Abstract
OBJECTIVE
T o determine the best treatment strategy for the management of patients presenting with symptoms consistent with uncomplicated heartburn.
METHODS
We performed a cost-utility analysis of 4 alternatives: empirical proton pump inhibitor, empirical histamine2-receptor antagonist, and diagnostic strategies consisting of either esophagogastroduodenoscopy (EGD) or an upper gastrointestinal series before treatment. The time horizon of the model was 1 year. The base case analysis assumed a cohort of otherwise healthy 45-year-old individuals in a primary care practice.
MAIN RESULTS
Empirical treatment with a proton pump inhibitor was projected to provide the greatest quality-adjusted survival for the cohort. Empirical treatment with a histamine2receptor antagonist was projected to be the least costly of the alternatives. The marginal cost-effectiveness of using a proton pump inhibitor over a histamine2-receptor antagonist was approximately $10,400 per quality-adjusted life year (QALY) gained in the base case analysis and was less than $50,000 per QALY as long as the utility for heartburn was less than 0.95. Both diagnostic strategies were dominated by proton pump inhibitor alternative.
CONCLUSIONS
Empirical treatment seems to be the optimal initial management strategy for patients with heartburn, but the choice between a proton pump inhibitor or histamine2-receptor antagonist depends on the impact of heartburn on quality of life.
Keywords: cost-utility analysis, heartburn, proton pump inhibitor, histamine2-receptor antagonist, quality of life
Heartburn affects a large segment of the U.S. population; approximately 10,000,000 Americans suffer from heartburn at least once a month.1,2 Although over-the-counter antacid medications are often effective, a significant percentage of patients seek care for persistent heartburn, making it a common complaint in primary care.
Most heartburn patients have gastroesophageal reflux disease (GERD)3; this syndrome includes disease states ranging from severe esophagitis with Barrett's esophagus to symptoms of acid reflux in the absence of mucosal inflammation or evidence of pathologic reflux.1–3 Ideally, one should tailor GERD management to the severity of symptoms and degree of mucosal injury. Patients with severe esophagitis require lifelong antacid therapy,4–7 whereas those with mild disease often respond to lifestyle modification and symptom-based therapy.4
Among GERD patients, neither symptoms nor patient characteristics reliably predict the underlying severity of disease.4,8–10 As a result, primary care providers face 2 opposing management strategies for the patient with symptoms of heartburn. One approach uses an initial empirical trial of acid reduction, reserving diagnostic testing for those patients not responding to the therapeutic trial; the two drug classes used in empiric trials are proton pump inhibitors and histamine receptor antagonists. The other approach starts with a diagnostic test, establishing a firm diagnosis, then proceeds to diagnosis-directed management. Commonly used diagnostic tests are esophagogastroduodenoscopy and barium upper gastrointestinal series, and less commonly used is ambulatory 24-hour esophageal pH monitoring.
An optimal decision, for both patients and primary care providers, requires careful assessment of the cost and benefits associated with the competing management strategies. Decision analytic techniques allow for the creation of models that explicitly and systematically consider all the variations associated with a clinical question.11 In this project we included simultaneous consideration of diagnostic test performance, efficacy of competing treatment modalities, and the costs associated with each diagnostic and treatment procedure. This analysis may enable providers and patients to make informed decisions regarding the most cost-effective approach to the management of heartburn in the primary care setting. We sought to answer the following question: Is it cost-effective to perform a diagnostic procedure and tailor therapy to a specific diagnosis (e.g., proton pump inhibitor for severe esophagitis) rather than treating all patients empirically?
METHODS
To represent potential management strategies and related health outcomes of patients presenting with heartburn, we utilized a decision tree.12 A total of 4 alternatives were considered under the following two strategies: the empirical strategy, either an initial trial of a histamine2-receptor antagonist or a proton pump inhibitor and the diagnostic strategy, either an initial esophagogastroduodenoscopy or upper gastrointestinal series.
We simulated a cohort of otherwise healthy 45-year-old men. We chose this age group as it is representative of patients who are most likely to present with heartburn in the primary care setting.13 We assumed that in this cohort of patients there would be a 3% chance of having peptic ulcer disease; gastrointestinal cancer was not considered in this analysis. We limited the time horizon of the simulation model to 1 year, reflecting currently available data on healing and relapse rates in GERD management. We extended the time horizon to 24 months in the sensitivity analyses. Costs represent 1998 U.S. dollars. Effectiveness is presented as quality-adjusted life months (QALMs); patients in perfect health for a year accumulate a maximum of 12 QALMs (1 QALM per month). Marginal cost-effectiveness ratios were calculated by comparing the cost and effectiveness among all 4 alternatives. All tree modeling and analysis was completed on TreeAge Data 3.05, a customized software for decision analysis (TreeAge Software, Inc., Williamstown, MA, 1996).
