Abstract
Herpes simplex virus (HSV) infects dendritic cells (DC) efficiently but with minimal replication. HSV, therefore, appears to have evolved the ability to enter DC even though they are nonpermissive for virus growth. This provides a potential utility for HSV in delivering genes to DC for vaccination purposes and also suggests that the life cycle of HSV usually includes the infection of DC. However, DC infected with HSV usually lose the ability to become activated following infection (M. Salio, M. Cella, M. Suter, and A. Lanzavecchia, Eur. J. Immunol. 29:3245-3253, 1999; M. Kruse, O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer, J. Virol. 74:7127-7136, 2000). We report that for DC to retain the ability to become activated following HSV infection, the virion host shutoff protein (vhs) must be deleted. vhs usually functions to destabilize mRNA in favor of the production of HSV proteins in permissive cells. We have found that it also plays a key role in the inactivation of DC and is therefore likely to be important for immune evasion by the virus. Here, vhs would be anticipated to prevent DC activation in the early stages of infection of an individual with HSV, reducing the induction of cellular immune responses and thus preventing virus clearance during repeated cycles of virus latency and reactivation. Based on this information, replication-incompetent HSV vectors with vhs deleted which allow activation of DC and the induction of specific T-cell responses to delivered antigens have been constructed. These responses are greater than if DC are loaded with antigen by incubation with recombinant protein.
Herpes simplex virus (HSV) infects and enters a latent state in sensory neurons from which it can intermittently reactivate, resulting in symptoms of either cold sores (HSV type 1 [HSV-1]) or genital herpes (HSV-2) (reviewed in reference 28). This follows an initial peripheral infection in the skin or mucosa, where initial interaction with host defenses might be anticipated to occur. HSV, as well as infecting neurons, has also on a number of occasions been shown to infect dendritic cells (DC) at high efficiency (5, 15, 22, 30). This has suggested that HSV (i) naturally infects DC as part of its life cycle and (ii) might be used as an effective vector to deliver genes to DC. DC are the body's most potent antigen-presenting cells (2) and as such have attracted considerable interest for the development of antitumor and other vaccines following the “loading” of DC with tumor-specific antigens by various means (6, 16, 18, 20, 25, 26, 43). The development of efficient and nontoxic vectors for DC is therefore likely to improve the chances of DC-based immunotherapy for cancer or other diseases entering routine clinical use.
While it appears likely that HSV infects DC as part of its natural life cycle (5, 15, 22, 30), suggesting its use as a vector for these cells, it has also become apparent that HSV-infected DC are significantly functionally compromised by the infection process (15, 30). Thus, DC infected with HSV fail to become activated, down-regulate a number of surface markers, and fail to produce a number of cytokines in response to activation stimuli, such that their T-cell-activating capabilities are minimal (15, 30). HSV infection of DC would be expected to occur early in the infection process (i.e., following infection at the skin or mucosa, sites rich in DC). This early interaction might therefore reduce cellular immune responses which could otherwise prevent the virus entering latency or clear the virus during episodes of reactivation. It is thus likely that the highly efficient infection of DC possible with HSV is a result of an HSV immune avoidance mechanism which serves to minimize cellular immune responses to the virus.
While some of the effects of HSV resulting in DC inactivation have been noted previously (15, 30), the means by which HSV inactivates DC has not been identified. A number of HSV genes which are associated with immune system avoidance by the virus have been identified, including ICP47, which blocks antigen processing via inhibition of the transporter associated with antigen presentation (TAP) (10); glycoprotein C, which blocks complement activation (7); and gE/gI, which binds immunoglobulin G (13). However, none of these has been shown to be responsible for the prevention of activation of DC by HSV.
The work reported here identifies the HSV virion host shutoff protein (vhs) as playing a key role in the DC inactivation process. While vhs has been reported to have effects on the immune response to HSV (see Discussion), previous work has not identified vhs as being important for inactivating DC. Indeed, in the context of a replication-incompetent vector, which inactivates DC as effectively as wild-type virus, the further removal of vhs abrogates all the effects on surface marker expression and cytokine production otherwise seen. These marked effects of vhs deletion in DC are in contrast to effects in other cells, where the contribution of vhs to vector toxicity is minimal (14). Such vectors also allow the stimulation of significant antigen-specific in vitro T-cell responses, which has not previously been possible with HSV (15, 30).
MATERIALS AND METHODS
Viruses.
