Abstract
Recent epidemiologic studies show increasing human immunodeficiency virus type 1 (HIV-1) transmission through oral-genital contact. This paper examines the possibility that normal human oral keratinocytes (NHOKs) might be directly infected by HIV or might convey infectious HIV virions to adjacent leukocytes. PCR analysis of proviral DNA constructs showed that NHOKs can be infected by CXCR4-tropic (NL4-3 and ELI) and dualtropic (89.6) strains of HIV-1 to generate a weak but productive infection. CCR5-tropic strain Ba-L sustained minimal viral replication. Antibody inhibition studies showed that infection by CXCR4-tropic viral strains is mediated by the galactosylceramide receptor and the CXCR4 chemokine coreceptor. Coculture studies showed that infectious HIV-1 virions can also be conveyed from NHOKs to activated peripheral blood lymphocytes, suggesting a potential role of oral epithelial cells in the transmission of HIV infection.
The majority of human immunodeficiency virus type 1 (HIV-1) infections occur via mucosal contact (40), and there are several reports indicating that the oral mucosa may be one route of exposure (25, 27). It is difficult to confirm that oral mucosa is a major transmission portal because of the correlation between oral-genital contact and other transmission risk behaviors (36). However, several studies of receptive oral intercourse suggest that it is an independent risk factor for HIV infection (36) and may contribute to the high transmission rates among men who have sex with men (37, 38; D. G. Ostrow and W. DiFranceisco, Abstr. XI Int. Conf. AIDS, abstr. Tu.D.365, 1996), people of low socioeconomic status (17), and crack cocaine users (17; J. A. Hoffman, H. Klein, and D. C. Clark, Abstr. 5th Pan-Am. Conf. AIDS, abstr. PCV267, 1997).
HIV-1 can be recovered from the saliva of infected individuals, but concentrations are generally lower than those observed in blood or genital secretions (28). Antiviral properties of saliva may limit the infectivity of HIV in the oral mucosa (18, 39). In vitro infection of lymphocytes is reduced in the presence of saliva (40) via mechanisms that include virus-specific antibodies (23), aggregation of viral particles by salivary mucins (5), and competition for viral or cellular targets by inhibitory endogenous proteins (35). Despite these salivary defense mechanisms, there are reports of postnatal oral HIV transmission to infants, suggesting that colostrum and breast milk may be vehicles for infection (41). In HIV-1-seropositive lactating mothers, HIV can be detected in milk monocytes and macrophages by in situ RNA hybridization and immunocytochemistry (S. Southern and P. J. Southern, HIV-1 Infect., Mucosal Immun., Pathogenesis, abstr. 57, 1997). Adhesion molecules in saliva may be important in transporting infected cells into tonsillar and intestinal crypts and may facilitate HIV transmission during breast feeding (36). Another indication of oral infectivity comes from studies of simian immunodeficiency virus (SIV) in which cell-free viral particles were capable of generating systemic infection of macaques when introduced by nontraumatic oral inoculation (42).
The biological mechanism of HIV transmission by the oral mucosa is not known, but possible pathways include entry through lesions in the epithelium and binding of free virions to lymphoid cells residing in the mucosal microenvironment (10). Studies of clinical specimens have identified HIV DNA and RNA in oral epithelial cells obtained from saliva (33). In mucosal tissue biopsies from HIV-infected patients, HIV-positive lymphocytes were localized in both submucosal and mucosal layers in the vicinity of epithelial cells bearing HIV-1 DNA sequences (32). By electron microscopy, HIV was detected in two-thirds of the buccal mucosal scrapings obtained from HIV-seropositive patients. The virus was localized in the interepithelial space bound by tight junctions, suggesting epithelial cell to epithelial cell contact as a route of transmission of HIV in mucosal linings. Consistent with this hypothesis, Qureshi et al. (32) reported histological studies of primary tissue samples indicating that epithelial cells were infected at the basal layer, migrated toward the superficial layers, and were then sloughed off into the oral cavity. Such results are consistent with in vitro data showing that epithelial cell lines can be productively infected with HIV-1 (9, 31, 43).
Clinical HIV isolates exhibit tropism for CD4+ monocytes and lymphocytes. Although CD4 functions as the major receptor for HIV-1 (29), infection of CD4-negative cells such as fibroblasts (44), oligodendrocytes (22), spermatozoa (4), and vaginal (19) or intestinal epithelial cells (46) indicates the existence of one or more alternate receptors for viral interaction. Several studies have shown that the glycosphingolipid galactosylceramide (GalCer) or its sulfated derivative 3′-sulfo-GalCer, can function as a primary receptor for HIV-1 in the absence of CD4 (2, 11, 22). GalCer has a strong affinity for the HIV-1 envelope glycoprotein gp120, and antibodies to GalCer block gp120 binding to GalCer in CD4− GalCer+ cells (16, 45, 47). Cell surface expression of GalCer in a CD4− human colon epithelial cell line increases vulnerability to HIV-1 infection (15), and inhibition of GalCer expression confers protection (48). Binding studies have shown that the recognition site for GalCer is located in the V3 loop of gp120 (47), although the C2 domain (amino acids 266 to 275) may also be involved (7).
In addition to CD4 and GalCer receptors, HIV requires an auxiliary chemokine coreceptor to infect human cells. These coreceptors are members of a large family of G protein-coupled receptors with seven-transmembrane domains (6) and include CXCR4, CCR2b, CCR3, CCR5, Bonzo (STRL33), BOB (GPR15), GPR1, and US28, although CCR5 and CXCR4 are those predominantly used by HIV-1 (50). Interaction with the CD4 receptor induces a conformational change in the viral envelope protein (34), which is required for subsequent interaction with the coreceptor (26). CXCR4 can also mediate CD4-independent entry of HIV-2 into some cells (13).
