN, Raskob G, Landefeld S, Kearon C. Hemorrhagic complications of anticoagulant treatment. Chest. 1998;114:511S–523S. doi: 10.1378/chest.114.5_supplement.511s. [DOI] [PubMed] [Google Scholar]
- 16.Weitz JI. Low-molecular weight heparins. N Engl J Med. 1997;337:688–98. doi: 10.1056/NEJM199709043371007. [DOI] [PubMed] [Google Scholar]
- 17.Hull RD, Raskob GE, Rosenbloom D, et al. Treatment of proximal vein thrombosis with subcutaneous low-molecular-weight heparin vs intravenous heparin: an economic perspective. Arch Intern Med. 1997;157:289–94. [PubMed] [Google Scholar]
- 18.van den Belt AG, Bossuyt PM, Prins MH, Gallus AS, Buller HR TASMAN Study Group. Replacing inpatient care by outpatient care in the treatment of deep venous thrombosis—an economic evaluation. Thromb Haemost. 1998;79:259–63. [PubMed] [Google Scholar]
- 19.Drummond MF, Davies L. Economic analysis alongside clinical trials: revisiting the methodological issues. Int J Technol Assess Health Care. 1991;7:561–73. doi: 10.1017/s0266462300007121. [DOI] [PubMed] [Google Scholar]
- 20.Ellwein LB, Drummond MF. Economic analysis alongside clinical trials: bias in the assessment of economic outcomes. Int J Technol Assess Health Care. 1996;12:691–7. doi: 10.1017/s0266462300010977. [DOI] [PubMed] [Google Scholar]
- 21.Sox HC, Blatt MA, Higgins MC, Marton KI. Medical Decision Making. Boston, Mass: Butterworths; 1988. [Google Scholar]
- 22.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 23.Haddix AC, Teutsch SM, Shaffer PA, Dunet DO. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 24.Jobe AC, Mansfield CJ. Health policy and economics. In: Mengel MB, Holleman WL, editors. Fundamentals of Clinical Practice: A Textbook on the Patient, Doctor, and Society. New York, NY: Plenum Medical Book Company; 1997. pp. 297–322. [Google Scholar]
- 25.van den Belt AG, Prins MH, Lensing AWA, et al. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism (Cochrane Review) UK: Oxford; 1998. In: The Cochrane Library, Issue 3 Update Software. [DOI] [PubMed] [Google Scholar]
- 26.Hull RD, Raskob GE, Hirsh J, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1986;315:1109–14. doi: 10.1056/NEJM198610303151801. [DOI] [PubMed] [Google Scholar]
- 27.Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis: a meta-analysis of randomized, controlled trials. Ann Intern Med. 1999;130:800–9. doi: 10.7326/0003-4819-130-10-199905180-00003. [DOI] [PubMed] [Google Scholar]
- 28.Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA. 1998;279:458–62. doi: 10.1001/jama.279.6.458. [DOI] [PubMed] [Google Scholar]
- 29.Hommes DW, Bura A, Mazzolai L, Buller HR, ten Cate JW. Subcutaneous heparin compared with continuous intravenous heparin administration in the initial treatment of deep vein thrombosis: a meta-analysis. Ann Intern Med. 1992;116:279–84. doi: 10.7326/0003-4819-116-4-279. [DOI] [PubMed] [Google Scholar]
- 30.Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332:1330–5. doi: 10.1056/NEJM199505183322003. [DOI] [PubMed] [Google Scholar]
- 31.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–5. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 32.Goldhaber SZ. Pulmonary embolism. N Engl J Med. 1998;339:93–103. doi: 10.1056/NEJM199807093390207. [DOI] [PubMed] [Google Scholar]
- 33.Lilienfeld DE, Godbold JH, Burke GL, Sprafka JM, Pham DL, Baxter J. Hospitalization and case fatality for pulmonary embolism in the twin cities: 1979-1984. Am Heart J. 1990;120:392–5. doi: 10.1016/0002-8703(90)90085-c. [DOI] [PubMed] [Google Scholar]
- 34.Siddique RM, Siddique MI, Rimm AA. Trends in pulmonary embolism mortality in the US elderly population: 1984 through 1991. Am J Public Health. 1998;88:478–80. doi: 10.2105/ajph.88.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen VS, Pollak EW. Fatal pulmonary embolism in cancer patients: is heparin prophylaxis justified? South Med J. 1980;73:841–3. doi: 10.1097/00007611-198007000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Kniffin Wd, Jr, Baron JA, Barrett J, Birkmeyer JD, Anderson Fa., Jr The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med. 1994;154:861–6. [PubMed] [Google Scholar]
