Abstract
Natural alpha interferon (IFN-α)-producing cells (IPCs) are now recognized as identical to plasmacytoid dendritic cell (DC) precursors in human blood and are thought to play an important role in antiviral immunity. In the present study, we examined the susceptibility as well as the cellular responses of IPCs to human immunodeficiency virus type 1 (HIV-1) infection. HLA-DR+ CD11c− lineage-negative cells (IPCs) were purified from peripheral blood mononuclear cells by magnetic-bead separation and cell sorting. We substantiated that IPCs expressing the major HIV-1 coreceptors, CXCR4 and CCR5, are susceptible to infection of both T-cell-line-tropic NL4-3 and macrophage-tropic JR-CSF HIV-1 by quantification of HIV-1 p24 in the culture supernatants and by provirus integration assay using human conserved Alu-HIV-1 long terminal repeat PCR. To evaluate the cellular response of IPCs to HIV-1, we examined IFN-α production and their differentiation into DCs. After incubation with either NL4-3 or JR-CSF, IPCs produced a large amount of IFN-α and at the same time underwent morphological differentiation into DCs with upregulation of CD80 and CD86. Heat inactivation of the supernatants containing HIV-1 did not affect the IFN-α production and maturation, whereas removal of virions by ultracentrifugation completely nullified both biological effects, indicating that these cellular responses do not require actual HIV-1 infection but are elicited by interaction with HIV-1 virions or certain viral components. In conclusion, these data strongly suggest that IPC can directly recognize and respond to HIV-1 with IFN-α production, which is crucial for preventing progress of HIV-1 infection and occurrence of opportunistic infection.
Dendritic cells (DCs) are a heterogeneous population with antigen-presenting capability that bridges the innate and adaptive immunity. In human peripheral blood, there are two types of DCs, immature myeloid DCs and plasmacytoid DC precursors, based on the expression of the β-integrin CD11c. Evidence has indicated that the two types belong to different lineages and have distinct functions (28, 36, 37). While CD11c+ DCs are precursors of Langerhans cells and interdigitating cells (19), CD11c− plasmacytoid DC precursors are now recognized as identical to natural alpha interferon (IFN-α)-producing cells (IPCs) (5, 34, 39). IPCs are present in blood and lymphoid organs and are ready to produce a large amount of IFN-α/β in response to viruses and other pathogens (11, 20). Intriguingly, they differentiate upon stimulation into type2 (lymphoid) DCs that can present antigens to T cells as myeloid DCs (16). Thus, IPCs play pivotal roles in control of viral infection by producing a large amount of IFN-α/β that inhibits viral replication and augments antiviral functions of effector cells (27, 35, 38) and by inducing adaptive immune response as DCs.
In human immunodeficiency virus type 1 (HIV-1) infection, CD4 T cells are the major target cells of HIV-1, and their progressive loss eventually leads to depression of adaptive immunity. However, a number of studies have revealed that some of the activities of innate immunity are also impaired in patients with progressive immunodeficiency. IFN-α/β accounts for an important part of innate immunity and IFN-α/β production by total peripheral blood mononuclear cells (PBMCs) has been reported to be decreased during the course of HIV-1 infection (24, 40). In accordance with this, plasmacytoid DCs (IPCs) in peripheral blood are reduced in AIDS patients and, on the contrary, increased in asymptomatic long-term survivors (7, 41), suggesting that the decrease in the number of IPCs and in the capability of IFN-α/β production is associated not only with a high incidence of opportunistic infections but also with enhanced HIV-1 viral replication. Thus, the delineation of the role of IPCs in HIV-1 infection is of particular importance for better understanding of the pathogenesis of AIDS.
The susceptibility and cellular response of DCs to HIV-1 infection have been investigated but still remain controversial (23, 25, 29, 31). This seems to be partly because the blood DC samples used in early studies unintentionally contained both myeloid DCs and IPCs. Therefore, what was described for DCs and HIV-1 should be redefined with each subset of DCs, especially IPCs. In the present study, we confirmed that HIV-1 can infect IPCs in our hands and then examined the cellular response of IPCs to HIV-1. Herewith we show that IPCs respond to HIV-1 with IFN-α production and their differentiation into DCs. Experiments with heat inactivation of HIV-1 and removal of HIV-1 virions indicate that the IFN-α production requires the interaction of IPCs with HIV-1 virions or viral components but not actual HIV-1 infection. The possible pathophysiological meaning of these findings in HIV-1 infection is discussed.
MATERIALS AND METHODS
Cells and culture conditions.