Basic Elements of the Model
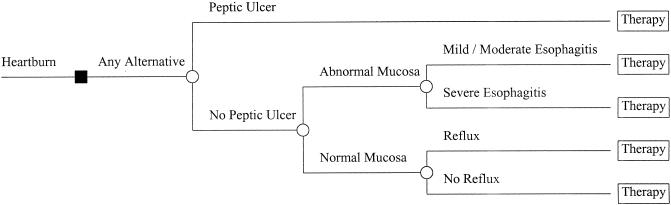
Five disease states were considered: peptic ulcer disease, severe esophagitis, mild/moderate esophagitis, heartburn with acid reflux, and heartburn without evidence of acid reflux (Fig. 1).
FIGURE 1.
Decision tree representation of disease states considered for all 4 alternatives considered in the model (proton pump inhibitor first, histamine2-receptor antagonist first, esophagogastroduodenoscopy first, and upper gastrointestinal series first).
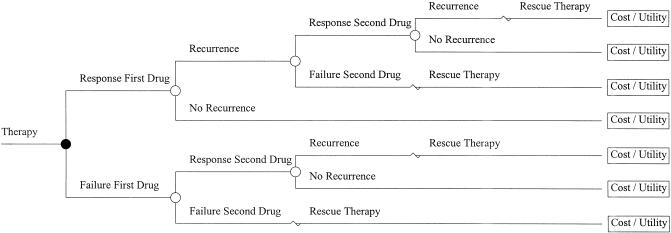
Two clinical responses were modeled: response to initial therapy (success or failure) and recurrence (yes or no). All cohort members were subjected to a two-drug sequential regimen (Fig. 2). A proton pump inhibitor at a standard dose always followed initiation of therapy with a histamine-receptor antagonist if initial therapy failed or recurrence occurred. Cohort members receiving a proton pump inhibitor first received the same agent at a higher dose in case of initial failure or later recurrence. Therapy in both diagnostic strategies was based on the results of the respective test. Both diagnostic tests were assumed to have perfect sensitivity and specificity for the diagnosis of peptic ulcer disease. Esophagogastroduodenoscopy was assumed to have perfect sensitivity and specificity for the presence of esophagitis including its severity. For the upper gastrointestinal series cohort, results were modeled (true or false positives, true or false negatives) based on the known test characteristic of this test.14,15 Cohort members in the esophagogastroduodenoscopy and upper gastrointestinal series diagnostic strategies were assumed to receive a histamine2-receptor antagonist first unless severe esophagitis was identified. Rescue therapy (after failure with two drugs) was assumed to be cisapride in addition to the most current successful drug. For all 4 alternatives, cohort members with a diagnosis of peptic ulcer disease were assumed to be treated with a 15-day course of omeprazole and clarithromycin.
FIGURE 2.
Decision tree representation of the two-drug sequence and the clinical events sequence.
Model Parameters
All parameter estimates are depicted in Table 1) together with the range of values used in the sensitivity analyses.
Table 1.
Probability, Cost, and Utility Estimates for the Model Parameters
| Parameter* | Value (range†) | References |
|---|---|---|
| Efficacy | ||
| Healing H2RA (normal) | 0.60 (0.50–0.70) | Expert opinion |
| Healing PPI (normal) | 0.80 (0.6–1.0) | 17–27 |
| Healing H2RA (Mild) | 0.45 (0.30–0.60) | 17–27 |
| Healing H2RA (severe) | 0.05 (0.02–0.20) | 17–27 |
| Healing PPI (mild) | 0.85 (0.75–0.95) | 17–27 |
| Healing PPI (severe) | 0.60 (0.40–0.80) | 17–27 |
| Healing H2RA (reflux) | 0.60 (0.45–0.75) | Expert opinion |
| Healing PPI (reflux) | 0.80 (0.60–1.0) | 17–27 |
| Healing H2RA (ulcer) | 0.95 | 32,33 |
| Healing PPI (ulcer) | 0.95 | 32,33 |
| Relapse H2RA (normal) | 0.10 (0.0–0.20) | Expert opinion |
| Relapse PPI (normal) | 0.00 (0.0–0.10) | Expert opinion |
| Relapse H2RA (mild) | 0.30 (0.10–0.50) | 5,27–29 |
| Relapse H2RA (severe) | 1.00 (0.90–1.0) | 5,27–29 |
| Relapse PPI (mild) | 0.05 (0.0–0.20) | 5,27–29 |
| Relapse PPI (severe) | 0.40 (0.20–0.60) | 5,27–29 |
| Relapse H2RA (reflux) | 0.15 (0.05–0.30) | Expert opinion |
| Relapse PPI (reflux) | 0.03 (0.0–0.20) | Expert opinion |
| Relapse H2RA (ulcer) | 0.60 | 32,33 |
| Relapse PPI (ulcer) | 0.60 | 32.33 |
| Epidemilogic | ||
| Mucosal injury | 0.45 (0.10–0.95) | 16 |
| Mild/moderate | 0.66 (0.50–0.80) | 16 |
| Severe | 0.34 (0.20–0.50) | 16 |
| Normal mucosa | 0.55 (0.20–0.60) | 9,10,16 |
| Heartburn with reflux | 0.34 (0.15-0.50) | 10,17 |
| Heartburn without reflux | 0.66 (0.50–0.80) | 10,17 |
| Peptic ulcer disease | 0.03 | 32,33 |
| Test characteristics | ||
| Sensitivity UGI | 0.70 (0.60–0.80) | 14,15 |
| Specificity UGI | 0.74 (0.65–0.85) | 14,15 |
| Costs (US$) | ||
| Monthly cisapride (brand) | 75.00 | Phone survey‡ |
| Monthly lansoprazole (brand) | 111.50 (94–127) | Phone survey |
| Monthly ranitidine (generic) | 65.00 (50–81) | Phone survey |
| Clarithromycin (brand; 2 wk) | 60.00 | Phone survey |
| Office visit | 39.00 | BC/BS§ |
| UGI | 150.00 (50–150) | BC/BS |
| Upper endoscopy | 1105.00 (300-1105) | BC/BS |
| 24-hour pH study | 646.00 (500-750) | BC/BS |
| Utilities | ||
| Well | 1.00 (0.92–1.0) | |
| Heartburn/regurgitation | 0.82 (0.75–0.95) | Panel |
| Ulcer | 0.79 | 33 |
H 2RA indicates histamine2-receptor antagonist; PPI, proton pump inhibitor; UGI, upper gastrointestinal series.