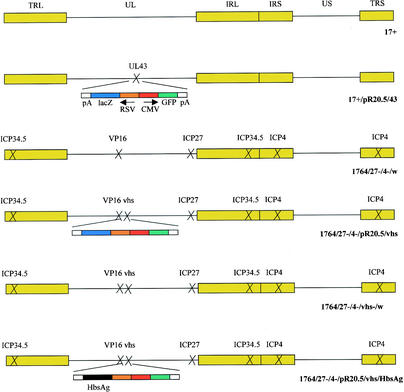
The majority of the viruses used in this study have been described elsewhere, and they are schematically represented in Fig. 1. All of the viruses are based on HSV-1 strain 17+ (GenBank file he1cg). Strain 17+/pR20.5/43 (38) contains a green fluorescent protein (GFP) and lacZ marker gene cassette inserted into the nonessential UL43 gene. Strains 17+ and 17+/pR20.5/43 were produced on BHK cells for this study. The other viruses are disabled so that they are replication incompetent and give only minimal HSV gene expression in noncomplementing cells (19), and they were grown on 27/12/M:4 cells, which complement the deficiencies in the viruses (39). Strain 1764/27−/4−/w has ICP34.5, ICP27, and ICP4 deleted and has an inactivating mutation in VP16 (1). Strain 1764/27−/4−/pR20.5/vhs also has vhs deleted (19). Virus strain 1764/27−/4−/vhs−/w was constructed by removal of the pR20.5 cassette from 1764/27−/4−/pR20.5/vhs using the plasmid pΔvhs, which completely removes the UL41 gene encoding vhs (nucleotides 91,168 to 92,642 were removed). Virus strain 1764/27−/4−/pR20.5/vhs/HbsAg contains the gene encoding hepatitis B virus surface antigen (HbsAg), excised from plasmid pHBV130 (9) with XhoI and NsiI, replacing the lacZ gene (excised with XbaI and EcoRI) in virus strain 1764/27−/4−/pR20.5/vhs constructed by standard techniques.
FIG. 1.
Viruses used in this study. In each case, the indicated genes have been deleted or inactivated. Further details are given in Materials and Methods.
DC preparation.
DC were prepared from peripheral blood as previously described (23, 29). Briefly, peripheral blood mononuclear cells were prepared from 60 ml of donor blood (including hepatitis B virus-vaccinated and unvaccinated individuals) using lymphoprep (Nycomed). After the removal of red cells, nonadherent cells were removed and collected for subsequent T-cell isolation. Adherent cells were cultured in RPMI medium supplemented with granulocyte-macrophage colony-stimulating factor (0.1 μg/ml) and interleukin 4 (IL-4) (0.05 μg/ml) and incubated for 7 days at 37°C and 5% CO2. After further lymphoprep purification, the cells were magnetically depleted (Dynal) using anti-CD19 (Harlan), anti-CD2 (Harlan), and anti-CD3 (Harlan) antibodies, and the DC were resuspended in complete RPMI medium for immediate use.
Isolation of CD4+ T cells.
Briefly, T and B cells were frozen at −80°C after isolation as described above. On day 8, the cells were defrosted and washed, and the T cells were magnetically isolated (Dynal) using anti-CD19, anti-CD14 (Serotec), and anti-HLA-DR (Serotec) monoclonal antibodies.
Infection of DC.
DC (5 × 105) were pelleted at 1,400 rpm for 5 min at room temperature. The DC were then infected at a multiplicity of infection (MOI) of 1, unless otherwise stated, by resuspension in 200 μl of RPMI medium containing 5 × 105 PFU of virus for 1 h at 37°C and 5% CO2. One milliliter of RPMI supplemented with granulocyte-macrophage colony-stimulating factor (0.1 μg/ml; Insight) and IL-4 (0.05 μg/ml; Insight) was then added, and the DC were incubated at 37°C and 5% CO2. For lipopolysaccharide (LPS) stimulation, RPMI also containing 100 ng of LPS (Sigma)/ml was added to the DC at the time of infection.
Trypan blue exclusion assay.
Ten microliters of DC suspension infected as described above was diluted in an equal volume of 0.04% trypan blue and observed immediately. White (live) cells and blue (dead) cells were then counted. The results presented are from experiments conducted three times with sample aliquots counted four times per sample.
Fluorescence-activated cell sorter (FACS) analysis.
DC were labeled with phycoerythrin-conjugated anti-immunoglobulin G1 (Serotec), CD40 (Insight), CD80 (Pharmingen), CD83 (Serotec), and CD86 (Serotec) by standard methods 24 h after mock infection or infection with various viruses (see Fig. 3), and the labeled cells were analyzed using a FACSCAN (Becton Dickinson, Mountain View, Calif.) and WinMDI software. HSV infection levels were measured using a polyclonal anti-HSV antibody (Dako).
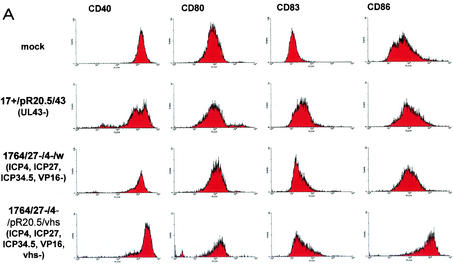
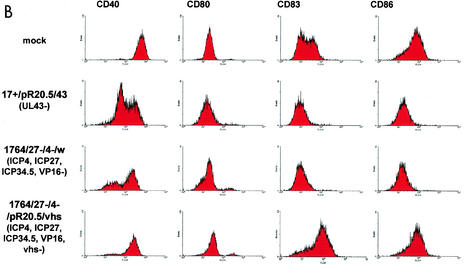
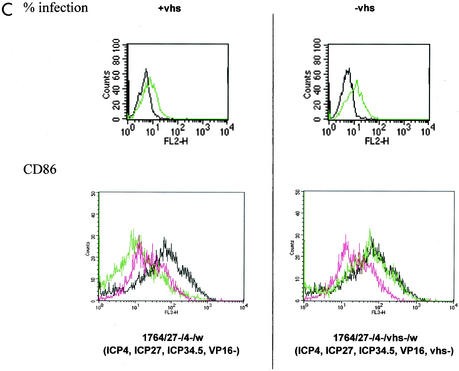
FIG. 3.