Buffy coats from healthy volunteers were obtained from the Kyoto Red Cross Blood Center. PBMCs were prepared from buffy coats by standard Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation. CD11c+ DCs and IPCs were isolated from PBMCs as previously described (16). Briefly, total PBMCs were depleted of lymphocytes and monocytes by treatment with a mixture of anti-CD3, anti-CD14, anti-CD16, anti-CD19, and anti-CD56 monoclonal antibodies (MAbs), and this was followed by separation with anti-mouse immunoglobulin G (IgG)-coated magnetic beads (Dynal AS, Oslo, Norway). CD4+ (or HLA-DR+) CD11c+ lineage-negative cells and CD4+ (or HLA-DR+) CD11c− lineage-negative cells were isolated by cell sorting as CD11c+ DCs and IPCs, respectively. Alternatively, IPCs were purified by using magnetic microbeads coated with anti-BDCA-4 MAb, which is a novel marker of IPCs (BDCA-4 cell isolation kit; Miltenyi Biotec, Auburn, Calif.) according to the manufacturer's instruction (8). Purified cells were cultured in RPMI 1640 (Life Technologies, Inc., Rockville, Md.) containing 20% fetal calf serum (Life Technologies, Inc.).
Virus stocks.
HIV-1 T-cell-line-tropic (T-tropic) NL4-3 and macrophage-tropic (M-tropic) JR-CSF viruses were prepared by transfection of pNL4-3 and pYK-JR-CSF into HEK293T cells (15, 33), respectively, all of which were obtained from the NIH AIDS Research and Reference Reagent Program (Rockville, Md.) (1, 3, 17, 22). Herpes simplex virus type 1 (HSV-1) (KOS strain) attenuated with gamma irradiation (a gift of Yong-Jun Liu, DNAX Research Institute, Palo Alto, Calif.) was also used at a final concentration of 106 PFU/ml as a positive control. The concentrations of viruses that induced optimal survival and IFN production were selected. Higher concentrations of viruses induced cell death, probably due to an overwhelming cytopathic effect (data not shown). We also used noninfectious HIV-1 that had been heat inactivated at 56°C for 1 h.
RT-PCR.
Total RNA was isolated from purified IPCs or CD11c+ DCs by using an RNeasy mini kit (Qiagen, Hilden, Germany). Reverse transcription (RT) was performed with oligo(dT) primer, pd(T)12-18 (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom). cDNA was amplified by PCR using the following primers: CXCR4 forward (GATGACAGATATATCTGTGACCGC) and CXCR4 reverse (TTAGCTGGAGTGAAAACTTGAAGA); CCR5 forward (ATGGTCATCTGCTACTCGGGAATC) and CCR5 reverse (TCACAAGCCCACAGATATTTCCTG); and DC-SIGN forward (TGTCCCTGGGAATGGACATTC) and DC-SIGN reverse (GGCTGGAGAAAGAAACTGTTC). A GeneAmp PCR system (Perkin-Elmer/Applied Biosystems) was used with an initial denaturation step of 94°C for 4 min, followed by 25 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 1 min and a final elongation step of 72°C for 10 min. PCR products were separated on a 1% agarose gel, stained with ethidium bromide, and visualized by a UV transilluminator.
Flow cytometric analysis.
To analyze the expression of HIV-1 coreceptors, CXCR4 and CCR5, and phenotypic changes after exposure to HIV-1 or HSV, freshly isolated as well as cultured IPCs were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CXCR4 or anti-CCR5 MAb (R & D Systems, Minneapolis, Minn.), anti-CD80 MAb (Immunotech Coulter, Marseille, France), or anti-CD86 MAb (BD PharMingen, San Diego, Calif.). All samples were analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.).
HIV-1 infection assay.
HIV-1 NL4-3 or JR-CSF virus supernatants adjusted to an equivalent of 500 ng of HIV-1-p24 were added to 106 CD11c+ DCs or IPCs/ml. After the virus adsorption at 37°C for 2 h, cells were washed with phosphate-buffered saline three times to remove free virions thoroughly, and the cultures were continued in fresh medium. On the indicated day after infection, culture supernatants were harvested and stored at −20°C. Productive virus infection was estimated by quantification of HIV-1 p24 in each supernatant using an enzyme-linked immunosorbent assay (ELISA) kit for the p24 antigen (RETRO-TEK; ZeptoMetrix, Buffalo, N.Y.). In some experiments, a neutralizing anti-IFN-α MAb (clone MMHA-2, peripheral blood lymphocyte) or a control mouse IgG (Sigma) was added to the final concentration of 2 μg/ml together with the viruses to evaluate the effect of endogenous IFN-α on HIV-1 replication.
HIV-1 provirus integration assay.