To define more clearly the molecular mechanisms by which oral mucosal cells might contribute to HIV-1 infection, we examined the capacity of normal human oral keratinocytes (NHOKs) to be productively infected by cell-free CXCR4-tropic, CCR5-tropic, and dualtropic HIV-1 strains in vitro. Recent evidence suggesting that dendritic cells may convey HIV-1 virions to lymphoid target cells without themselves being productively infected (14) also led us determine whether NHOKs might transfer HIV-1 to lymphocytes. To determine the mechanism of such effects, we surveyed NHOKs for the expression of CD4, chemokine coreceptors, and the alternative primary receptor GalCer. The results indicate that both CCR5- and CXCR4-tropic strains of HIV-1 establish early proviral DNA sequences in oral keratinocytes. For CXCR4- and dualtropic strains, full replication ensues to generate weak but productive infection of NHOKs. CCR5-tropic strains of HIV-1 generate minimal productive infection of NHOKs, apparently because of limited entry. However, coculture studies show that NHOKs can efficiently convey infectious HIV-1 virions to adjacent T lymphocytes, suggesting that the oral mucosa may represent a significant transmission reservoir in vivo.
MATERIALS AND METHODS
Cell culture.
NHOKs were cultured from tissue obtained during routine third-molar extraction from healthy, HIV-seronegative patients. Primary cultures were established as previously described (30). Briefly, the epithelial layer from the excised oral tissue was digested for 60 min at 37°C with collagenase type II (1.0 mg/ml; Millipore Corp., New Bedford, Mass.) and dispase grade II (2.4 mg/ml; Boehringer Mannheim, Indianapolis, Ind.) to separate the epithelium from the underlying submucosa. Epithelial sheets were then dispersed into individual cells by using trypsin. The cells were washed with phosphate-buffered saline (PBS), resuspended in keratinocyte growth medium (Clonetics, Corp., San Diego, Calif.), and 104 cells were plated on a 60-mm-diameter plastic tissue culture dish. Human peripheral blood lymphocytes (PBLs) from normal, HIV-seronegative donors were separated by Ficoll-Hypaque gradient centrifugation and cultured in RPMI 1640 medium supplemented with 10% pooled human AB serum (Gemini Bioproducts, Inc., Calabasas, Calif.), penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM). When required, the cells were costimulated with an anti-CD3 monoclonal antibody (MAb; 0.1 μg/ml) immobilized on goat anti-mouse antibody-coated flasks (Southern Biotechnology, Birmingham, Ala.) and soluble anti-CD28 (0.1 μg/ml; Biodesign, Kennebunkport, Maine) 2 days before infection.
Virus stocks.
HIV-1 strains NL4-3 (1) and 89.6 (12) have been previously described. CCR5-tropic strains JR-CSF (24), Ada-M (21), and Ba-L (20) and CXCR4-tropic strain ELI were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Rockville, Md.). Virus stocks were propagated by electroporation of infectious proviral DNA (30 μg) into CEM cells, followed by coculture with uninfected PBLs. To assess viral infection, measurements of p24gag in culture supernatants were performed by enzyme-linked immunosorbent assay (ELISA; Coulter, Hialeah, Fla.). Determination of numbers of infectious units per milliliter of virus stocks was achieved by infection of PBLs and followed by PCR for proviral sequences 24 h postinfection (49). Values were calculated by comparison to standard curves of known amounts of both HIV-1 plasmid DNA and human genomic DNA by phosphorimager analysis (Storm 860; Molecular Dynamics). Under our culture conditions, 1 ng of p24 is equivalent to 125 infectious units in PBLs.
HIV infection.
NHOKs were exposed to 400 ng of virus for 2 h at 37°C in the presence of 10 μg of Polybrene per ml. Virus stocks were filtered and treated with RNase-free DNase I (1 μg/ml; Worthington Biochemical Corp., Lakewood, N.J.) for 30 min at room temperature in the presence of 0.01 M MgCl2 to degrade contaminating HIV-1 DNA. Costimulated PBLs were used as a positive control for virus infection. Cells were rinsed and cultured for 16 h prior to DNA extraction for PCR quantification. As a control, cells were also infected with heat-inactivated virus, which was prepared by incubation at 65°C for 1 h.
Coculture assays.
For coculture assays, NHOKs were infected as described above and washed twice to remove any residual cell-free virus prior to the addition of anti-CD3- and anti-CD28-costimulated PBLs at a 1:1 ratio. After overnight incubation, PBLs were removed and washed extensively before culturing for 11 days postinfection. Increasing p24 concentrations in the culture supernatant and quantitative PCR of proviral DNA were used to demonstrate productive infection of NHOKs prior to the addition of uninfected PBLs. Since NHOKs and PBLs utilize different culture media, control experiments using both keratinocyte growth medium and RPMI 1640 medium supplemented with 10% human AB serum were conducted to determine cell viability during coculture.
Quantitative PCR.
To determine viral entry, infected cell pellets were resuspended in urea lysis buffer (4.7 M urea, 1.3% sodium dodecyl sulfate, 0.23 M NaCl, 0.67 mM Tris-HCl, pH 8.0) and the DNA was extracted with phenol and chloroform prior to ethanol precipitation. Quantitative PCR was performed on purified DNA samples by using primers that recognize the R/U5 region of the viral long terminal repeat (LTR) (M667 and AA55) to detect initiation of reverse transcription (49). Amplification of human β-globin sequences was also included as a control for cellular DNA input. After 25 cycles of PCR with 32P-end-labeled primers (49), the products were fractionated on a 6% polyacrylamide gel and quantified by comparison to standard curves of known amounts of both HIV-1 and human genomic DNA by phosphorimager analysis.