Effects of HSV and HSV mutants on DC surface marker expression with and without stimulation with LPS. (A and B) Levels of indicated surface markers were measured by FACS (see Materials and Methods) following infection of DC at an MOI of 1 with the indicated viruses or mock infection either without (A) or with (B) LPS stimulation. (C) Further analysis of the effect of vhs deletion; infection levels (green) and CD86 expression levels (black, LPS; green, virus plus LPS; pink, DC alone) with (+vhs; virus 1764/27−/4−/w) or without (−vhs; virus 1764/27−/4−/vhs−/w) vhs are compared. Details of the viruses used are given in Materials and Methods and in Fig. 1.
Cytokine analysis.
IL-6 and tumor necrosis factor alpha levels were measured in DC culture supernatants using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). Prior to ELISA, supernatants were collected 42 h after the infection of DC with various viruses (see Fig. 3) and stored at −20°C before use.
T-cell proliferation assays.
DC and CD4+ T cells were isolated from hepatitis B virus-vaccinated and unvaccinated individuals and treated as described above. The DC were used at dilutions from 105 to 104/ml, and the CD4+ T cells were used at a dilution of 106 cells/ml. Experiments at each DC concentration were performed in triplicate; 100 μl of DC and 100 μl of CD4+ T cells were added to each assay well. HbsAg (Austral) was added to the wells at a final concentration of 1 μg/well. HSV-1-infected (MOI = 1) and uninfected DC were cultured with CD4+ T cells for 6 days at 37°C and 5% CO2. [3H]thymidine (1 μCi/well; Amersham) was then added, and 18 h later, the cells were harvested and [3H]thymidine incorporation was measured.
RESULTS
HSV infects DC at high efficiency but without significant virus growth.
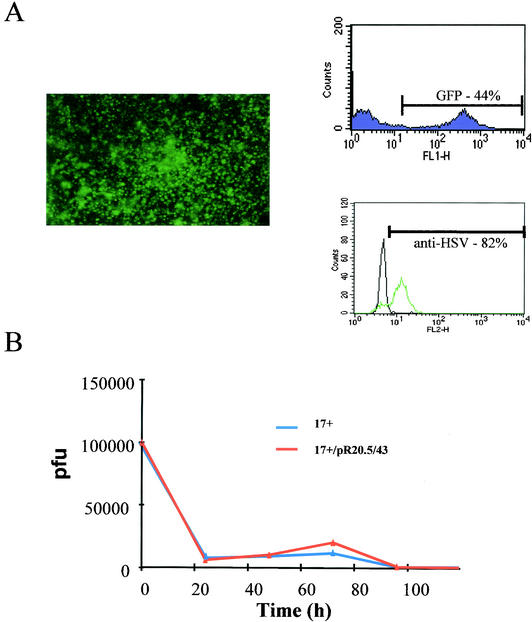
Figure 1 shows the viruses used in this study. Figure 2A shows that wild-type HSV with a GFP/lacZ insertion into UL43 infects human peripheral blood-derived DC at high efficiency at a low MOI of 1. UL43 is a nonessential gene which has previously been shown not to affect the kinetics of HSV reactivation and latency (21), and it is used here as a nondisabling site for gene insertion, as in a previous work (4). Infection rates of 40 to 50% are achievable at this low MOI, as measured by GFP expression. If cells are assessed by measurement of HSV antigen on their surfaces using a polyclonal anti-HSV antibody, ∼80% stain positive for infection. This suggests that <100% of the infected cells express detectable levels of GFP, and thus infection levels are higher than if the assessment is made on the basis of GFP fluorescence alone.
FIG. 2.
HSV infects human DC efficiently but with minimal replication. (A) DC photographed under UV fluorescence 24 h following infection with 17+/pR20.5/43 at an MOI of 1 (left) and analyzed by FACS for GFP (purple) and HSV antigen expression (green, infected cells; black, uninfected control) (right). (B) Growth curves of 17+ and 17+/pR20.5/43 in human DC infected at an MOI of 0.5. The yield (pfu) was determined by titration on BHK cells at the indicated time points.