IPCs or PHA-stimulated PBMCs were incubated with HIV-1 NL4-3 or JR-CSF virus supernatants overnight and washed three times with phosphate-buffered saline. Total DNA was purified by using QIAamp mini kits (Qiagen) from each sample. The first PCR was carried out using an LA PCR kit (Takara, Otsu, Japan) with a sense primer, Alu-long terminal repeat (LTR) from conserved sequences of human Alu (5′-TCC CAG CTA CTC GGG AGG CTG AGG-3′) (6), and an antisense primer from conserved HIV-1 LTR sequences, M661 (5′-CTT GCG TCG AGA GAT CTC CTC TG-3′) (26). Using the first PCR products as templates, second nested PCR was carried out using Ex Taq (Takara) with a sense primer, M667 (5′-GGC TAA CTA GGG AAC CCA CTG-3′), and an antisense primer, AA55 (5′-CTG CTA GAG ATT TTC CAC ACT GAC-3′) (26). Purified DNA from IPCs or PHA-stimulated PBMCs cultured with heat-inactivated HIV-1, NL4-3 or JR-CSF, and medium alone were used for negative control.
Mesurement of cytokine secretion by ELISA.
IPCs were cultured at 2 × 105/200 μl with HIV-1 adjusted to an equivalent of 100 ng of HIV-1 p24 or HSV, and the culture supernatants were collected after 48 h. IFN-α in the supernatants was measured by a human IFN-α ELISA kit (BioSource International, Camarillo, Calif.). In some experiments, HIV-1 was added together with 2 μg/ml of anti-CD4 MAb (Leu-3a [Becton Dickinson]; PRA-T4 [eBioscience, San Diego, Calif.]) or control IgG to examine the involvement of CD4 in HIV-1-induced IFN-α production.
Morphological examination.
Cytospin preparations of cultured cells were made and subjected to microscopic examination after May-Giemsa staining.
RESULTS
Expression of HIV-1 coreceptors and DC-SIGN on IPCs.
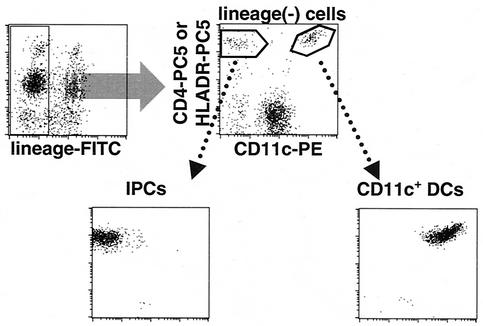
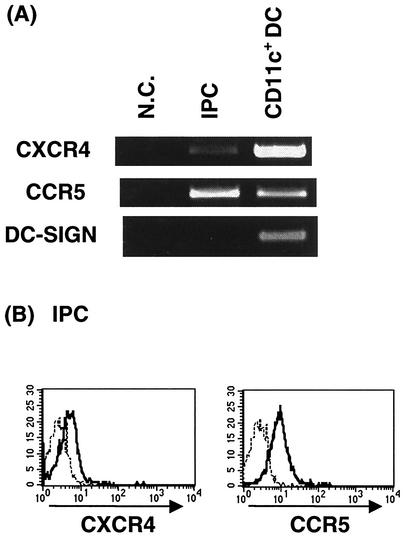
We first examined and compared the expression of CXCR4, CCR5, and DC-SIGN in IPCs and CD11c+ DCs. These two subsets of blood DCs were prepared from fresh PBMCs by cell sorting as shown in Fig. 1. The purity of the isolated cells was always >98% in reanalysis. Expression of CXCR4 as well as CCR5 mRNA was detected by RT-PCR in both IPCs and CD11c+ DCs. On the other hand, expression of DC-SIGN mRNA was detected in CD11c+ DCs but not in IPCs (Fig. 2A). Flow cytometric analysis also demonstrated the expression of CXCR4 and CCR5 on the cell surface of IPCs (Fig. 2B). We repeated these experiments with samples from five different donors and obtained essentially the same results, indicating that together with CD4, IPCs express HIV-1 coreceptors which are required for HIV-1 infection.
FIG. 1.
Purification of blood DCs by cell sorting. Total PBMCs were depleted of lymphocytes and monocytes with a mixture of anti-CD3, anti-CD14, anti-CD16, anti-CD19, and anti-CD56 MAbs and with magnetic beads. Subsequently, CD4+ (or HLA− DR+) CD11c+ lineage-negative cells and CD4+ (or HLA-DR+) CD11c− lineage-negative cells were isolated as CD11c+ DCs and IPCs, respectively, by cell sorting.
FIG. 2.
Expression of HIV-1 coreceptors and DC-SIGN on IPCs. (A) Detection of mRNA for CXCR4, CCR5, and DC-SIGN by RT-PCR in blood DC subsets. Cells were isolated by cell sorting to the purity of >99%. N.C., negative control. (B) Expression of CXCR4 and CCR5 on purified IPCs. IPCs were stained with FITC-conjugated anti-CXCR4 or anti-CCR5 MAb. The dotted lines represent staining with isotype-matched control MAbs.