Range utilized in the sensitivity analysis.
Survey of 5 pharmacies in the Birmingham metropolitan area.
Blue Cross/Blue Shield of Alabama.
Probability Estimates
Main probability estimates for esophageal diseases were derived from a community-based study that systematically completed esophagogastroduodenoscopy in self-medicated healthy volunteers with episodic heartburn16,17; in this study, 45% of patients had evidence of mucosal injury, of which approximately two thirds exhibited mild or moderate esophagitis. Epidemiologic studies were used to estimate the probability of reflux among the 45% of patients without mucosal injury.10,17 Randomized clinical trials provided probability estimates of healing after 8 weeks18–28; we also used these studies to estimate the probability of different grades of esophagitis. Probability estimates for relapse rates were obtained from randomized studies of patients categorized as healed at endoscopy who were then allocated to maintenance therapy with a histamine2-receptor antagonist, proton pump inhibitor, or placebo.5,29–31 We also obtained the following probability estimates from the literature: healing rate on standard dose proton pump inhibitor following failure to heal with a histamine2-receptor antagonist,26,27 healing rate with high-dose proton pump inhibitor after failure to heal on standard-dose proton pump inhibitor,32 and healing and relapse of peptic ulcer disease managed exclusively with either a proton pump inhibitor or histamine2-receptor antagonist alone.33,34
We could not identify the following probability estimates in the literature: healing rate of high-dose proton pump inhibitor after symptom recurrence on standard-dose proton pump inhibitor; and recurrence rate on high-dose proton pump inhibitor after healing with that dose. We assumed that the healing rates with high-dose proton pump inhibitor after failure to heal on standard-dose proton pump inhibitor equaled the healing rates of standard-dose proton pump inhibitor following histamine2-receptor antagonist healing failure. We believe this is a reasonable assumption as available data on therapeutic failure with proton pump inhibitors correlate with inadequate acid suppression.35 Similarly, recurrence rates of high-dose proton pump inhibitor therapy were assumed to be identical to recurrence rates on standard-dose proton pump inhibitor, assuming that therapeutic success follows acid suppression.
Utility Estimates
Utility estimates for heartburn based on patient preferences do not exist in the published literature. As such, we obtained utility estimates for daily heartburn symptoms using a modification of the Delphi technique36; the consensus value was 0.82. Members of the panel included 2 gastroenterologists, 2 general internists, and a clinical psychologist with expertise in quality of life. Patients without heartburn were assigned a utility of 1.0 (perfect health) for the baseline analysis; this value was varied to 0.92 in the sensitivity analyses to reflect published data on individuals of similar age reporting no other health problems.37
Cost Estimates
We took the perspective of a third-party payer. Cost estimates for all procedures (i.e., esophagogastroduodenoscopy, upper gastrointestinal series, physician visit, and pH study) represent reimbursement from Alabama's Blue Cross/Blue Shield (BC/BS) for the corresponding procedures. The facility reimbursement figures were obtained from the billing office of the Hospital of the University of Alabama–Birmingham (UAB); the physician fees were obtained from the billing office of Health Services Foundation, the UAB faculty practice group.
We calculated the actual cost to BC/BS of prescription drugs using 3 steps: (1) survey of 5 local drugstores by telephone to obtain an estimate of the average retail price of all 3 medications in the Birmingham metropolitan area; (2) apply the full amount of the annual deductible for prescription toward the purchase of one of the 3 drugs; and (3) calculate the annual cost after the deductible is applied.
In the sensitivity analyses, we used the average wholesale price of the medications for the upper bound of medication costs. These latter estimates came from the computerized form of the 1998 Red Book.38 We also used the national purchasing prices for the Department of Veteran Affairs for the lower bound of medication costs.
Sensitivity Analyses
Sensitivity analyses assessed the impact of varying probability, cost, and utility values on the results. We allowed for a wider range of values around the parameter estimate for those variables for which no published data were available.