Figure 2B shows that, while some growth of HSV in DC can be demonstrated, the growth burst which occurs ∼40 to 60 h after absorption is complete (60 to 80 h after infection) results in the production of fewer progeny virus than the original input virus used, and further bursts of virus growth do not then occur over time. Very similar results were found for the growth of strain 17+ (i.e., without the marker gene insertion in UL43 [Fig. 1]). Thus, while growth of HSV in DC has previously been reported (22), the replication level is very low and results in a steadily declining rather than increasing amount of virus in the culture over time. Overall, therefore, HSV appears to have evolved the ability to infect DC at high efficiency, as evidenced by either GFP expression or the presence of HSV antigen, even though significant virus growth does not occur.
HSV infection does not activate DC and blocks activation by LPS.
When DC infected with HSV are assessed for activation as measured by the expression of a number of surface markers (Fig. 3), several effects are observed. Without artificial stimulation (LPS treatment), CD40 expression levels are reduced and CD83 and CD86 expression levels remain constant compared to those in uninfected cells. These results are similar to those of previous work (15, 22, 30), showing that HSV infection per se does not activate DC. CD40 is a key T-cell activator, CD83 is a surface marker which is up-regulated during DC maturation and activation (although with an unknown function [42]), and CD86 (B7.1) is a potent T-cell costimulatory molecule which is also up-regulated on activated DC.
LPS treatment usually activates DC. This results in greatly elevated levels of CD83 and CD86 (Fig. 3B, mock). However, if DC are infected with HSV and treated with LPS, this does not occur, and CD40 levels are reduced even further than with HSV infection alone.
DC infected with HSV are therefore likely to be impaired in the ability to induce cellular immune responses, as they do not become activated by the infection process. Moreover, HSV infection also blocks activation of DC by other stimuli, such as treatment with LPS, as in this case. This suggests that HSV actively inhibits DC activation rather than having evolved so as not to activate DC itself.
Removal of HSV immediate-early (IE) genes increases DC survival following infection but does not prevent HSV-mediated inhibition of DC activation.
The effects described above and observed previously could have a number of causes. They could be due to a generalized toxic effect of HSV on DC function, particular HSV structural proteins which prevent activation of DC, or the expression of particular HSV genes in DC following infection. The effects observed could also be due to a combination of these possibilities.
In an attempt to reduce any generalized toxic effects on DC mediated by the expression of HSV genes, an HSV mutant in which IE gene expression has been reduced to very low levels was tested for its effects on DC survival and surface marker expression in comparison to the virus described above. This virus has ICP4, ICP27, and ICP34.5 deleted, has an inactivating mutation in VP16 (19), and expresses only very low levels of HSV proteins in cells which do not complement the ICP4, ICP27, and VP16 mutations. It has previously been shown that such HSV mutants, in which IE gene expression is minimized or prevented, are nontoxic in the cells so far tested (14, 31). The HSV virion itself is therefore generally nontoxic to cells in the absence of HSV gene expression.
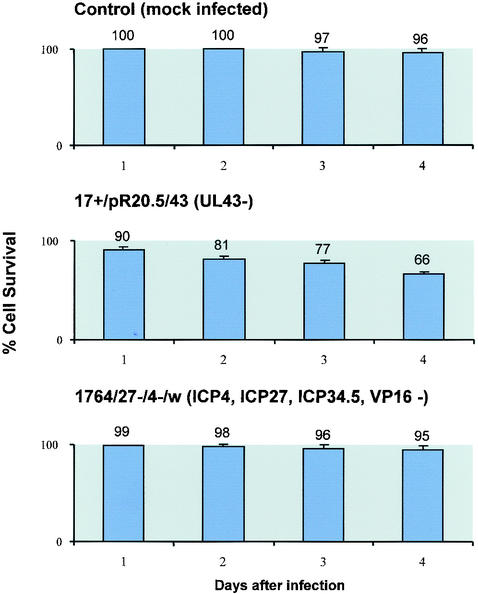
When effects on cell survival were observed using trypan blue staining following infection of DC with the virus with UL43 deleted or the IE gene-deficient virus, as expected, cell survival was improved with the IE gene-deficient virus (Fig. 4). Here, while only ∼66% of DC survived 96 h after infection with the virus with UL43 deleted, 96% of cells survived in mock-infected cultures compared to 95% in cultures infected with the IE gene-deficient virus. However, when effects on surface markers were assessed with or without LPS stimulation, the IE gene-deficient virus gave results generally similar to those with the virus with UL43 deleted (Fig. 3). Here, with or without the addition of LPS, no activation of the DC occurred (levels of CD40, CD80, CD83, and CD86 were not increased). While the virus with UL43 deleted reduced levels of CD40 with and without LPS, the IE gene-deficient virus reduced CD40 levels only when the DC were also treated with LPS. These results suggested that the effects on surface markers showing DC activation to be blocked by infection with HSV are not the result of a generalized toxic effect of HSV, as they would be expected to have been minimized through the use of the IE gene-deficient virus, as is the case in other cell types. It would appear likely, therefore, that the block to activation is either mediated through the expression of a specific HSV gene or genes in DC or due to effects of the HSV virion itself. Thus, either the expression of particular HSV genes must be activated in DC outside of the usual IE gene-regulated gene expression mechanism to cause the effects or, unlike in other cells, the toxic effects must be mediated by the virion itself.