Identification of cell surface receptors on NHOKs.
Cell surface expression of known HIV-1 receptors was quantified by flow cytometry. Cultured NHOKs were harvested with 0.5 mM EDTA and washed once with PBS prior to the incubation of 2 × 105 cells for 15 min in 50 μl of fluorescence-activated cell sorter buffer (50% human AB serum, 50% PBS) containing 10 μl of phycoerythrin (PE)-conjugated MAbs. PE-conjugated MAbs against the major HIV-1 receptor, CD4, were obtained from Becton Dickinson Immunocytometry (Mountain View, Calif.), and PE-conjugated MAbs against the chemokine coreceptors CXCR4 and CCR5 were obtained from PharMingen (San Diego, Callif.). Cells stained with MAbs against GalCer (Galactocerebroside; Boehringer Mannheim) and Epithelium Pan (Chemicon International Inc.) were counterstained with PE-conjugated goat anti-mouse immunoglobulin G1 (IgG1) and goat F(ab′)2 anti-mouse IgG (heavy and light chains; Caltag Laboratories, Burlingame, Calif.), respectively. Following incubation, cells were washed with 1% fetal bovine serum in PBS and fixed in 2% paraformaldehyde. Mouse anti-human IgG1 (Becton Dickinson) was used as an antibody isotype control for MAbs against CD4 and GalCer and mouse IgG2a (Becton Dickinson) for MAbs against CXCR4 and CCR5. Flow cytometric data were accumulated with a FACScan instrument and analyzed with CellQuest software (Becton Dickinson Immunocytometry) with gating to exclude dead cells and debris on the basis of forward versus side scatter profiles. For the inhibition studies, NHOKs were pretreated with 10 μg of recombinant soluble CD4 (Life Science Products) per ml, 100 ng of SDF-1α (R&D Systems, Minneapolis, Minn.) per ml, 50 μg of anti-CCR5 MAb (PharMingen) per ml, or 2.5 μg of anti-GalCer MAb (Boehringer Mannheim) per ml for 1 h at 37°C. The cells were then exposed to HIV-1NL4-3 for an additional hour at 37°C in the continued presence of the inhibitors.
RESULTS
Analysis of reverse transcription in primary NHOK cells infected with HIV-1.
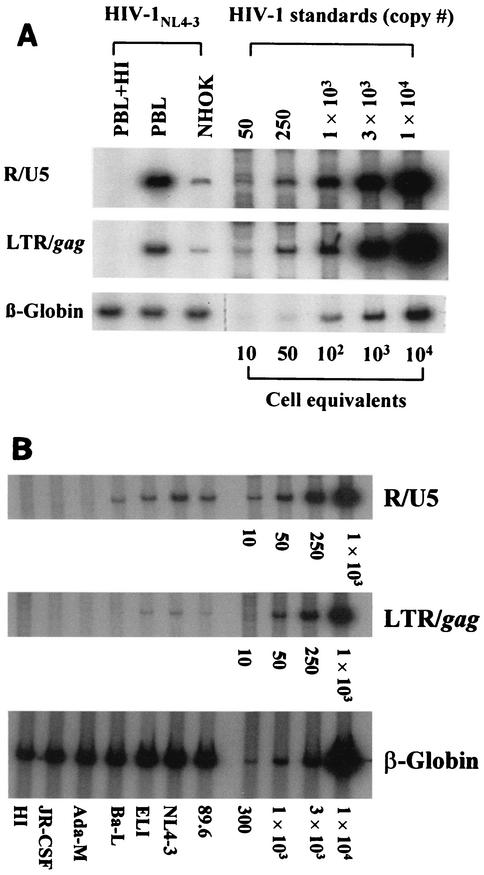
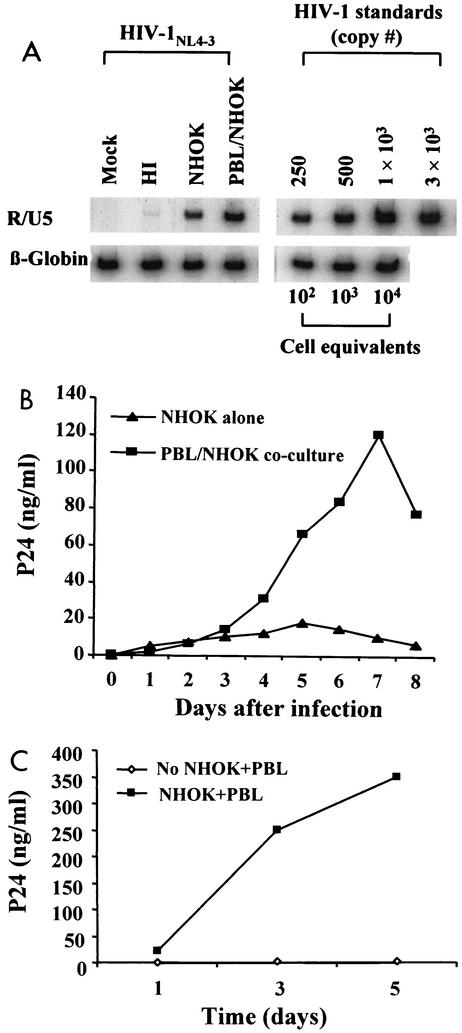
Sixteen hours after exposure to cell-free HIV-1, NHOKs were assayed for proviral DNA sequences by PCR amplification of R/U5 (M667 and AA55) and LTR/gag structures. Results showed that cell-free CXCR4-tropic NL4-3 entered NHOKs in vitro with an efficiency approximately one-fourth of that with which it entered an equivalent number of PBLs (Fig. 1A). CXCR4-tropic ELI (Fig. 1B), CCR5-tropic Ba-L, and the dualtropic 89.6 strain also infected NHOKs with an efficiency approximately one-eighth of that with which they infected PBLs, although full-length viral DNA was minimal for Ba-L (Fig. 1B). PCR failed to detect proviral DNA sequences in NHOKs following exposure to other CCR5-tropic strains of HIV-1, such as Ada-M or JR-CSF (Fig. 1B). Thus, NHOKs are substantially less vulnerable to infection by CCR5-tropic strains of HIV-1 than to infection by CXCR4-tropic strains. Proviral DNA sequences were absent from NHOKs incubated with heat-inactivated virus, confirming that the cells were initially free of HIV and that contaminating DNA in the virus stock was minimal.