Susceptibility of IPCs to HIV-1 infection.
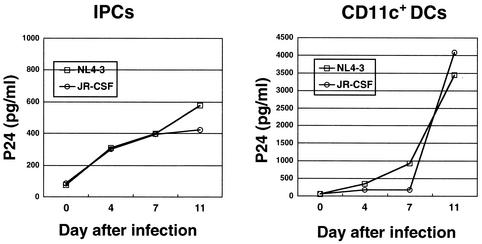
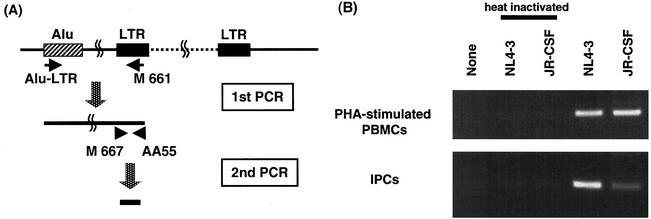
Although a number of studies have reported that HIV-1 can infect DCs (23, 30), there remains some controversy as to which particular subset is susceptible to HIV-1 infection and whether the infection is productive or not. Therefore, we next examined the infectivity of HIV-1 on IPCs in our hands. IPCs and CD11c+ DCs purified from PBMCs were incubated with T-tropic NL4-3 or M-tropic JR-CSF virus. After 2 h, cells were washed three times and cultured for 11 days, and then the supernatants were harvested. HIV-1 productive infection was analyzed by measuring HIV-1 p24 antigen in the supernatants. We found that both NL4-3 and JR-CSF could productively infect IPCs as well as CD11c+ DCs (Fig. 3). It was, however, observed that infection of IPCs yielded less HIV-1 p24 than did infection of CD11+ DCs. To verify HIV-1 infection of IPCs, we carried out the HIV-1 integration assay using Alu-LTR nested PCR as previously described (2, 6, 26, 43) (Fig. 4A). The first PCR product was serially diluted and subjected to the second PCR. Three independent experiments were done and gave similar results. We detected the specific band of integrated HIV-1 proviral DNA in IPCs cultured with either HIV-1 NL4-3 or JR-CSF, but not in those cultured with medium alone or heat-inactivated viruses (Fig. 4B), which substantiates the argument that both T-tropic and M-tropic HIV-1 can infect IPCs.
FIG. 3.
Productive infection of NL4-3 or JR-CSF in IPCs or CD11c+ DCs. The levels of HIV-1 p24 antigen in supernatants were measured by ELISA.
FIG. 4.
HIV-1 provirus integration assay. (A) Detection of the integrated form of viral DNA by using Alu-LTR PCR. Genomic DNAs from IPCs or phytohemagglutinin-stimulated PBMCs were subjected to in duplicate to PCR with nested 5′ primers from conserved human Alu and 3′ primers from conserved HIV-1 LTR sequences. The diluted PCR product was further subjected to the second PCR by using nested HIV-1 LTR specific primers. (B) Results of Alu-LTR PCR of genomic DNAs from pre-DC2s and phytohemagglutinin (PHA)-stimulated PBMCs. Viruses heat inactivated at 56°C for 1 h were used as a negative control.
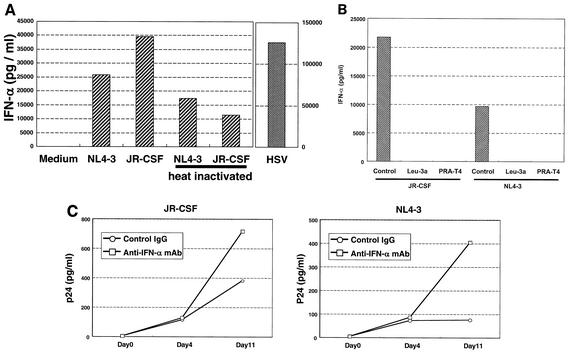
Production of IFN-α by IPCs stimulated with HIV-1.
In order to define the cellular responses of IPCs to HIV-1, we measured IFN-α production by IPCs after exposure to HIV-1, because IPCs or plasmacytoid DCs have been reported to produce a large amount of IFN-α upon stimulation with certain viruses such as HSV (39), influenza virus (5), and Sendai virus (9). We repeated this assay three times, and the data of a representative experiment is shown in Fig. 5. IPCs produced a large amount of IFN-α within 48 h in response to HSV-1 and, to a lesser extent, NL4-3 or JR-CSF (Fig. 5A). CD11c+ DCs were not induced to produce detectable levels of IFN-α by HSV-1 or HIV-1 (data not shown). Interestingly, IFN-α production was observed in IPCs stimulated with heat-inactivated NL4-3 and JR-CSF. Removal of HIV-1 virions by ultracentrifugation (data not shown) or addition of anti-CD4 MAb (Fig. 5B) resulted in complete elimination of IFN-α production, suggesting that IFN-α production requires interaction of IPCs with HIV-1 virions or viral components via CD4 but not actual HIV-1 infection. We then examined whether IPC-derived endogenous IFN-α suppressed HIV-1 replication. As shown in Fig. 5C, treatment with neutralizing anti-IFN-α MAb resulted in an increase in production of p24, indicating that IPC-derived IFN-α had suppressive effects on HIV-1 infection.