FIG. 4.
Increased survival of DC infected with HSV IE gene mutants compared to that of DC infected with wild-type virus. DC were infected with the indicated viruses at an MOI of 1, and the percentage of surviving cells was assessed by trypan blue exclusion at the indicated times after infection. The error bars indicate standard error.
Removal of the HSV vhs protein allows activation of DC.
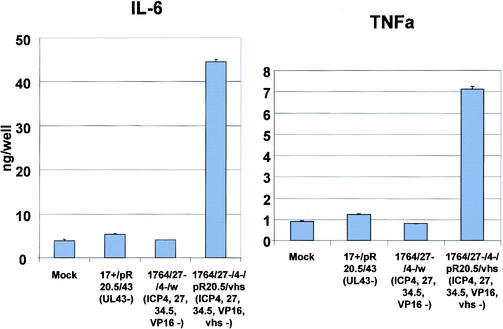
The vhs protein is contained within the HSV virion and functions to destabilize host RNA in favor of the translation of more rapidly produced viral RNAs during lytic infection (17, 34). Toxic effects of vhs have not previously been observed with replication-defective HSV mutants (14). However, as vhs is contained within the virion, we tested an IE gene-deficient mutant virus identical to the virus described above but with the additional removal of vhs (a lacZ/GFP insertion into vhs). This virus was used to infect DC, and the effects on surface marker expression levels were again assessed. This showed that the additional removal of vhs from an already IE gene-deficient virus prevented the block to LPS activation of DC which usually occurs following infection with HSV. Moreover, the virus itself, without LPS stimulation, at least partially activated DC, as evidenced by greatly increased levels of CD86 (Fig. 3A). Thus, in LPS-stimulated cells infected with the vhs− virus, the expression levels of CD40 and CD86 are essentially identical to those in uninfected, LPS-stimulated cells, and CD83 expression levels are greater than those in cells which are LPS stimulated alone (Fig. 3B). Moreover, in unstimulated cells, vhs− virus infection alone induces production of IL-6 and tumor necrosis factor alpha, also indicative of DC activation, which did not occur when vhs was not deleted (Fig. 5).
FIG. 5.
Cytokine secretion from HSV-infected DC. DC were infected with the indicated viruses, and cytokine levels were measured by ELISA 42 h later. TNFa, tumor necrosis factor alpha. The error bars indicate standard error.
The above-mentioned experiments were carried out with a virus in which a GFP/lacZ expression cassette had been inserted into vhs. It was thus possible that this insertion, rather than the inactivation of vhs itself, could have caused the effects observed. To address this, a further virus was produced in which vhs was deleted with no accompanying insertion, and this virus was tested for the ability to activate DC. As can be seen in Fig. 3C, the vhs− virus increases CD86 expression to levels similar to those caused by LPS treatment, whereas infection with an identical virus in which vhs was not deleted does not. A further possibility was that removal of vhs reduced the infection level of DC. This was tested using the vhs+ and vhs− viruses, and infection levels were found to be essentially identical (Fig. 3C).
The HSV vhs protein therefore appears to be a key mediator of the inactivating effects of HSV on DC. Removal of vhs from an already IE gene-deficient virus provides a virus which removes the usual HSV-mediated block to activation of DC by LPS.
HSV vectors with the IE gene and vhs deleted allow the stimulation of antigen-specific T-cell responses.
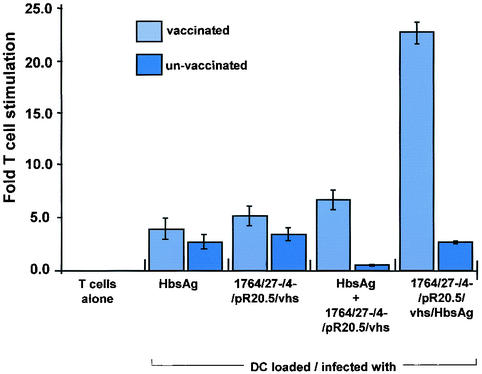
The results described above suggested that IE gene-deficient HSV vectors in which vhs is also removed might be used as vectors for DC due to their ability to infect DC at high efficiency and because the inactivating effects of HSV on DC would have been prevented. Indeed, DC infected with such HSV mutants appear to be at least partially activated in response to infection, as measured by CD86 up-regulation and the secretion of certain cytokines. To test whether HSV mutants with vhs deleted might be used to direct antigen-specific immune responses following the delivery of antigen-encoding genes to DC, experiments were performed using DC and T cells prepared from hepatitis B virus-vaccinated and unvaccinated individuals. Here, a virus was first constructed in which an HbsAg expression cassette was inserted into the vhs-encoding gene of the IE gene-deficient virus (Fig. 1). T-cell proliferation assays were then performed in which DC from vaccinated or unvaccinated individuals were either untreated, loaded with antigen by mixing them with recombinant HbsAg protein, infected with the vector containing the control marker gene, infected with the control vector and also mixed with recombinant HbsAg, or infected with the vector expressing HbsAg. The DC were then mixed with T cells derived from the vaccinated or unvaccinated individuals, respectively, and effects on T-cell proliferation observed.
