A recent paper by H. J. Ezelle and coworkers reported the generation of hepatitis C virus (HCV)-like particles by using a recombinant vesicular stomatitis virus (VSV) vector (4). The authors inserted the contiguous HCV Core, E1, and E2 coding region into the VSV genome, and recombinant VSV producing the three HCV structural proteins were used to infect the BHK-21 cell line. With this strategy, the authors claimed to have obtained the complete budding of HCV-like particles, visualized by transmission electron microscopy (TEM) in cytoplasmic vacuoles derived from the rough endoplasmic reticulum (ER). In their Fig. 3 (panels C and D) they indicate “HCV-like virions,” described as 40- to 80-nm particles exhibiting an electron-dense core with an envelope, fully released into the ER lumen (4). Unfortunately, we believe that these particles represent the endogenous viruses of BHK-21 cells known as intracisternal R-type particles that have been widely described elsewhere (3, 6, 7).
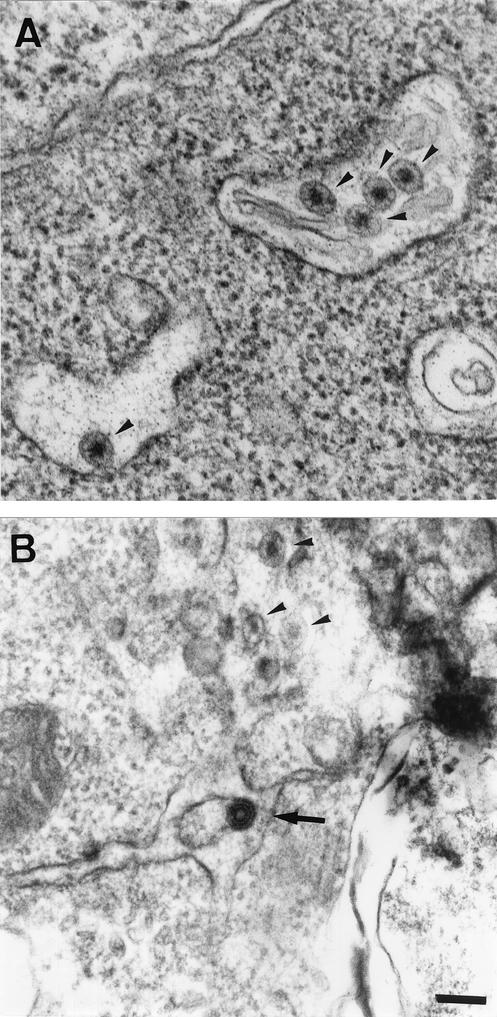
Our group is experienced in the transfection of BHK-21 cells with various constructs encoding viral structural proteins (1, 2, 5). We have frequently observed these intracisternal R-type particles in untransfected BHK-21 studied by TEM (arrowheads in Fig. 1A). In our most recent study, we used a recombinant Semliki forest virus (SFV) replicon to express, in BHK-21 cells, the genes encoding HCV structural proteins (1). The self-assembly of HCV proteins at the ER membrane was associated with the budding of HCV-like particles towards the ER lumen. These HCV-like particles could not be confused with the particles endogenous to BHK-21, as seen in Fig. 1B. HCV-like virions consist of a core-like particle, 30 to 35 nm in diameter, surrounded by an electron-dense ER-derived envelope, yielding a much darker particle with a total diameter of 50 to 60 nm (Fig. 1B, arrow). In our study, these HCV-like particles appeared to display abortive budding in BHK-21 cells. Indeed, few particles were fully released from the ER membrane (1). This is consistent with the low levels of HCV structural proteins detected in the transfected cell supernatant. A similar absence of HCV structural protein secretion was reported by H. J. Ezelle and coworkers in studies of BHK-21 cells infected with recombinant VSV (4).
FIG. 1.
Electron micrographs of ultrathin sections of untransfected BHK-21 cells (A) and BHK-21 cells electroporated with an SFV vector encoding the HCV Core, E1, and E2 structural proteins (B). The bar in panel B (for both panels A and B) represents 100 nm. The arrowheads in panels A and B indicate the endogenous viruses known as intracisternal R-type particles, which are frequently encountered in the ER lumen of BHK-21 cells. The arrow in panel B indicates an HCV-like particle budding towards the ER lumen.
Despite the inefficiency of particle secretion, both the SFV and VSV expression systems may be valuable tools for studies of virus assembly mechanisms and virus-host cell interactions. Nevertheless, we would like to emphasize that no system in which the complete budding of HCV-like particles is observed has yet been developed.
REFERENCES
- 1.Blanchard, E., D. Brand, S. Trassard, A. Goudeau, and P. Roingeard. 2002. Hepatitis C virus-like particle morphogenesis. J. Virol. 76:4073-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand, D., F. Lemiale, G. Thibault, B. Verrier, S. Lebigot, P. Roingeard, L. Buzelay, and F. Barin. 2000. Antigenic properties of recombinant envelope glycoproteins derived from T-cell-line adapted isolates or primary human immunodeficiency virus isolates and their relationship to immunogenicity. Virology 271:350-362. [DOI] [PubMed] [Google Scholar]
- 3.Compans, R. W., K. V. Holmes, S. Dales, and P. W. Choppin. 1966. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology 30:411-426. [DOI] [PubMed] [Google Scholar]
- 4.Ezelle, H. J., D. Markovic, and G. N. Barber. 2002. Generation of hepatitis C virus-like particles by use of a recombinant vesicular stomatitis virus vector. J. Virol. 76:12325-12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hourioux, C., D. Brand, P.-Y. Sizaret, F. Lemiale, S. Lebigot, F. Barin, and P. Roingeard. 2000. Identification of the glycoprotein 41TM cytoplasmic tail domains of human immunodeficiency virus type 1 that interact with Pr 55 gag particles. AIDS Res. Hum. Retrovir. 16:1141-1147. [DOI] [PubMed] [Google Scholar]
- 6.Shipman, C., Jr., G. C. Vander Weide, and B. I. Ma. 1969. Prevalence of type R virus-like particles in clones of BHK-21 cells. Virology 38:707-710. [DOI] [PubMed] [Google Scholar]
- 7.Wang, G., M. J. Mulligan, D. N. Baldwin, and M. L. Linial. 1999. Endogenous virus of BHK-21 cells complicates electron microscopy studies of foamy virus maturation. J. Virol. 73:8917. [DOI] [PMC free article] [PubMed] [Google Scholar]