FIG. 5.
(A) IFN-α production by IPCs cultured with HIV-1 or HSV. IPCs were stimulated with HIV-1 or HSV for 48 h, and the concentrations of IFN-α in the supernatants were measured by ELISA. (B) Effect of anti-CD4 MAbs on HIV-1-induced IFN-α production by IPCs. (C) Effect of anti-IFN-α MAb on HIV-1 production.
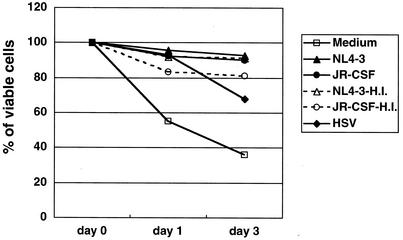
Survival of IPCs in response to HIV-1.
As previously reported (20), IPCs died rapidly and few viable cells remained after 3 days of culture in medium alone (Fig. 6). It is known that IFN-α/β (20) and interleukin-3 (16) could prolong survival of IPCs, and stimulation with certain viruses has similar effects, probably mediated by induction of IFN-α/β production. Accordingly, we examined the effect of HIV-1 on the survival of IPCs and found that HIV-1 or HSV-1 could extend the survival of IPCs compared with medium alone. Representative data of three experiments are shown in Fig. 6. It is paradoxical that HIV-1 infection sustains cellular viability instead of exerting cytopathic effects, the former of which seems to be mediated by induction of IFN-α. Corresponding to the ability to induce IFN-α production, the heat-inactivated HIV-1 could also sustain the viability of IPCs.
FIG. 6.
Viability of IPCs cultured with HIV-1 or HSV. Viable cells were counted by trypan blue staining. Percentages of viable IPCs cultured under different conditions were indicated. H.I., heat inactivated.
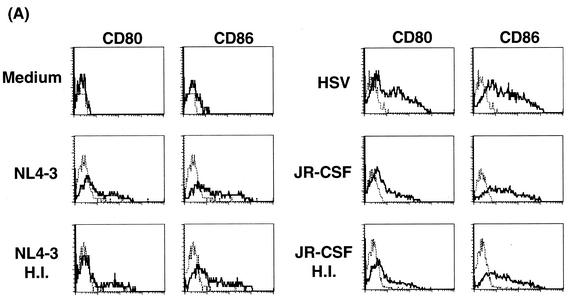
Differentiation of IPCs into DCs in response to HIV-1.
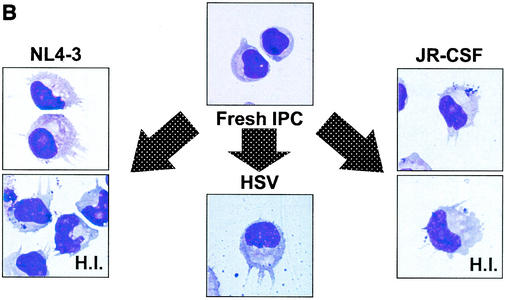
To examine whether HIV-1 induces the differentiation of IPCs, the expressions of CD80 and CD86 were examined by flow cytometry after culture with HIV-1 or HSV-1 for 48 h without washing. As shown in Fig. 7A, both CD80 and CD86 were upregulated in IPCs cultured with either NL4-3 or JR-CSF regardless of whether the viruses were infection competent or heat inactivated. The magnitude of upregulation by HIV-1 was somewhat weak compared with that by HSV-1, and again removal of HIV-1 virions by ultracentrifugation resulted in elimination of the effects in line with the results of the IFN-α production (data not shown). At the same time, we examined the morphological changes of IPCs after stimulation with HIV-1 by May-Giemsa staining of the cytospin preparations. Freshly isolated IPCs showed plasmacytoid morphology and after 2 days of culture with T-tropic or M-tropic HIV-1 as well as HSV-1 underwent morphological differentiation into DCs with dendrites and larger cytoplasm (Fig. 7B). These experiments were repeated three times and gave similar results.
FIG. 7.