We also conducted Monte Carlo simulations, a special class of sensitivity analyses.39 For these simulations, we used a normal distribution for the following parameters: cost of omeprazole, cost of ranitidine, utility of heartburn, cost of upper gastrointestinal series, and cost of esophagogastroduodenoscopy; means and standard deviations were calculated after 1,000 iterations.
RESULTS
Cost-effectiveness for Base Case Model
Overall results from the cost-effectiveness analysis indicated that the empiric strategy had an alternative that was either least expensive (histamine2-receptor antagonist first) or more effective (proton pump inhibitor first) compared with either diagnostic strategy (Table 2).
Table 2.
Overall Results for the Baseline Analysis
| Strategy | Cost, $ | Effectiveness,QALMs* | Marginal Cost-Utility Ratio,$/QALY† |
|---|---|---|---|
| Empiric | |||
| Histamine2-receptor antagonist | 1,230 | 11.556 | |
| Proton pump inhibitor | 1,411 | 11.765 | 10,440 |
| Diagnostic | |||
| Upper gastrointestinal series | 1,598 | 11.570 | 305,000 |
| Esophagogastroduodenoscopy | 2,159 | 11.603 | 240,000 |
QALMs indicates quality-adjusted life months.
Dollars per additional quality-adjusted life years; calculated with reference to the histamine 2-receptor antagonist first alternative.
Within each strategy there were important advantages in either the cost or effectiveness among the competing alternatives. Within the empiric strategy, treatment with a histamine2-receptor antagonist first was projected to be less expensive but accrued fewer QALMs than the proton pump inhibitor alternative. The marginal cost-effectiveness (cost per additional unit of QALM) was $870 if a proton pump inhibitor was to be used over a histamine2-receptor antagonist (annualized figure $10,400 per additional quality-adjusted life year [QALY]). The strategy of using a proton pump inhibitor first dominated (was both more efficacious and less expensive than) the esophagogastroduodenoscopy and upper gastrointestinal series alternatives.
In the diagnostic strategy the esophagogastroduodenoscopy alternative was slightly more efficacious; however, the upper gastrointestinal series alternative was significantly less expensive. The annualized marginal cost-effectiveness of esophagogastroduodenoscopy compared with upper gastrointestinal series was $120,000 per QALY. The esophagogastroduodenoscopy first alternative was more effective but more expensive than the histamine2-receptor antagonist first strategy, while the upper gastrointestinal series alternative was more expensive and similarly efficacious. The annualized marginal cost-effectiveness of the esophagogastroduodenoscopy alternative compared with histamine2-receptor antagonist first was high, exceeding $100,000 per QALY.
Impact of Quality of Life and Clinical End Points on Cost-effectiveness
To estimate the impact of heartburn resolution on quality of life, we determined QALMs in the symptomatic state assuming no therapeutic intervention for the duration of the full model. As expected, the average QALMs in each alternative were identical (9.84). Compared with proton pump inhibitor first (11.76 QALMs), untreated patients would suffer approximately a 2-month decline in quality of life over a 1-year period. Even if we assumed no medical costs for those not treated (i.e., no outpatient visits), the marginal cost-effectiveness of a histamine2-receptor antagonist compared with no therapy would be $6,400 per QALY and that of a proton pump inhibitor would be $7,050 per QALY.
Our model used randomized trial data for healing and recurrence estimates. Using endoscopy healing as the main outcome generated these efficacy data. We approximated clinical efficacy by assuming a one-third reduction in recurrence rates when using symptom improvement as the outcome of interest.40 The overall increase in efficacy for each of the 4 alternatives was small (0.02 QALM or the equivalent of another 7 hours per month) when using symptoms as opposed to endoscopic outcomes.
Sensitivity Analyses
In the one-way sensitivity analyses, we found 2 cost parameters to which the model was sensitive: (1) the monthly cost of a proton pump inhibitor, and (2) the monthly cost of a histamine2-receptor antagonist. The proton pump inhibitor first alternative dominated under 2 cost circumstances: cost per month of omeprazole or lansoprazole less than $55, or histamine2-receptor antagonist greater than $76 per month. Use of prices (ranitidine at $4.20 compared with lansoprazole at $40 per month) from high-volume purchasers such as the Department of Veterans Affairs had an interesting effect on the results. The marginal cost-utility ratio of a proton pump inhibitor (lansoprazole) compared with a histamine2-receptor antagonist (ranitidine) decreased from $10,500 to $8,500 per QALY. This decrease reflected the large impact of a proton pump inhibitor in the management of patients with heartburn. When comparing monthly average wholesale prices of brand products for the most expensive proton pump inhibitor (omeprazole, $83.75) and the least expensive histamine2-receptor antagonist (famotidine, $39.00), we found an annualized marginal cost-effectiveness of $13,200 if the proton pump inhibitor was used first.
