Abstract
We present a biochemical and morphological characterization of recycling endosomes containing the transferrin receptor in the epithelial Madin-Darby canine kidney cell line. We find that recycling endosomes are enriched in molecules known to regulate transferrin recycling but lack proteins involved in early endosome membrane dynamics, indicating that recycling endosomes are distinct from conventional early endosomes. We also find that recycling endosomes are less acidic than early endosomes because they lack a functional vacuolar ATPase. Furthermore, we show that recycling endosomes can be reached by apically internalized tracers, confirming that the apical endocytic pathway intersects the transferrin pathway. Strikingly, recycling endosomes are enriched in the raft lipids sphingomyelin and cholesterol as well as in the raft-associated proteins caveolin-1 and flotillin-1. These observations may suggest that a lipid-based sorting mechanism operates along the Madin-Darby canine kidney recycling pathway, contributing to the maintenance of cell polarity. Altogether, our data indicate that recycling endosomes and early endosomes differ functionally and biochemically and thus that different molecular mechanisms regulate protein sorting and membrane traffic at each step of the receptor recycling pathway.
INTRODUCTION
Receptor-mediated endocytosis involves sequential passage through distinct endosomal compartments. Internalized molecules first arrive in early endosomes, where a mildly acidic luminal pH favors uncoupling of ligands and receptors (Geuze et al., 1983; Yamashiro and Maxfield, 1984). Ligands destined for degradation, such as low-density lipoprotein (LDL), are then forwarded toward late endosomes and lysosomes, whereas housekeeping receptors, such as LDL-receptor (LDLR) and transferrin receptor (TfR), are recycled back to the plasma membrane via recycling endosomes to undergo further rounds of internalization (Gruenberg and Maxfield, 1995). Organelles involved in degradation or recycling exhibit strikingly different characteristics. The luminal pH decreases by more than a pH unit along the degradative pathway but was shown to increase along the recycling pathway in nonpolarized Chinese hamster ovary (CHO) cells (Yamashiro et al., 1984). Whereas endosomes along the degradative pathway contain numerous internal membranes, recycling endosomes consist of networks of 60-nm tubules organized around the microtubule-organizing center in some cell types. Early endosomes, which are common to both pathways, exhibit a complex cisternal, tubular, and vesicular organization. Although clear morphological differences can be observed between organelles on the two legs of the endocytic pathway, the molecular basis and the functional significance of these differences are not understood.
Segregation of ligands destined for degradation or recycling takes place with rapid kinetics (half-life < 3 min) in the early endosome (Yamashiro and Maxfield, 1987). A few sequence motifs responsible for sorting of proteins destined for late endosomes have been identified (Green et al., 1994; Subtil et al., 1997; Blagoveshchenskaya et al., 1998; Piguet et al., 1999). Formation of transport intermediates along the degradative pathway depends on the acidic pH of the early endosome (Clague et al., 1994), on the small GTP-binding protein ARF1 (Gu and Gruenberg, 2000), and on COPI coat proteins (Whitney et al., 1995; Aniento et al., 1996), and their transport is facilitated by polymerized microtubules (Gruenberg et al., 1989). Finally, N-ethylmaleimide sensitive factor (Robinson et al., 1997) and perhaps the small GTPase rab7 (Feng et al., 1995) are necessary for their docking and/or fusion with late endosomes. The molecular mechanisms responsible for receptor recycling remain unclear. Until now, no sorting signals have been identified in the cytoplasmic domain of recycling proteins. Therefore, it has been proposed that recycling receptors may be sorted by iterative fractionation (Dunn et al., 1989; Mayor et al., 1993). Several molecules were shown to play a role in TfR recycling, including cellubrevin, SNAP23, syntaxin13, calmodulin, the unconventional myosin myr4, EFA6, rab11bp, and the small GTPases rab4, rab11, rhoA, rhoD, and ARF6 (van der Sluijs et al., 1992; Apodaca et al., 1994a; Galli et al., 1994; D'Souza-Schorey et al., 1995; Murphy et al., 1996; Ullrich et al., 1996; Leung et al., 1998, 1999; Prekeris et al., 1998; Franco et al., 1999; Zeng et al., 1999; Huber et al., 2000), but their precise functions along the recycling pathway are not clear.