(A) The expression of CD80 and CD86 on IPCs. IPCs cultured with HIV-1, HSV, or medium alone for 48 h were stained with FITC-conjugated anti-CD80 or anti-CD86 MAbs and subjected to flow cytometric analysis. (B) Morphological changes of IPCs cultured with HIV-1 or HSV by using Giemsa staining. Freshly isolated IPCs showed plasmacytoid morphology, whereas IPCs cultured with HIV-1 and HSV for 2 days acquired DC morphology with prominent dendrites. H.I., heat inactivated.
DISCUSSION
Despite recent progress in AIDS research, neither the host defense mechanism against HIV-1 nor the pathogenesis of AIDS has been fully understood. While adaptive immunity of virus-specific cytotoxic T lymphocyte and neutralizing antibody has been extensively investigated, the role of innate immunity in HIV-1 infection remains largely unknown. A number of studies suggested that DCs, a constituent of innate immunity, play key roles both in induction of antiviral immune responses and in viral transmission to CD4+ T cells. However, there has been some controversy as to the susceptibility of DCs to HIV-1 infection and IFN-α production by DCs. Some studies have presented negative data on productive HIV-1 infection of DCs (18, 30). Likewise, it has not been settled which subset of DCs or other cell type in PBMC respond to HIV-1 with IFN-α production (13, 14). The controversy seems to be partly due to the preparations of DCs since recent evidence has disclosed that peripheral blood DCs consist of at least two different cell types which can be distinguished phenotypically as well as functionally. It is, therefore, urgently required to reevaluate what was known about DCs and HIV-1 from the current point of view.
We first confirmed previous data (32) that IPCs as well as CD11c+ DCs express CXCR4 and CCR5 together with CD4, which strongly suggests that IPCs are susceptible to cell entry of both T-tropic and M-tropic HIV-1. On the other hand, as we showed, IPCs do not express DC-SIGN, which is a C-type lectin known to be expressed on CD11c+ DCs and involved in transmission of HIV-1 to surrounding CD4+ T cells (12). Absence of DC-SIGN on IPCs may imply that they are less important in viral transmission than CD11c+ DCs. However, more detailed analysis of expression of other C-type lectins including DC-SIGNR (32) is needed to draw any conclusion on this issue.
Regarding the susceptibility to HIV-1, Patterson et al. have recently reported that plasmacytoid DCs (virtually identical to IPCs) can be infected by HIV-1 productively based on the results of nested limiting-dilution PCR of proviral DNA (32). The authors also performed a second-round infection assay to test for the release of infectious virus. The data presented varied considerably among the experiments but suggested that plasmacytoid DCs are susceptible to productive HIV-1 infection and allow more-efficient viral replication than CD11c+ DCs. Our results of quantification of HIV-1 p24 in the culture supernatants are compatible with their results in that both T-tropic and M-tropic HIV-1 can infect IPCs productively. However, in our hands, the levels of p24 were reproducibly lower in IPCs than in CD11c+ DCs. This may be due to different culture conditions and infection procedures. Nonetheless, we think that our results are consistent with the function of IPCs because we have presented data suggesting that IFN-α produced upon interaction with HIV-1 suppressed viral replication. We also carried out PCR-based provirus integration assay to verify infectivity of HIV-1 to IPCs. This technique is more specific to the detection of provirus integration than simple nested PCR of proviral DNA, which may also amplify preintegration proviral DNA. Since the IPC samples we used were highly purified, our data present additional evidence that IPCs are susceptible to productive infection of both T-tropic and M-tropic HIV-1.
Early studies described that PBMCs (4) and certain subpopulations of PBMCs could produce IFN-α upon stimulation with HIV-1 infection. For example, Szebeni et al. showed that IFN-α was produced in monocyte-macrophage culture (42), and afterwards Ferbas et al. reported that DCs were producers of IFN-α in response to HIV-1 infection (10). However, the DC preparations they used were mixtures of CD11c+ myeloid DCs and IPCs. Here, we separated DCs into the two subsets and unambiguously showed that the major source of IFN-α is IPCs but not CD11c+ DCs. In the subsequent paper, the same group claimed that DCs did not show any differentiation or maturation upon HIV-1 infection in terms of morphology as well as expression of CD80 and CD86 (14). This forms a sharp contrast to our data, because we observed not only prolonged survival but also differentiation of IPCs into DCs with upregulation of CD80 and CD86 after exposure to either T-tropic or M-tropic HIV-1. We speculate that the majority of DCs described in the above-mentioned paper were CD11c+ myeloid DCs that were poorly responsive to HIV-1. Differentiation of IPCs into DCs is important since it leads to antigen presentation to T cells and induction of adaptive immune response to HIV-1. In any case, the present study has first demonstrated that IPCs but not CD11c+ DCs are producers of IFN-α in response to HIV-1 infection.