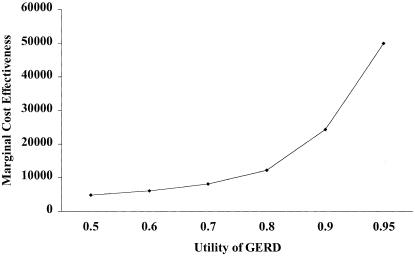
The model was relatively robust to changes in utility. Varying the utility from 0.50 to 0.95 did not change the rankings, in terms of cost-utility ratios, among the 4 alternatives modeled. However, at a utility of 0.75 for heartburn, the annualized marginal cost-effectiveness for lansoprazole with respect to ranitidine decreased to approximately $7,500 from $10,500 per QALY (Fig. 3). Of interest, the marginal cost-effectiveness remained below $50,000 per QALY with a utility value up to 0.95. From there on the curve is rather steep, with a marginal cost-effectiveness of $185,000 per QALY at a utility of 0.99.
FIGURE 3.
Sensitivity analysis on the marginal cost-effectiveness of the empiric alternatives (proton pump inhibitor vs histamine2-receptor antagonist) based on changes in the utility value for heartburn.
The histamine2-receptor antagonist strategy remained less expensive than proton pump inhibitor first when varying the following parameters: probability of having esophagitis, probability of having mild esophagitis, and healing and recurrence rates for both omeprazole and ranitidine.
None of the parameters related to either esophagogastroduodenoscopy or upper gastrointestinal series changed the results. Decreasing the cost of upper gastrointestinal series to $65, increasing the sensitivity to 0.99, or increasing the specificity to 0.99 did not make the upper gastrointestinal series first strategy more cost-effective than the histamine2-receptor antagonist first strategy. Even if esophagogastroduodenoscopy could be done for $300, the histamine2-receptor antagonist alternative remained less expensive.
Extending the time horizon of the model to 24 months yielded interesting results. The ranking for effectiveness remained unaltered (proton pump inhibitor best, histamine2-receptor antagonist worst), but the upper gastrointestinal series alternative became nearly equivalent in cost to using a proton pump inhibitor first. However, upper gastrointestinal series first remained more expensive than histamine2-receptor antagonist first while being of relatively equal effectiveness.
Results of the Monte Carlo simulations are depicted in Table 3. The rankings for both effectiveness and cost remained unchanged compared with the results obtained from the direct calculation of the model.
Table 3.
Results of Monte Carlo Simulation
| Cost (SD), $ | Utility (SD) | Marginal Cost-Utility Ratio,*$/QALY | |
|---|---|---|---|
| Histamine2-receptor antagonist | 1,195 (915) | 11.56 (0.27) | |
| Proton pump inhibitor | 1,412 (760) | 11.76 (0.26) | 13,000 |
| Upper gastrointestinal series | 1,516 (1065) | 11.58 (0.29) | 190,000 |
| Esophagogastroduodenoscopy | 2,145 (642) | 11.61 (0.25) | 230,000 |
Dollars per additional quality-adjusted life year; all comparisons against the histamine 2-receptor antagonist alternative.
DISCUSSION
Our decision analytic model indicated that an initial empiric trial of acid reduction therapy is the most cost-effective approach to a patient with newly diagnosed heartburn; of the 2 empirical strategies, a proton pump inhibitor was projected to provide greater quality-adjusted survival at a reasonable increased cost relative to a histamine2-receptor antagonist if heartburn was assigned a utility value less than or equal to 0.95. Among the 2 diagnostic strategies, upper gastrointestinal series was estimated to be the most cost-effective. Both diagnostic alternatives were estimated to be less efficacious and more expensive than the proton pump inhibitor alternative.
Use of a histamine2-receptor antagonist was estimated to be least effective of all alternatives considered in the model. This finding reflects the superior efficacy of a proton pump inhibitor in both the healing and maintenance phases of treatment for GERD. Faced with the dilemma of starting a histamine2-receptor antagonist or performing a diagnostic test, choosing either an upper gastrointestinal series or an esophagogastroduodenoscopy would cost over $100,000 per QALY. Most policymakers would agree that such cost is excessive compared with other accepted health care interventions (e.g., mammography for breast cancer screening).
Calculation of overall effectiveness by using clinical outcomes (i.e., symptom resolution) as compared with endoscopic outcomes for recurrence rates revealed an improvement of 0.02 QALM over the time horizon of the model. This arguably clinically unimportant improvement represents a ceiling effect of current therapy. In other words, therapy is already so successful (approximately 11.6–11.8 QALMs out of a maximum total of 12 QALMs) that small to moderate changes in recurrence rates are unlikely to have an important impact on overall effectiveness.
A primary research question in this study was the estimated impact of a diagnostic approach on patients' health-related quality of life. Health-related quality of life was represented by utility estimates derived through consensus methodology (using a modified Delphi procedure). In the present model, these surrogate utility estimates were conceptualized as the presence or absence of heartburn-related symptoms. Unfortunately, there are no empirical data on patient-reported utilities in heartburn populations. Existing health-related quality-of-life data in GERD populations have been based on health status measures (i.e., SF-36),41 which conceptually address the multidimensional nature of health-related quality of life, but cannot be used in decision analytic models. As such, the evaluation of patient-reported health-related quality of life in a manner that allows for utility values (i.e., standard gamble or time trade-off methodology) is needed to increase the precision of decision analytic models.