FIG. 1.
(A) Quantitative PCR analysis of HIV-infected NHOKs and PBLs. NHOKs were exposed to 400 ng of live HIV-1NL4-3 or heat-inactivated (HI) virus for 2 h at 37°C in the presence of 10 μg of Polybrene per ml. Cells were rinsed and cultured for 16 h prior to DNA extraction. Viral DNA was PCR amplified with the R/U5 primer pair (M667 and AA55, 140 bp) to detect early viral LTR reverse transcripts and LTR/gag (M667 and M661, 200 bp) for full-length reverse transcripts. The amplified products were resolved on a 6% nondenaturing polyacrylamide gel and visualized by autoradiography. Each lane represents DNA from approximately 5 × 103 cells. β-Globin primers were used in parallel for a loading control. Heat inactivation of the virus was done for 1 h at 65°C, and activated PBLs were used as a positive control for virus infection. This is a representative result of more than five independent experiments. (B) Quantitative PCR analysis of NHOKs infected with primary HIV-1 strains.
HIV-1 replication in NHOKs in vitro.
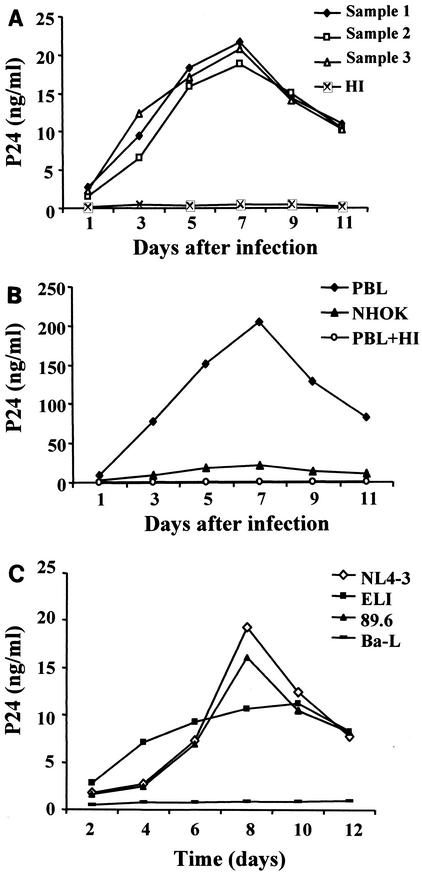
To determine whether proviral DNA structures were capable of sustaining productive viral gene expression, we assayed levels of p24gag in cell culture supernatants by ELISA. Following infection of NHOKs with cell-free NL4-3, p24 levels increased over 7 days in three independent experiments (Fig. 2A). p24 levels peaked at 20 ng/ml and subsequently declined after 7 days in culture (Fig. 2A). Similar growth profiles were observed in activated PBLs infected in parallel (Fig. 2B), but the peak p24 level was 10-fold higher (200 ng/ml) than that in NHOKs (20 ng/ml). As expected, p24 was not released from either NHOKs or PBLs infected with the heat-inactivated virus (Fig. 2A and B). Similar results were observed when dualtropic strain 89.6 was used (Fig. 2C), but no detectable replication was observed when CCR5-tropic strains JR-CSF, Ada-M, and Ba-L were used.
FIG. 2.
(A) p24gag antigen accumulation in the culture medium of HIV-1NL4-3-infected NHOKs. The cell-free culture medium of HIV-infected NHOKs was removed at the times indicated for measurement of p24gag levels by ELISA. The graph shows p24 levels versus the number of days postinfection, and the results are averages of three independent experiments. HI, heat-inactivated virus. (B) Experiment similar to that in panel A, except that the replication of HIV-1NL4-3 was compared between NHOKs and PBLs by assaying p24 levels by the ELISA method. (C) Summary of p24 levels in T-lymphotropic NL4-3-, ELI-, dualtropic 89.6-, and CCR5-tropic Ba-L-infected NHOKs.
Identification of HIV receptor molecules on NHOKs.
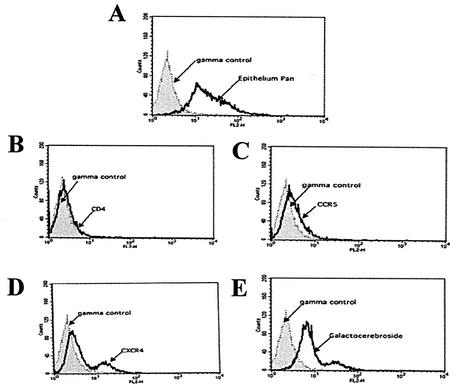
To identify cellular receptors for HIV-1 infection of NHOKs, cells were analyzed by flow cytometry for the primary receptors CD4 and GalCer and the coreceptors CXCR4 and CCR5. As shown in Fig. 3A, flow cytometry with the Epithelium Pan cell marker (human specific) indicated that the culture contained more than 90% epithelial cells. Analysis failed to detect any evidence of CD4 on NHOKs (Fig. 3B). However, the alternate receptor GalCer was expressed on NHOKs (Fig. 3E), as was the coreceptor CXCR4 (Fig. 3D). Small but detectable quantities of CCR5 were also observed (Fig. 3C).