It is noteworthy that heat-inactivated HIV-1 that lost infectivity as shown by provirus integration assay elicited biological effects on IPCs similar to infection-competent viruses, which indicates that these biological effects do not require actual HIV-1 infection. The mechanism of interaction between IPCs and HIV-1 virions or viral components is presently unclear except that CD4 seems to be involved in this interaction. It is possible that heat-inactivated virus could enter IPCs without proviral integration and trigger IFN-α production through a viral double-stranded RNA intermediate. We do not exclude this possibility but think it is rather unlikely, because poly(I:C), a synthetic double-stranded RNA, has been reported to induce CD11c+ DCs to produce IFN but has little effects on IPCs (21). Further studies including possible involvement of Toll-like receptors are needed to elucidate the molecular mechanism of cellular response of IPCs to HIV-1. In conclusion, the present study strongly suggests that IPCs play an important role in protecting the host from HIV-1 infection and viral propagation by producing IFN-α and by differentiating themselves into DCs. Increase in cell number as well as functional activation of IPCs should be one of the targets of a therapeutic approach to HIV-1 infection.
Acknowledgments
A. Yonezawa and R. Morita contributed equally to this work.
We thank Yoshio Koyanagi for technical advice on HIV-1 integration assays. We acknowledge Malcom Martin (pNL4-3) and Irvin S. Y. Chen and Yoshio Koyanagi (pYK-JR-CSF) for reagents obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This study was supported by grants-in-aid from the Ministry of Education, Science, Sports, Culture, and Technology of Japan.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler, S. L., M. S. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7:631-634. [DOI] [PubMed] [Google Scholar]
- 3.Cann, A. J., J. A. Zack, A. S. Go, S. J. Arrigo, Y. Koyanagi, P. L. Green, S. Pang, and I. S. Chen. 1990. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J. Virol. 64:4735-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capobianchi, M. R., F. De Marco, P. Di Marco, and F. Dianzani. 1988. Acid-labile human interferon alpha production by peripheral blood mononuclear cells stimulated by HIV-infected cells. Arch. Virol. 99:9-19. [DOI] [PubMed] [Google Scholar]
- 5.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. [DOI] [PubMed] [Google Scholar]
- 6.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c(+) myeloid and CD11c(-) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574-2576. [DOI] [PubMed] [Google Scholar]
- 8.Dzionek, A., A. Fuchs, P. Schmidt, S. Cremer, M. Zysk, S. Miltenyi, D. W. Buck, and J. Schmitz. 2000. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165:6037-6046. [DOI] [PubMed] [Google Scholar]
- 9.Feldman, S. B., M. C. Milone, P. Kloser, and P. Fitzgerald-Bocarsly. 1995. Functional deficiencies in two distinct interferon alpha-producing cell populations in peripheral blood mononuclear cells from human immunodeficiency virus seropositive patients. J. Leukoc. Biol. 57:214-220. [DOI] [PubMed] [Google Scholar]
- 10.Ferbas, J. J., J. F. Toso, A. J. Logar, J. S. Navratil, and C. R. Rinaldo, Jr. 1994. CD4+ blood dendritic cells are potent producers of IFN-alpha in response to in vitro HIV-1 infection. J. Immunol. 152:4649-4662. [PubMed] [Google Scholar]
- 11.Fitzgerald-Bocarsly, P. 1993. Human natural interferon-alpha producing cells. Pharmacol. Ther. 60:39-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 13.Gendelman, H. E., L. M. Baca, C. A. Kubrak, P. Genis, S. Burrous, R. M. Friedman, D. Jacobs, and M. S. Meltzer. 1992. Induction of IFN-alpha in peripheral blood mononuclear cells by HIV-infected monocytes. Restricted antiviral activity of the HIV-induced IFN. J. Immunol. 148:422-429. [PubMed] [Google Scholar]
- 14.Ghanekar, S., L. Zheng, A. Logar, J. Navratil, L. Borowski, P. Gupta, and C. Rinaldo. 1996. Cytokine expression by human peripheral blood dendritic cells stimulated in vitro with HIV-1 and herpes simplex virus. J. Immunol. 157:4028-4036. [PubMed] [Google Scholar]
- 15.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 16.Grouard, G., M. C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y. J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haltiner, M., T. Kempe, and R. Tjian. 1985. A novel strategy for constructing clustered point mutations. Nucleic Acids Res. 13:1015-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausser, G. A., C. Hultgren, K. Akagawa, Y. Tsunetsugu-Yokota, and A. Meyerhans. 1995. Infection of cultured immature dendritic cells with human immunodeficiency virus type 1. Adv. Exp. Med. Biol. 378:477-479. [DOI] [PubMed] [Google Scholar]