Exploration of the utility value in the sensitivity analyses yielded important information to aid clinicians in clinical practice. At very low utility values (0.50 and less), the proton pump inhibitor first strategy became more cost-effective than histamine2-receptor antagonist first, while at relatively high values (0.95 and greater) the cost per QALY for the proton pump inhibitor strategy became steep (see Fig. 3). This suggests that patients with mild and infrequent symptoms should be treated first with a histamine2-receptor antagonist; however, for those individuals in whom heartburn is disruptive on a daily basis, strong consideration should be given empiric proton pump inhibitor therapy.
The optimal duration of therapy for patients with heartburn is unknown. In patients with esophagitis, a strategy of treating only when symptomatic appears to be optimal for patients with grade 3 or less severe esophagitis, while patients with severe esophagitis would benefit from continued therapy.7 Our model assumed continuous therapy for all cohort members; this strategy resulted in overtreatment of approximately 85% of patients, based on the known 15% frequency of severe esophagitis. Therefore, the model overestimates the true costs associated with the use of a proton pump inhibitor first. The efficacy of using intermittent therapy with a histamine2-receptor antagonist is not known, but it is likely to create a larger number of individuals that require therapy owing to the lower efficacy of this agent compared with a proton pump inhibitor. As such, we believe that the overestimate in costs is smaller for a histamine2-receptor antagonist alternative.
Not surprisingly, medication cost was the most sensitive parameter in the sensitivity analysis. It was also the most unstable parameter as cost associated with either drug was highly dependent on the perspective of the analysis and the purchaser of the medication. Our telephone survey revealed a moderate cost differential between lansoprazole and ranitidine ($82.50 vs $45.50, respectively); for our base case analysis, use of a proton pump inhibitor first should be considered for patients with severe symptoms. However, from the perspective of the Department of Veterans Affairs, negotiated prices for ranitidine are low enough compared with omeprazole to have a significant impact on the annual pharmacy budget. In terms of pharmacy outlays, the Department of Veterans Affairs would have to pay an additional $180 per patient if a proton pump inhibitor were chosen routinely; this translates into approximately $1,800,000 per year for every 10,000 patients with heartburn treated with lansoprazole first. Although the marginal cost-effectiveness of proton pump inhibitor over histamine2-receptor antagonist remains reasonable (approximately $8,500 per QALY), constraints in resources make for difficult decisions among policymakers due to the large impact on choosing a proton pump inhibitor first.
The up-front cost of either diagnostic strategy (esophagogastroduodenoscopy or upper gastrointestinal series) was responsible for their unfavorable cost-effectiveness ratio compared with either a proton pump inhibitor or a histamine2-receptor antagonist first. The high clinical efficacy of either drug overshadowed the diagnostic stratification provided by either diagnostic test. Extending the time horizon to 24 months made the upper gastrointestinal series alternative cost equivalent to the proton pump inhibitor alternative, but the histamine2-receptor antagonist alternative remained significantly less expensive. To the extent that practitioners are compelled to treat symptoms, using a diagnostic test first will seldom be a reasonable strategy from a cost-effectiveness perspective unless other symptoms, such as dysphagia, accompany heartburn. However, if clinicians are willing to treat nonsevere esophagitis with intermittent proton pump inhibitor therapy and use a low-dose histamine2-receptor antagonist for patients with only reflux or heartburn symptoms without esophagitis or reflux, then the cost of testing might be justified.
A recently published decision analytic model has also concluded that empirical therapy should be considered as first-line therapy for patients suspected of having GERD.42 The approach of these authors differed from ours: they used a threshold model and not a cost-utility model; sequential drug therapy was not modeled; utilities were not considered in the modeling; uncertainty about the diagnosis of GERD was explicitly modeled; and GERD was modeled as one disease entity in contrast to our approach using 4 clinical states. Our approach helps us to better understand the role of quality of life and the consequences of a full spectrum of disease states underlying GERD on decision making. In addition, our model provides a framework that is usable by policy makers.
As with other decision analytic models, several cautions are warranted. First, this model provides guidance for patients with uncomplicated heartburn; individuals with concomitant weight loss or dysphagia might be harboring a malignant process and, as such, should be investigated vigorously from the onset. Second, we limited the time frame of the model to 2 years; it is plausible that with a longer follow-up period, the initial investment of a diagnostic test would be diluted amid all other medication and physician costs. Third, estimates of efficacy were derived from randomized clinical trial data. To the extent that patients in the community are not compliant, the efficacy estimates would be overestimated; however, we do not expect a substantial impact of noncompliance on the model results as both a proton pump inhibitor and a histamine2-receptor antagonist generally are well tolerated. Furthermore, it is unlikely that compliance differences between either drug would be substantial owing to their comparable safety and tolerability profile.
In summary, faced with a patient with heartburn, the clinician should consider the following issues. First, refrain from using a diagnostic test first unless the patient has worrisome symptoms. Second, gauge the impact of heartburn on quality of life: for patients with severe symptoms that affect daily living, the initial use of a proton pump inhibitor seems rational. For individuals with either intermittent or mild to moderate symptoms, the use of a histamine2-receptor antagonist would result in significant savings. Organizations that are high-volume purchasers might find the histamine2-receptor antagonist attractive as a result of the current price differential compared with a proton pump inhibitor.