FIG. 3.
Expression of HIV receptors on NHOKs. After culture for 2 weeks, 106 NHOKs were incubated with PE-conjugated MAbs (CD4, GalCer, CXCR4, and CCR5) and assayed by flow cytometry. Panels: A, Epithelium Pan control; B, CD4; C, CCR5; D, CXCR4; E, Galactocerebroside.
HIV-1 infection of NHOKs requires GalCer and CXCR4.
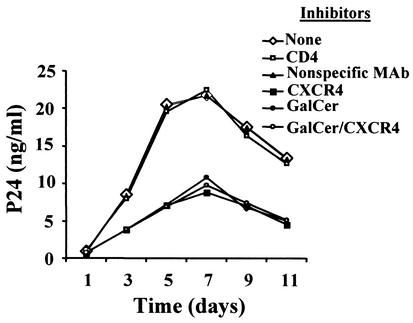
Inhibition studies were conducted to identify the cell surface receptors required for HIV-1 infection of NHOKs. Preincubation of cells for 1 h with soluble recombinant CD4 failed to block viral replication (Fig. 4). The control experiment with a nonspecific MAb or no inhibitor had no effect on HIV infection. However, HIV-1 replication was inhibited by approximately 50% in cells preincubated with SDF-1α or a MAb against GalCer. Complete inhibition of HIV-1 replication was not observed in the presence of both SDF-1α and the anti-GalCer MAb, suggesting that other viral coreceptors may play a role.
FIG. 4.
HIV-1NL4-3 requires GalCer and/or CXCR4 for NHOK infection. NHOKs were pretreated with various HIV-1 entry inhibitors for 1 h at 37°C and then exposed to HIV-1NL4-3 for 1 h at 37°C. The final concentrations of the inhibitors were 10 μg of recombinant soluble CD4 per ml, 100 ng of SDF-1α per ml, 50 μg of nonspecific MAb per ml, and 2.5 μg of anti-GalCer MAb per ml. Viral replication was analyzed by determining the p24 antigen concentration in the postinfection culture supernatant.
Transmission of HIV-1 from NHOKs to leukocytes.
To determine if NHOKs might convey infectious HIV-1 virions to adjacent lymphoid cells, oral keratinocytes were incubated with cell-free viral culture supernatants (400 ng of p24/ml) for 2 h, washed twice in PBS, and cocultured with PBLs overnight. PCR analysis of the viral early reverse transcripts at 12 h showed that 5 × 103 cell equivalents of NHOKs contained approximately 200 copies of HIV-1 (as in Fig. 1). Coculture with equal numbers of activated T lymphocytes resulted in a productive infection marked by an increasing prevalence of proviral DNA (Fig. 5A) and increasing supernatant p24 levels (Fig. 5B). Peak p24 production of approximately 10 ng/ml was observed in NHOKs at day 5 (Fig. 5B). p24 levels in NHOK/PBL coculture peaked at day 7 (120 ng/ml) at levels five time higher than those of NHOK alone. Similar results were obtained with CCR5-tropic HIV-1Ba-L (Fig. 5C). Thus, infected NHOKs can efficiently transfer infectious HIV-1 to adjacent PBLs even if there is little productive viral gene expression in the oral cells themselves (e.g., CCR5-tropic strains).
FIG. 5.
(A) Viral transmission from NHOKs to PBLs during coculture. NHOKs (5 × 103) were incubated for 2 h in culture supernatants containing approximately 300 ng of HIV-1NL4-3 per ml. Following incubation, NHOKs were washed extensively and cultured overnight with an equal number of uninfected activated PBLs. Nonadherent PBLs were then harvested, washed extensively, and incubated. After 48 h of culture, HIV-1 infection of cocultured PBLs was assessed by PCR amplification of viral early reverse transcripts. HI, heat inactivated. (B) HIV-1NL4-3 replication in coculture assays. p24 levels were assayed by ELISA in supernatants from the final 48 h of PBL culture in the experiment shown in panel A. Triangles, p24 levels of originally infected NHOKs before coculture; squares, p24 levels of rescuing PBLs after overnight incubation with infected NHOKs. (C) Experiment parallel to those in panels A and B following exposure of NHOKs to 300 ng of HIV-1Ba-L per ml for 2 h.
DISCUSSION
The present data show that normal human oral epithelial cells can be productively infected by HIV-1 and can transfer the infection to adjacent leukocytes in vitro. Infection by CXCR4-tropic viral strains is mediated in part by expression of CXCR4 and the glycolipid GalCer on NHOKs and results in productive viral expression with an efficiency approximately one-fourth of that in normal human PBLs. Small but detectable levels of CCR5 were also expressed by NHOKs. Some CCR5-tropic viral strains established reverse-transcribed DNA sequences, but productive replication was limited. However, coculture experiments showed that NHOKs could transfer infectious CCR5-tropic virions to adjacent PBLs, suggesting that oral epithelial cells may constitute an infectious reservoir for HIV-1, as has previously been documented for myeloid dendritic cells (14). These data suggest that expression of chemokine receptors and the alternative primary receptor GalCer may render NHOKs a cellular portal for HIV infection via oral mucosal exposure.