These experiments showed (Fig. 6) that while HbsAg recombinant protein and the control HSV vector could induce a small T-cell-proliferative response in vaccinated individuals (the HSV response probably indicating proliferation of T cells specific to HSV structural proteins) and the control vector mixed with recombinant HbsAg could elicit a slightly greater response, the HbsAg-expressing vector induced a significantly greater response than any of these. Thus, delivery of HbsAg directly into DC using an HSV vector allows a specific T-cell-proliferative response to be induced which is greater than that achieved by mixing DC with recombinant antigen alone. While the results presented here are from a single HbsAg-vaccinated individual for whom the experiments were repeated three times, similar results have been obtained from three separate HbsAg-vaccinated individuals from whom DC and T cells have been prepared.
FIG. 6.
Antigen-specific T-cell responses can be generated following transduction of DC with vhs− HSV. T-cell proliferation was measured following infection of DC with the indicated viruses at an MOI of 1 or following mock infection and incubation with T cells isolated from hepatitis B virus-vaccinated or unvaccinated individuals. The error bars indicate standard error.
DISCUSSION
HSV has evolved to enter a latent state in infected individuals from which it can reactivate intermittently to cause disease. During these processes, the virus must avoid the host immune response, which might otherwise result in clearance of the virus either before latency has become established, during latency, or during reactivation. HSV initially infects the skin or mucosa, where interaction with the host immune system would first be anticipated to occur. DC are present in such tissues and are important inducers of cellular immune responses to incoming pathogens. It appears from the work described above and previously that HSV has not only evolved to infect DC at high efficiency but has also evolved efficient means to prevent their activation following infection (5, 15, 22, 30). The mediator of this inactivation had not previously been identified. We have shown that the HSV vhs protein, delivered to DC as part of the HSV virion, is important for this inactivation process to occur.
It thus appears that HSV encodes at least two proteins to minimize the induction of an adaptive immune response. ICP47 is expressed at IE times after the infection of a cell and inhibits the processing and presentation of antigens on the cell surface by inhibition of TAP (10). vhs, on the other hand, is a virion protein which appears (from this work) to be important in preventing the activation of DC. If DC were significantly activated following infection, a vigorous cellular immune response would be anticipated which might prevent the virus entering latency or result in clearance of the virus during reactivation.
The HSV vhs protein destabilizes mRNA in infected cells so that host protein synthesis is reduced in favor of translation from more rapidly produced viral mRNA (17, 34). Surprisingly therefore, the effects of the removal of vhs on virus growth in vitro are minimal (36), as are the effects on toxicity to cultured cells if vhs is removed from disabled HSV vectors (14). However, in vivo, vhs mutants are nonpathogenic, while they induce a robust immune response which is protective against subsequent wild-type virus challenge (41). Thus, we may speculate that these in vivo effects are due, at least in part, to the removal of vhs, allowing improved activation of DC and the induction of a more effective cellular immune response.
The UL41 gene encoding vhs has been identified on a number of occasions as having potential effects on the host immune response to HSV. However, none of this work has implicated vhs as being important for the interaction of HSV with DC and thus in minimizing cellular immune responses to the virus. Thus, vhs has been shown to aid the prevention of the transport of class 1 HLA molecules to the surfaces of infected fibroblasts and their recognition by cytotoxic T lymphocytes due to inhibition of de novo protein synthesis by vhs (11, 40). vhs deletion was also shown to increase the sensitivity of HSV-infected human embryonic fibroblast (HEL) cells to alpha and beta interferon and to increase levels of IL-1b, IL-8, and MIP-1a produced by infected HEL cells, U937 macrophage-like cells, and NB69 neuroblastoma cells (37).
DC are the most potent antigen-presenting cells and have been demonstrated to have great promise for the induction of immune responses to tolerized antigens (27, 32, 33). Such antigens are often present in cancer and chronic infectious disease so that an effective immune response is not mounted by the host, but this might be overcome if immune priming with disease-associated antigens could be mediated through DC.
A number of strategies aimed at antitumor vaccination have been used to deliver antigens to DC, with some success. These include the mixing of DC with tumor extracts or peptides derived from tumor antigen sequences (6, 18, 20, 25, 26, 43) and the direct fusion of DC with tumor cells (16). Adenovirus, alphavirus, and poxvirus vectors have also been used (3, 8, 24, 35, 44), although adenovirus requires high virus doses (MOI = 30 to 1,000), and toxicity results following infection of DC with alphavirus or poxvirus vectors (8, 12).
The discovery that HSV infects DC at high efficiency (5) suggested that HSV might be developed as a vector for these cells. However, DC infected with HSV, including replication-incompetent HSV vectors, generally become inactivated following infection and transduction (references 15, 22, and 30 and this work). The identification of vhs as a key mediator of this inactivation process has allowed the construction of HSV vectors which give high-level transduction of DC at low MOI and which also allow activation of the DC, which is necessary for the induction of an effective immune response. As a result, HSV vectors with vhs deleted are promising for use in vaccination strategies requiring the antigen loading of DC.