- 19.Ito, T., M. Inaba, K. Inaba, J. Toki, S. Sogo, T. Iguchi, Y. Adachi, K. Yamaguchi, R. Amakawa, J. Valladeau, S. Saeland, S. Fukuhara, and S. Ikehara. 1999. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J. Immunol. 163:1409-1419. [PubMed] [Google Scholar]
- 20.Kadowaki, N., S. Antonenko, J. Y. Lau, and Y. J. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadowaki, N., S. Antonenko, and Y. J. Liu. 2001. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c-type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J. Immunol. 166:2291-2295. [DOI] [PubMed] [Google Scholar]
- 22.Koyanagi, Y., S. Miles, R. T. Mitsuyasu, J. E. Merrill, H. V. Vinters, and I. S. Chen. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819-822. [DOI] [PubMed] [Google Scholar]
- 23.Langhoff, E., E. F. Terwilliger, H. J. Bos, K. H. Kalland, M. C. Poznansky, O. M. Bacon, and W. A. Haseltine. 1991. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proc. Natl. Acad. Sci. USA 88:7998-8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez, C., P. A. Fitzgerald, and F. P. Siegal. 1983. Severe acquired immune deficiency syndrome in male homosexuals: diminished capacity to make interferon-alpha in vitro associated with severe opportunistic infections. J. Infect. Dis. 148:962-966. [DOI] [PubMed] [Google Scholar]
- 25.Macatonia, S. E., R. Lau, S. Patterson, A. J. Pinching, and S. C. Knight. 1990. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology 71:38-45. [PMC free article] [PubMed] [Google Scholar]
- 26.Masuda, T., V. Planelles, P. Krogstad, and I. S. Chen. 1995. Genetic analysis of human immunodeficiency virus type 1 integrase and the U3 att site: unusual phenotype of mutants in the zinc finger-like domain. J. Virol. 69:6687-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 28.O'Doherty, U., M. Peng, S. Gezelter, W. J. Swiggard, M. Betjes, N. Bhardwaj, and R. M. Steinman. 1994. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology 82:487-493. [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson, S., N. R. English, H. Longhurst, P. Balfe, M. Helbert, A. J. Pinching, and S. C. Knight. 1998. Analysis of human immunodeficiency virus type 1 (HIV-1) variants and levels of infection in dendritic and T cells from symptomatic HIV-1-infected patients. J. Gen. Virol. 79:247-257. [DOI] [PubMed] [Google Scholar]
- 30.Patterson, S., J. Gross, P. Bedford, and S. C. Knight. 1991. Morphology and phenotype of dendritic cells from peripheral blood and their productive and non-productive infection with human immunodeficiency virus type 1. Immunology 72:361-367. [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson, S., and S. C. Knight. 1987. Susceptibility of human peripheral blood dendritic cells to infection by human immunodeficiency virus. J. Gen. Virol. 68:1177-1181. [DOI] [PubMed] [Google Scholar]
- 32.Patterson, S., A. Rae, N. Hockey, J. Gilmour, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75:6710-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perussia, B., V. Fanning, and G. Trinchieri. 1985. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat. Immun. Cell Growth Regul. 4:120-137. [PubMed] [Google Scholar]
- 35.Pfeffer, L. M., C. A. Dinarello, R. B. Herberman, B. R. Williams, E. C. Borden, R. Bordens, M. R. Walter, T. L. Nagabhushan, P. P. Trotta, and S. Pestka. 1998. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 58:2489-2499. [PubMed] [Google Scholar]
- 36.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183-1186. [DOI] [PubMed] [Google Scholar]
- 37.Robinson, S. P., S. Patterson, N. English, D. Davies, S. C. Knight, and C. D. Reid. 1999. Human peripheral blood contains two distinct lineages of dendritic cells. Eur. J. Immunol. 29:2769-2778. [DOI] [PubMed] [Google Scholar]
- 38.Santini, S. M., C. Lapenta, M. Logozzi, S. Parlato, M. Spada, T. Di Pucchio, and F. Belardelli. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 40.Siegal, F. P., C. Lopez, P. A. Fitzgerald, K. Shah, P. Baron, I. Z. Leiderman, D. Imperato, and S. Landesman. 1986. Opportunistic infections in acquired immune deficiency syndrome result from synergistic defects of both the natural and adaptive components of cellular immunity. J. Clin. Investig. 78:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98:906-912. [DOI] [PubMed] [Google Scholar]
- 42.Szebeni, J., C. Dieffenbach, S. M. Wahl, C. N. Venkateshan, A. Yeh, M. Popovic, S. Gartner, L. M. Wahl, M. Peterfy, R. M. Friedman, et al. 1991. Induction of alpha interferon by human immunodeficiency virus type 1 in human monocyte-macrophage cultures. J. Virol. 65:6362-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]