The present data are consistent with previous studies showing CD4-independent infection of epithelial cells derived from the intestine (15, 46) and oligodendrocytes (8). NHOKs bear GalCer and CXCR4, and inhibition studies show that both GalCer and CXCR4 play a role in mediating HIV-1 entry. However, the effects of these two molecules were not additive because treatment of NHOKs with both SDF-1α and an anti-GalCer MAb failed to fully block HIV-1 entry. These results suggest that other receptors may also be involved in HIV-1 entry into NHOKs under in vitro conditions, and the identification of such molecules remains an important topic for further research. The present data document low but detectable levels of CCR5 on NHOKs, and HIV-1 virions establish reverse-transcribed DNA structures in these cells. However, efficiency of entry was low compared to that into PBLs and productive expression of the HIV genome was minimal for CCR5-tropic strains. The poor efficiency of HIV-1 replication in NHOKs is likely due to the limited expression of CCR5 and CD4. In general, CCR5-tropic HIV-1 infection in activated PBLs results in approximately 10-fold higher p24gag levels in the supernatant. In the HT-29 colonic epithelial cell line expressing GalCer and CXCR4, the p24 level is approximately 1 ng/ml after 16 h of viral exposure (11). However, HIV-1 infection of the same HT-29 cell line expressing an exogenous CD4+ antigen resulted in about 10 ng of p24gag per ml, which was comparable to the level in activated PBLs.
Expression of GalCer, CXCR4, and, to a lesser extent, CCR5 on the surface of oral epithelial cells suggests that the oral mucosa may represent a significant portal for HIV-1 transmission. Given the limited efficiency of viral replication in NHOKs, the oral epithelium is unlikely to contribute significantly to the whole-body viral load of individuals who are already infected. However, to the extent that NHOKs can sustain low levels of productive infection, these cells may provide a foothold for HIV-1 in the body and subsequently transfer infection to more productive cell types, such as leukocytes trafficking through the oral mucosa (e.g., CD4+ T lymphocytes or monocytes/macrophages). Consistent with this hypothesis, we observed transfer of infection from NHOKs exposed to cell-free HIV-1 virions to peripheral blood leukocytes in coculture experiments. Such results are consistent with macaque studies showing that nontraumatic oral exposure to cell-free SIV resulted in viral infection and the subsequent development of simian AIDS (3). Similar patterns of viremia were observed in orally and intravenously infected macaques, and transmission of cell-free SIV was considerably more efficient via the oral route than via the intrarectal route.
Human saliva contains several types of anti-HIV-1 activity, that may help protect an individual against a small virus inoculum (39). However, if individuals are exposed to inocula containing a heavy viral load, it is conceivable that the oral epithelium could be infected and thus serve as a beachhead for HIV-1 infection. This is particularly true in cases of oral inflammation, but the present studies suggest that even uninflamed oral mucosal cells may sustain productive infection with CXCR4-tropic virus and transmit CCR5-tropic virus to circulating CD4+ T lymphocytes and macrophages. These results provide a cellular and molecular basis for understanding in vivo results from animal models and human case reports documenting HIV-1 seroconversion after oral-genital sex only (27). Further studies will help identify factors that influence oral keratinocytes' vulnerability to HIV-1 infection and define more fully their role in conveying infection to migrating leukocytes. It is hoped that these results will lead to the development of effective strategies for preventing oral HIV-1 transmission.
Acknowledgments
X.L. was supported by National Institute of Dental and Craniofacial Research, NIH, grant DE00430 and RCMI grants P20 RR11145 and 5U42RR14616; J.Z. was supported by NIH training grant T32-DE07296; J.A.Z. was supported by NIH grant AI36059; and S.W.C. was supported by NIH grants AI49135 and AI52737.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfsen, A., and M. Bomsel. 2002. HIV-1 gp41 envelope residues 650-685 exposed on native virus act as a lectin to bind epithelial cell galactosyl ceramide. J. Biol. Chem. 12:25649-25659. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., A. M. Trichel, L. An, V. Liska, L. N. Martin, M. Murphey-Corb, and R. M. Ruprecht. 1996. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science 272:1486-1489. [DOI] [PubMed] [Google Scholar]
- 4.Baccetti, B., A. Benedetto, A. G. Burrini, G. Collodel, E. C. Ceccarini, N. Crisa, A. Di Caro, M. Estenoz, A. R. Garbuglia, A. Massacesi, et al. 1994. HIV-particles in spermatozoa of patients with AIDS and their transfer into the oocyte. J. Cell Biol. 127:903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergey, E. J., M. I. Cho, B. M. Blumberg, M. L. Hammarskjold, D. Rekosh, L. G. Epstein, and M. J. Levine. 1994. Interaction of HIV-1 and human salivary mucins. J. Acquir. Immune Defic. Syndr. 7:995-1002. [PubMed] [Google Scholar]