REFERENCES
- 1.Ace, C. I., T. A. McKee, J. M. Ryan, J. M. Cameron, and C. M. Preston. 1989. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J. Virol. 63:2260-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Brown, M., Y. Zhang, S. Dermine, E. A. de Wynter, C. Hart, H. Kitchener, P. L. Stern, M. A. Skinner, and S. N. Stacey. 2000. Dendritic cells infected with recombinant fowlpox virus vectors are potent and long-acting stimulators of transgene-specific class I restricted T lymphocyte activity. Gene Ther. 7:1680-1689. [DOI] [PubMed] [Google Scholar]
- 4.Coffin, R. S., A. R. MacLean, D. S. Latchman, and S. M. Brown. 1996. Gene delivery to the central and peripheral nervous systems of mice using HSV1 ICP34.5 deletion mutant vectors. Gene Ther. 3:886-891. [PubMed] [Google Scholar]
- 5.Coffin, R. S., S. K. Thomas, N. S. Thomas, C. E. Lilley, A. R. Pizzey, C. H. Griffiths, B. J. Gibb, M. J. Wagstaff, S. J. Inges, M. H. Binks, B. M. Chain, A. J. Thrasher, K. Rutault, and D. S. Latchman. 1998. Pure populations of transduced primary human cells can be produced using GFP expressing herpes virus vectors and flow cytometry. Gene Ther. 5:718-722. [DOI] [PubMed] [Google Scholar]
- 6.Fong, L., D. Brockstedt, C. Benike, L. Wu, and E. G. Engleman. 2001. Dendritic cells injected via different routes induce immunity in cancer patients. J. Immunol. 166:4254-4259. [DOI] [PubMed] [Google Scholar]
- 7.Fries, L. F., H. M. Friedman, G. H. Cohen, R. J. Eisenberg, C. H. Hammer, and M. M. Frank. 1986. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 137:1636-1641. [PubMed] [Google Scholar]
- 8.Gardner, J. P., I. Frolov, S. Perri, Y. Ji, M. L. MacKichan, M. J. Zur, M. Chen, B. A. Belli, D. A. Driver, S. Sherrill, C. E. Greer, G. R. Otten, S. W. Barnett, M. A. Liu, T. W. Dubensky, and J. M. Polo. 2000. Infection of human dendritic cells by a Sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 74:11849-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gough, N. M., and K. Murray. 1982. Expression of the hepatitis B virus surface, core and E antigen genes by stable rat and mouse cell lines. J. Mol. Biol. 162:43-67. [DOI] [PubMed] [Google Scholar]
- 10.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 11.Hill, A. B., B. C. Barnett, A. J. McMichael, and D. J. McGeoch. 1994. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J. Immunol. 152:2736-2741. [PubMed] [Google Scholar]
- 12.Jenne, L., C. Hauser, J. F. Arrighi, J. H. Saurat, and A. W. Hugin. 2000. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 7:1575-1583. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, D. C., and V. Feenstra. 1987. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J. Virol. 61:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, P. A., M. J. Wang, and T. Friedmann. 1994. Improved cell survival by the reduction of immediate-early gene expression in replication-defective mutants of herpes simplex virus type 1 but not by mutation of the virion host shutoff function. J. Virol. 68:6347-6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 74:7127-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kugler, A., G. Stuhler, P. Walden, G. Zoller, A. Zobywalski, P. Brossart, U. Trefzer, S. Ullrich, C. A. Muller, V. Becker, A. J. Gross, B. Hemmerlein, L. Kanz, G. A. Muller, and R. H. Ringert. 2000. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat. Med. 6:332-336. [DOI] [PubMed] [Google Scholar]
- 17.Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau, R., F. Wang, G. Jeffery, V. Marty, J. Kuniyoshi, E. Bade, M. E. Ryback, and J. Weber. 2001. Phase I trial of intravenous peptide-pulsed dendritic cells in patients with metastatic melanoma. J. Immunother. 24:66-78. [DOI] [PubMed] [Google Scholar]
- 19.Lilley, C. E., F. Groutsi, Z. Han, J. A. Palmer, P. N. Anderson, D. S. Latchman, and R. S. Coffin. 2001. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J. Virol. 75:4343-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodge, P. A., L. A. Jones, R. A. Bader, G. P. Murphy, and M. L. Salgaller. 2000. Dendritic cell-based immunotherapy of prostate cancer: immune monitoring of a phase II clinical trial. Cancer Res. 60:829-833. [PubMed] [Google Scholar]
- 21.MacLean, C. A., S. Efstathiou, M. L. Elliott, F. E. Jamieson, and D. J. McGeoch. 1991. Investigation of herpes simplex virus type 1 genes encoding multiply inserted membrane proteins. J. Gen. Virol. 72:897-906. [DOI] [PubMed] [Google Scholar]