- 37.AbuRahma AF, Boland JP, Witsberger T. Diagnostic and therapeutic strategies of white clot syndrome. Am J Surg. 1991;162:175–9. doi: 10.1016/0002-9610(91)90183-e. [DOI] [PubMed] [Google Scholar]
- 38.Stanton Pe, Jr, Evans JR, Lefemine AA, et al. White clot syndrome. South Med J. 1988;81:616–20. doi: 10.1097/00007611-198805000-00018. [DOI] [PubMed] [Google Scholar]
- 39.King DJ, Kelton JG. Heparin-associated thrombocytopenia. Ann Intern Med. 1984;100:535–40. doi: 10.7326/0003-4819-100-4-535. [DOI] [PubMed] [Google Scholar]
- 40.Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996;101:502–7. doi: 10.1016/s0002-9343(96)00258-6. [DOI] [PubMed] [Google Scholar]
- 41.Osterkorn D, Schramm W, Szucs T. Health care economics evaluation of inpatient treatment of venous thrombosis with unfractionated heparin versus subcutaneous low molecular weight heparin at home. Med Klin. 1996;91:607–9. [PubMed] [Google Scholar]
- 42.Teo CP, Lim HL, Kueh YK. Cost effectiveness and ease of administration of low molecular weight heparin in deep vein thrombosis. Thromb Haemost. 1994;72:328–9. Letter. [PubMed] [Google Scholar]
- 43.Sarasin FP, Eckman MH. Management and prevention of thromboembolic events in patients with cancer-related hypercoagulable states: a risky business. J Gen Intern Med. 1993;8:476–86. doi: 10.1007/BF02600108. [DOI] [PubMed] [Google Scholar]
- 44.Hull RD, Feldstein W, Pineo GF, Raskob GE. Cost effectiveness of diagnosis of deep vein thrombosis in symptomatic patients. Thromb Haemost. 1995;74:189–96. [PubMed] [Google Scholar]
- 45.Oudkerk M, van Beek EJ, van Putten WL, Buller HR. Cost-effectiveness analysis of various strategies in the diagnostic management of pulmonary embolism. Arch Intern Med. 1993;153:947–54. [PubMed] [Google Scholar]
- 46.Hillner BE, Philbrick JT, Becker DM. Optimal management of suspected lower-extremity deep vein thrombosis: an evaluation with cost assessment of 24 management strategies. Arch Intern Med. 1992;152:165–75. [PubMed] [Google Scholar]
- 47.Bergqvist D, Jendteg S, Johansen L, Persson U, Odegaard K. Cost of long-term complications of deep venous thrombosis of the lower extremities: an analysis of a defined patient population in Sweden. Ann Intern Med. 1997;126:454–7. doi: 10.7326/0003-4819-126-6-199703150-00006. [DOI] [PubMed] [Google Scholar]
- 48.Hawkins DW, Langley PC, Krueger KP. Pharmacoeconomic model of enoxaparin versus heparin for prevention of deep vein thrombosis after total hip replacement. Am J Health Syst Pharm. 1997;54:1185–90. doi: 10.1093/ajhp/54.10.1185. [DOI] [PubMed] [Google Scholar]
- 49.Lloyd AC, Aitken JA, Hoffmeyer UK, Kelso EJ, Wakerly EC, Barber ND. Economic evaluation of the use of nadroparin in the treatment of deep-vein thrombosis in Switzerland. Ann Pharmacother. 1997;31:842–6. doi: 10.1177/106002809703100705. [DOI] [PubMed] [Google Scholar]
- 50.Bergqvist D, Jendteg S, Lindgren B, Matzsch T, Persson U. The economics of general thromboembolic prophylaxis. World J Surg. 1988;12:349–55. doi: 10.1007/BF01655669. [DOI] [PubMed] [Google Scholar]
- 51.Udvarhelyi IS, Colditz GA, Rai A, Epstein AM. Cost-effectiveness and cost-benefit analyses in the medical literature: are the methods being used correctly? Ann Intern Med. 1992;116:238–44. doi: 10.7326/0003-4819-116-3-238. [DOI] [PubMed] [Google Scholar]
- 52.Gould MK, Dembitzer AD, Sanders GD, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A cost-effectiveness analysis. Ann Intern Med. 1999;130:789–99. doi: 10.7326/0003-4819-130-10-199905180-00002. [DOI] [PubMed] [Google Scholar]
- 53.Rodger M, Bredeson C, Wells PS, Beck J, Kearns B, Huebsch LB. Cost-effectiveness of low-molecular-weight heparin and unfractionated heparin in treatment of deep vein thrombosis. CMAJ. 1998;159:931–8. [PMC free article] [PubMed] [Google Scholar]
- 54.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338:409–15. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 55.Hull RD, Feldstein W, Stein PD, Pineo GF. Cost-effectiveness of pulmonary embolism diagnosis. Arch Intern Med. 1996;156:68–72. [PubMed] [Google Scholar]
- 56.Bergqvist D, Benoni G, Bjorgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335:696–700. doi: 10.1056/NEJM199609053351002. [DOI] [PubMed] [Google Scholar]