- 6.Berson, J. F., D. Long, B. J. Doranz, J. Rucker, F. R. Jirik, and R. W. Doms. 1996. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J. Virol. 70:6288-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat, S., R. V. Mettus, E. P. Reddy, K. E. Ugen, V. Srikanthan, W. V. Williams, and D. B. Weiner. 1993. The galactosyl ceramide/sulfatide receptor binding region of HIV-1 gp120 maps to amino acids 206-275. AIDS Res. Hum. Retrovir. 9:175-181. [DOI] [PubMed] [Google Scholar]
- 8.Bhat, S., S. L. Spitalnik, F. Gonzalez-Scarano, and D. H. Silberberg. 1991. Galactosyl ceramide or a derivative is an essential component of the neural receptor for human immunodeficiency virus type 1 envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 88:7131-7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourinbaiar, A. S., and D. M. Phillips. 1991. Transmission of human immunodeficiency virus from monocytes to epithelia. J. Acquir. Immune Defic. Syndr. 4:56-63. [PubMed] [Google Scholar]
- 10.Chehimi, J., X. Ma, S. Chouaib, A. Zyad, T. Nagshunmugam, L. Wojcik, S. Chehimi, L. Nissim, and I. Frank. 1996. Differential production of interleukin 10 during human immunodeficiency virus infection. AIDS Res. Hum. Retrovir. 12:1141-1149. [DOI] [PubMed] [Google Scholar]
- 11.Delezay, O., N. Koch, N. Yahi, D. Hammache, C. Tourres, C. Tamalet, and J. Fantini. 1997. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. AIDS 11:1311-1318. [DOI] [PubMed] [Google Scholar]
- 12.Doranz, B. J., J. Rucker, Y. Yi, R. J. Smyth, M. Samson, S. C. Peiper, M. Parmentier, R. G. Collman, and R. W. Doms. 1996. A dualtropic primary HIV-1 isolate that uses fusion and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149-1158. [DOI] [PubMed] [Google Scholar]
- 13.Endres, M. J., P. R. Clapham, M. Marsh, M. Ahuja, J. D. Turner, A. McKnight, J. F. Thomas, B. Stoebenau-Haggarty, S. Choe, P. J. Vance, T. N. Wells, C. A. Power, S. S. Sutterwala, R. W. Doms, N. R. Landau, and J. A. Hoxie. 1996. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell 87:745-756. [DOI] [PubMed] [Google Scholar]
- 14.Engering, A., S. J. Van Vliet, T. B. Geijtenbeek, and Y. Van Kooyk. 2002. Subset of DC-SIGN+ dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood 1:1780-1786. [DOI] [PubMed] [Google Scholar]
- 15.Fantini, J., D. G. Cook, N. Nathanson, S. L. Spitalnik, and F. Gonzalez-Scarano. 1993. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc. Natl. Acad. Sci. USA 90:2700-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fantini, J., D. Hammache, O. Delezay, N. Yahi, C. Andre-Barres, I. Rico-Lattes, and A. Lattes. 1997. Synthetic soluble analogs of galactosylceramide (GalCer) bind to the V3 domain of HIV-1 gp120 and inhibit HIV-1-induced fusion and entry. J. Biol. Chem. 14:7245-7252. [DOI] [PubMed] [Google Scholar]
- 17.Faruque, S., B. R. Edlin, C. B. McCoy, C. O. Word, S. A. Larsen, D. S. Schmid, J. C. Von Bargen, and Y. Serrano. 1996. Crack cocaine smoking and oral sores in three inner-city neighborhoods. J. Acquir. Immune Defic. Syndr. 13:87-92. [DOI] [PubMed] [Google Scholar]
- 18.Fultz, P. 1986. Components of saliva inactivate human immunodeficiency virus. Lancet ii:1215. [DOI] [PubMed] [Google Scholar]
- 19.Furuta, Y., K. Eriksson, B. Svennerholm, P. Fredman, P. Horal, S. Jeansson, A. Vahlne, J. Holmgren, and C. Czerkinsky. 1994. Infection of vaginal and colonic epithelial cells by the human immunodeficiency virus type 1 is neutralized by antibodies raised against conserved epitopes in the envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 91:12559-12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gartner, S., P. Markovits, D. M. Markovitz, R. F. Betts, and M. Popovic. 1986. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA 256:2365-2371. [PubMed] [Google Scholar]
- 21.Gendelman, H. E., J. M. Orenstein, L. M. Baca, B. Weiser, H. Burger, D. C. Kalter, and M. S. Meltzer. 1989. The macrophage in the persistence and pathogenesis of HIV infection. AIDS 3:475-495. [DOI] [PubMed] [Google Scholar]
- 22.Harouse, J. M., S. Bhat, S. L. Spitalnik, M. Laughlin, K. Stefano, D. H. Silberberg, and F. Gonzalez-Scarano. 1991. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science 253:320-323. [DOI] [PubMed] [Google Scholar]
- 23.Janoff, E. N., R. W. Scamurra, T. C. Sanneman, K. Eidman, and J. R. Thurn. 1999. Human immunodeficiency virus type 1 and mucosal humoral defense. J. Infect. Dis. 179:S475-S479. [DOI] [PubMed] [Google Scholar]
- 24.Koyanagi, Y., S. Miles, R. T. Mitsuyasu, J. E. Merrill, H. V. Vinters, and I. S. Chen. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819-822. [DOI] [PubMed] [Google Scholar]
- 25.Lafferty, W. E., J. P. Hughes, and H. H. Handsfield. 1997. Sexually transmitted diseases in men who have sex with men: acquisition of gonorrhea and nongonococcal urethritis by fellatio and implications for STD/HIV prevention. Sex. Transm. Dis. 24:272-278. [DOI] [PubMed] [Google Scholar]
- 26.Lapham, C. K., J. Ouyang, B. Chandrasekhar, N. Y. Nguyen, D. S. Dimitrov, and H. Golding. 1996. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science 274:602-605. [DOI] [PubMed] [Google Scholar]