- 22.Mikloska, Z., L. Bosnjak, and A. L. Cunningham. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morse, M. A., H. K. Lyerly, E. Gilboa, E. Thomas, and S. K. Nair. 1998. Optimization of the sequence of antigen loading and CD40-ligand-induced maturation of dendritic cells. Cancer Res. 58:2965-2968. [PubMed] [Google Scholar]
- 24.Motta, I., F. Andre, A. Lim, J. Tartaglia, W. I. Cox, L. Zitvogel, E. Angevin, and P. Kourilsky. 2001. Cross-presentation by dendritic cells of tumor antigen expressed in apoptotic recombinant canarypox virus-infected dendritic cells. J. Immunol. 167:1795-1802. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, G. P., B. A. Tjoa, S. J. Simmons, J. Jarisch, V. A. Bowes, H. Ragde, M. Rogers, A. Elgamal, G. M. Kenny, O. E. Cobb, R. C. Ireton, M. J. Troychak, M. L. Salgaller, and A. L. Boynton. 1999. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate 38:73-78. [DOI] [PubMed] [Google Scholar]
- 26.Nestle, F. O., S. Alijagic, M. Gilliet, Y. Sun, S. Grabbe, R. Dummer, G. Burg, and D. Schadendorf. 1998. Vaccination of melanoma patients with pep. Nat. Med. 4:328-332. [DOI] [PubMed] [Google Scholar]
- 27.Nestle, F. O., J. Banchereau, and D. Hart. 2001. Dendritic cells: On the move from bench to bedside. Nat. Med. 7:761-765. [DOI] [PubMed] [Google Scholar]
- 28.Roizman, B., and A. Sears. 1996. Herpes simplex viruses and their replication, p. 2231-2295. In B. Fields (ed.), Field's virology. Raven Press, Philadelphia, Pa.
- 29.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 31.Samaniego, L. A., L. Neiderhiser, and N. A. DeLuca. 1998. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J. Virol. 72:3307-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreurs, M. W., A. A. Eggert, A. J. de Boer, J. L. Vissers, T. van Hall, R. Offringa, C. G. Figdor, and G. J. Adema. 2000. Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model. Cancer Res. 60:6995-7001. [PubMed] [Google Scholar]
- 33.Shimizu, Y., L. G. Guidotti, P. Fowler, and F. V. Chisari. 1998. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J. Immunol. 161:4520-4529. [PubMed] [Google Scholar]
- 34.Smibert, C. A., D. C. Johnson, and J. R. Smiley. 1992. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J. Gen. Virol. 73:467-470. [DOI] [PubMed] [Google Scholar]
- 35.Song, W., H. L. Kong, H. Carpenter, H. Torii, R. Granstein, S. Rafii, M. A. Moore, and R. G. Crystal. 1997. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 186:1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzutani, T., M. Nagamine, T. Shibaki, M. Ogasawara, I. Yoshida, T. Daikoku, Y. Nishiyama, and M. Azuma. 2000. The role of the UL41 gene of herpes simplex virus type 1 in evasion of non-specific host defence mechanisms during primary infection. J. Gen. Virol. 81:1763-1771. [DOI] [PubMed] [Google Scholar]
- 38.Thomas, S. K., G. Gough, D. S. Latchman, and R. S. Coffin. 1999. Herpes simplex virus latency-associated transcript encodes a protein which greatly enhances virus growth, can compensate for deficiencies in immediate-early gene expression, and is likely to function during reactivation from virus latency. J. Virol. 73:6618-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas, S. K., C. E. Lilley, D. S. Latchman, and R. S. Coffin. 1999. Equine herpesvirus 1 gene 12 can substitute for vmw65 in the growth of herpes simplex virus (HSV) type 1, allowing the generation of optimized cell lines for the propagation of HSV vectors with multiple immediate-early gene defects. J. Virol. 73:7399-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tigges, M. A., S. Leng, D. C. Johnson, and R. L. Burke. 1996. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J. Immunol. 156:3901-3910. [PubMed] [Google Scholar]
- 41.Walker, J., and D. A. Leib. 1998. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine 16:1-5. [DOI] [PubMed] [Google Scholar]
- 42.Weissman, D., Y. Li, J. M. Orenstein, and A. S. Fauci. 1995. Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expresses CD80 can pass infection to unstimulated CD4+ T cells. J. Immunol. 155:4111-4117. [PubMed] [Google Scholar]
- 43.Yu, J. S., C. J. Wheeler, P. M. Zeltzer, H. Ying, D. N. Finger, P. K. Lee, W. H. Yong, F. Incardona, R. C. Thompson, M. S. Riedinger, W. Zhang, R. M. Prins, and K. L. Black. 2001. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 61:842-847. [PubMed] [Google Scholar]
- 44.Zhong, L., A. Granelli-Piperno, Y. Choi, and R. M. Steinman. 1999. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur. J. Immunol. 29:964-972. [DOI] [PubMed] [Google Scholar]