REFERENCES
- 1.Richter JE. Long term management of gastroesophageal reflux disease and its complications. Am J Gastroenterol. 1997;92(4):30S–35S. [PubMed] [Google Scholar]
- 2.DeVault KR, Castell DO. Guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Arch Intern Med. 1995;155(13):2165–73. [PubMed] [Google Scholar]
- 3.Heading RC. Epidemiology of esophageal reflux disease. Scand J Gastroenterol. 1989;24(suppl 168):33–7. [PubMed] [Google Scholar]
- 4.Howden CW, Castell DO, Cohen S, et al. The rationale for continuous maintenance treatment of reflux esophagitis. Arch Intern Med. 1995;155:1465–71. [PubMed] [Google Scholar]
- 5.Vigneri S, Termini R, Leandro G, et al. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333(17):1106–10. doi: 10.1056/NEJM199510263331703. [DOI] [PubMed] [Google Scholar]
- 6.Klinkenberg-Knol EC, Festen HP, Jansen JB, et al. Long term treatment with omeprazole for refractory reflux esophagitis: efficacy and safety. Ann Intern Med. 1994;121(3):161–7. doi: 10.7326/0003-4819-121-3-199408010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Robinson M, Lanza F, Avner D, et al. Effective maintenance treatment of reflux esophagitis with low-dose lansoprazole. Ann Intern Med. 1996;124(10):859–67. doi: 10.7326/0003-4819-124-10-199605150-00001. [DOI] [PubMed] [Google Scholar]
- 8.Johnsson F, Joelsson B, Gudmundsson K, et al. Symptoms and endoscopic findings in the diagnosis of gastroesophageal reflux disease. Scand J Gastroenterol. 1987;22:714–8. doi: 10.3109/00365528709011148. [DOI] [PubMed] [Google Scholar]
- 9.Johnston BT, McFarland RJ, Collins JSA, et al. Symptom index as a marker of gastro-oesophageal reflux disease. Br J Surg. 1992;79:1054–5. doi: 10.1002/bjs.1800791022. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Richter JE, Bradley LA, et al. The symptom index: Differential usefulness in suspected acid-related complaints of heartburn and chest pain. Dig Dis Sci. 1993;38(8):1402–8. doi: 10.1007/BF01308595. [DOI] [PubMed] [Google Scholar]
- 11.Sox HC, Blatt MA, Higgins MC, et al. In: Medical Decision Making. Stoneham, Mass: Butterworths Publishing; 1988. [Google Scholar]
- 12.Gold MR, Siegel JE, Russell LB, et al. In: Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Publishing; 1996. [Google Scholar]
- 13.Spiro HM. Clinical Gastroenterology. 4th Ed. New York, NY: McGraw-Hill; 1993. [Google Scholar]
- 14.Thompson JK, Koehler RE, Richter JE. Detection of gastroesophageal reflux: value of barium studies compared with 24-hr pH monitoring. AJR. 1994;162:621–6. doi: 10.2214/ajr.162.3.8109509. [DOI] [PubMed] [Google Scholar]
- 15.Ott DJ. Gastroesophageal reflux: what is the role of barium studies? AJR. 1994;162(3):627–9. doi: 10.2214/ajr.162.3.8109510. [DOI] [PubMed] [Google Scholar]
- 16.Robinson M, Earnest D, Rodriguez-Stanley S, et al. Heartburn requiring frequent antacid use may indicate significant illness. Arch Intern Med. 1998;158:2373–6. doi: 10.1001/archinte.158.21.2373. [DOI] [PubMed] [Google Scholar]
- 17.Joelsson B, Johnsson F. Heartburn—the acid test. Gut. 1989;30:1523–5. doi: 10.1136/gut.30.11.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehn TC, Shepard HA, Colins-Jones D, et al. Double blind comparison of omeprazole (40mg qd) versus cimetidine (400 mg qd) in the treatment of symptomatic erosive reflux oesophagitis, assessed endoscopically, histologically and by 24 h pH monitoring. Gut. 1990;31:509–13. doi: 10.1136/gut.31.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinkenberg EC, Jansen JM, Festen HP, et al. Double-blind multicentre comparison of omeprazole and ranitidine in the treatment of reflux oesophagitis. Lancet. 1987:349–51. doi: 10.1016/s0140-6736(87)91726-0. [DOI] [PubMed] [Google Scholar]
- 20.Vantrappen G, Rutgeerts L, Schurmans P, et al. Omeprazole (40mg) is superior to ranitidine in short-term treatment of ulcerative reflux esophagitis. Dig Dis Sci. 1988;33(5):523–9. doi: 10.1007/BF01798351. [DOI] [PubMed] [Google Scholar]
- 21.Sandmark S, Carlsson R, Fausa O, et al. Omeprazole or ranitidine in the treatment of reflux esophagitis: results of a double-blind, randomized, Scandinavian multicenter study. Scand J Gastroenterol. 1988;23:625–32. doi: 10.3109/00365528809093923. [DOI] [PubMed] [Google Scholar]