- 27.Lifson, A. R., P. M. O'Malley, N. A. Hessol, S. P. Buchbinder, L. Cannon, and G. W. Rutherford. 1990. HIV seroconversion in two homosexual men after receptive oral intercourse with ejaculation: implications for counseling concerning safe sexual practices. Am. J. Public Health 80:1509-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liuzzi, G., A. Chirianni, M. Clementi, P. Bagnarelli, A. Valenza, P. T. Cataldo, and M. Piazza. 1996. Analysis of HIV-1 load in blood, semen and saliva: evidence for different viral compartments in a cross-sectional and longitudinal study. AIDS 10:F51-F56. [DOI] [PubMed] [Google Scholar]
- 29.Maddon, P. J., A. G. Dalgleish, J. S. McDougal, P. R. Clapham, R. A. Weiss, and R. Axel. 1986. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47:333-348. [DOI] [PubMed] [Google Scholar]
- 30.Park, N.-H., B. M. Min, S. L. Li, M. Z. Huang, H. M. Cherick, and J. Doniger. 1991. Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis 12:1627-1631. [DOI] [PubMed] [Google Scholar]
- 31.Phillips, D. M., and A. S. Bourinbaiar. 1992. Mechanism of HIV spread from lymphocytes to epithelia. Virology 186:261-273. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi, M. N., C. E. Barr, I. Hewlitt, R. Boorstein, F. Kong, O. Bagasra, L. E. Bobroski, and B. Joshi. 1997. Detection of HIV in oral mucosal cells. Oral Dis. 3:S73-S78. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi, M. N., C. E. Barr, T. Seshamma, J. Reidy, R. J. Pomerantz, and O. Bagasra. 1995. Infection of oral mucosal cells by human immunodeficiency virus type 1 in seropositive persons. J. Infect. Dis. 171:190-193. [DOI] [PubMed] [Google Scholar]
- 34.Rizzuto, C. D., R. Wyatt, N. Hernandez-Ramos, Y. Sun, P. D. Kwong, W. A. Hendrickson, and J. Sodroski. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949-1953. [DOI] [PubMed] [Google Scholar]
- 35.Robinovitch, M. R., J. M. Iversen, and L. Resnick. 1993. Anti-infectivity activity of human salivary secretions toward human immunodeficiency virus. Crit. Rev. Oral Biol. Med. 4:455-459. [DOI] [PubMed] [Google Scholar]
- 36.Rothenberg, R. B., M. Scarlett, C. del Rio, D. Reznik, and C. O'Daniels. 1998. Oral transmission of HIV. AIDS 12:2095-2105. [DOI] [PubMed] [Google Scholar]
- 37.Samuel, M. C., N. Hessol, S. Shiboski, R. R. Engel, T. P. Speed, and W. Winkelstein, Jr. 1993. Factors associated with human immunodeficiency virus seroconversions in homosexual men in three San Francisco cohort studies. J. Acquir. Immune Defic. Syndr. 6:303-312. [PubMed] [Google Scholar]
- 38.Schwarcz, S. K., T. A. Kellogg, R. P. Kohn, M. H. Katz, G. F. Lemp, and G. A. Bolan. 1995. Temporal trends in human immunodeficiency virus seroprevalence and sexual behavior at the San Francisco municipal sexually transmitted disease clinic, 1989-1992. Am. J. Epidemiol. 142:314-322. [DOI] [PubMed] [Google Scholar]
- 39.Shine, N., K. Konopka, and N. Duzgunes. 1997. The anti-HIV-1 activity associated with saliva. J. Dent. Res. 76:634-640. [DOI] [PubMed] [Google Scholar]
- 40.Shugars, D. C., and S. M. Wahl. 1998. The role of the oral environment in HIV-1 transmission. J. Am. Dent. Assoc. 129:851-858. [DOI] [PubMed] [Google Scholar]
- 41.Spencer, J. D., N. Latt, P. J. Beeby, E. Collins, J. B. Saunders, G. W. McCaughan, and Y. E. Cossart. 1997. Transmission of hepatitis C virus to infants of human immunodeficiency virus-negative intravenous drug-using mothers: rate of infection and assessment of risk factors for transmission. J. Viral Hepatitis 4:395-409. [DOI] [PubMed] [Google Scholar]
- 42.Stahl-Hennig, C., R. M. Steinman, K. Tenner-Racz, M. Pope, N. Stolte, K. Matz-Rensing, G. Grobschupff, B. Raschdorff, G. Hunsmann, and P. Racz. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285:1261-1265. [DOI] [PubMed] [Google Scholar]
- 43.Tan, X., R. Pearce-Pratt, and D. M. Phillips. 1993. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J. Virol. 67:6447-6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tateno, M., F. Gonzalez-Scarano, F., and J. A. Levy. 1989. Human immunodeficiency virus can infect CD4-negative human fibroblastoid cells. Proc. Natl. Acad. Sci. USA 86:4287-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber, K. T., D. Hammache, J. Fantini, and B. Ganem. 2000. Synthesis of glycolipid analogues that disrupt binding of HIV-1 gp120 to galactosylceramide. Bioorg. Med. Chem. Lett. 15:1011-1014. [DOI] [PubMed] [Google Scholar]
- 46.Yahi, N., S. Baghdiguian, H. Moreau, and J. Fantini. 1992. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J. Virol. 66:4848-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yahi, N., J. M. Sabatier, S. Baghdiguian., F. Gonzalez-Scarano, and J. Fantini. 1995. Synthetic multimeric peptides derived from the principal neutralization domain (V3 loop) of human immunodeficiency virus type 1 (HIV-1) gp120 bind to galactosylceramide and block HIV-1 infection in a human CD4-negative mucosal epithelial cell line. J. Virol. 69:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yahi, N., S. L. Spitalnik, K. A. Stefano, P. De Micco, F. Gonzalez-Scarano, and J. Fantini. 1994. Interferon-gamma decreases cell surface expression of galactosyl ceramide, the receptor for HIV-1 GP120 on colonic epithelial cells. Virology 204:550-557. [DOI] [PubMed] [Google Scholar]
- 49.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, L., T. He, Y. Huang, Z. Chen, Y. Guo, S. Wu, K. J. Kunstman, R. C. Brown, J. P. Phair, A. U. Neumann, D. D. Ho., and S. M. Wolinsky. 1998. Chemokine coreceptor usage by diverse primary isolates of human immunodeficiency virus type 1. J. Virol. 72:9307-9312. [DOI] [PMC free article] [PubMed] [Google Scholar]