- 22.Hatlebakk J, Berstad A, Carling L, et al. Lansoprazole versus omeprazole in short-term treatment of reflux oesophagitis: results of a Scandinavian multicentre trial. Scand J Gastroenterol. 1993;28:224–8. doi: 10.3109/00365529309096076. [DOI] [PubMed] [Google Scholar]
- 23.Hetzel D, Dent J, Reed W, et al. Healing and relapse of severe peptic esophagitis after treatment with omeprazole. Gastroenterology. 1988;95:903–12. doi: 10.1016/0016-5085(88)90162-x. [DOI] [PubMed] [Google Scholar]
- 24.Sontag S J, Hirschowitz BI, Holt S, et al. Two doses of omeprazole versus placebo in symptomatic erosive esophagitis: The U.S. Multicenter Study. Gastroenterology. 1992;102:109–18. doi: 10.1016/0016-5085(92)91790-b. [DOI] [PubMed] [Google Scholar]
- 25.Dent J, Hetzel DJ, Hetzel MA, et al. Evaluation of omeprazole in reflux oesophagitis. Scand J Gastroenterol. 1989;24(suppl 166):76–82. doi: 10.3109/00365528909091249. [DOI] [PubMed] [Google Scholar]
- 26.Robinson M, Campbell DR, Sontag S, et al. Treatment of erosive reflux esophagitis resistant to H2-receptor antagonist therapy: lansoprazole, a new proton pump inhibitor. Dig Dis Sci. 1995;40(3):590–7. doi: 10.1007/BF02064376. [DOI] [PubMed] [Google Scholar]
- 27.Feldman M, Harford W, Fisher R, et al. Treatment of reflux esophagitis resistant to H2-receptor antagonist with lansoprazole, a new H+/K+-ATPase inhibitor: a controlled, double-blind study. Am J Gastroenterol. 1993;88(8):1212–7. [PubMed] [Google Scholar]
- 28.Gough AL, Long RG, Cooper BT, et al. Lansoprazole versus ranitidine in the maintenance treatment of reflux oesophagitis. Ailment Pharmacol Ther. 1996;10:529–39. doi: 10.1046/j.1365-2036.1996.14156000.x. [DOI] [PubMed] [Google Scholar]
- 29.Dent J, Yeomans N, Mackinnon M, et al. Omeprazole v ranitidine for prevention of relapse in reflux oesophagitis: a controlled double blind trial of their efficacy and safety. Gut. 1994;35:590–8. doi: 10.1136/gut.35.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundell L, Backman L, Ekstrom P, et al. Prevention of relapse of reflux esophagitis after endoscopic healing: the efficacy and safety of omeprazole compared with ranitidine. Scand J Gastroenterol. 1991;26:248–56. doi: 10.3109/00365529109025038. [DOI] [PubMed] [Google Scholar]
- 31.Lundell L. Prevention of relapse of reflux oesophagitis after endoscopic healing: the efficacy and safety of omeprazole compared with ranitidine. Digestion. 1990;47(suppl 1):72–5. doi: 10.1159/000200522. [DOI] [PubMed] [Google Scholar]
- 32.Lind T, Havelund R, Carlsson R, et al. Effect of omeprazole (OME) 20 mg and 10 mg daily on heartburn in patients with endoscopy negative reflux disease (ENRD) treated on an on demand basis. Gastroenterology. 1996;110(suppl 6):A178. [Google Scholar]
- 33.Soll AH. Medical treatment of peptic ulcer disease. JAMA. 1996;275:622–9. [Google Scholar]
- 34.Walsh JH, Peterson WL. The treatment of Helicobacter pylori infection in the management of peptic ulcer disease. N Engl J Med. 1995;333:984–91. doi: 10.1056/NEJM199510123331508. [DOI] [PubMed] [Google Scholar]
- 35.Bell NJV, Hunt RH. Role of gastric acid suppression in the treatment of gastrooesophageal reflux disease. Gut. 1992;33:118–24. doi: 10.1136/gut.33.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feinstein AR. Clinimetrics. New Haven, Conn: Yale University Press; 1987. [Google Scholar]
- 37.Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 38. 1996 Drug Topics Red Book. Montvale, NJ: Medical Economics; 1996.
- 39.Doubilet P, Begg CB, Weinstein MC, et al. Probabilistic sensitivity analyses using Monte Carlo simulation. Med Decis Making. 1985;5:157–77. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 40.Harris RA, Kupperman M, Richter JE. Prevention of recurrences of erosive reflux esophagitis: a cost-effectiveness analysis of maintenance proton pump inhibition. Am J Med. 1997;102:78–88. doi: 10.1016/s0002-9343(96)00301-4. [DOI] [PubMed] [Google Scholar]
- 41.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, Mass: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 42.Sonnenberg A, Delco F, El-Serag H. Empirical therapy versus diagnostic tests in gastroesophageal reflux disease: a medical decision analysis. Dig Dis Sci. 1998;43:1001–8. doi: 10.1023/a:1018874516807. [DOI] [PubMed] [Google Scholar]