Polarized epithelial cells, such as Madin-Darby canine kidney (MDCK) cells, present additional complexity, because endocytosis occurs from both apical and basolateral plasma membrane domains (Parton, 1991; Mostov and Cardone, 1995). Whereas distinct sets of early endosomes are associated with each plasma membrane domain, late endosomes and lysosomes are common to both pathways (Bomsel et al., 1989, 1990; Parton et al., 1989). More recent studies indicate that communication also exists between apical and basolateral early endosomes, including along the routes followed by the TfR and the polymeric immunoglobulin (Ig) A receptor (Hughson and Hopkins, 1990; Apodaca et al., 1994b; Barroso and Sztul, 1994; Hunziker and Peters, 1998; Fialka et al., 1999). Whereas TfR constitutively cycles between the basolateral plasma membrane and the early endosomal system, ligand-bound polymeric IgR is transcytosed from the basolateral to the apical cell surface (Fuller and Simons, 1986; Mostov and Cardone, 1995). Despite their different final destinations and intracellular trafficking routes, both receptors can be observed within a network of thin tubules clustered in the apical region of the cell, which can also be reached by membrane-bound markers internalized from the apical surface (Apodaca et al., 1994b; Barroso and Sztul, 1994; Hunziker and Peters, 1998). These elements, which closely resemble the recycling endosomes observed in some nonpolarized cells (Yamashiro and Maxfield, 1984; Marsh et al., 1995), have sometimes been referred to as the apical recycling compartment (ARC) (Apodaca et al., 1994b). It is not clear whether passage through this apically located recycling endosome is obligatory for basolaterally recycling molecules or whether a specialized transcytosis compartment exists downstream of the ARC (Gibson et al., 1998). Importantly, protein sorting must occur in the ARC, because both transcytosing and recycling molecules have been shown to colocalize within the same structures before being selectively transported to opposite plasma membrane domains.
To better characterize recycling endosomes in polarized MDCK cells at the molecular level, we have established a subcellular fractionation protocol to specifically isolate the compartment. We show that the protein composition of recycling endosomes is distinct from that of sorting endosomes, providing biochemical evidence that the two organelles are physically separated from each other. Our data show that the luminal pH of recycling endosomes in polarized cells is less acidic than that of sorting endosomes and suggest that this pH difference is due to the absence of functional vacuolar ATPase in recycling endosomes. Last, we show that lipids and proteins generally believed to associate into membrane microdomains are highly enriched in recycling endosomes, probably creating a unique lipid environment within recycling endosomes. We propose that this environment may contribute to protein and lipid sorting along the recycling and/or transcytotic pathways.
MATERIALS AND METHODS
Reagents
M450 sheep anti-mouse Dynabeads were from Dynal (Oslo, Norway). Fluorescent probes were from Molecular Probes Europe (Leiden, The Netherlands). Human holo-Tf conjugated with HRP, apo-Tf, HRP, filipin, and fish skin gelatin were from Sigma (Division of Fluka Chemie, Buchs, Switzerland). Immobilized pH gradient strips were from Amersham Pharmacia Biotech (Dübendorf, Switzerland). All chemical reagents were from Fluka Chemie or Merck (Dietikon, Switzerland). Tissue culture media were from Life Technologies (Basel, Switzerland).
Antibodies
The anti-myc hybridoma cell line (MYC1-9E10.2; CRL1729) was obtained from the American Type Culture Collection (Rockville, MD). Antibodies against the luminal (B3/25) and cytoplasmic (H68.4) domains of the human TfR were purchased from Boehringer Mannheim (Rotkreuz, Switzerland) and Zymed Laboratories (San Francisco, CA), respectively. Anti-ZO1 and anti-caveolin-1 antibodies were purchased from Chemicon International (Temecula, CA) and Transduction Laboratories (Basel, Switzerland), respectively. Antibodies against β1 and β2 adaptins were purchased from Sigma. Anti-peptide antibodies against p23, Rab4, and Rab7 were raised in rabbits and affinity purified as described (Rojo et al., 1997). Other antibodies were generous gifts: annexin I and annexin II (V. Gerke, University of Münster, Münster, Germany), vacuolar ATPase A subunit (D. Stone, University of Texas Southwestern Medical Center, Dallas, TX), cellubrevin and rab5 (R. Jahn, Max Planck Institute, Göttingen, Germany), rab11 (R. Parton, University of Queensland, Brisbane, Australia), EEA1 (H. Stenmark, Norwegian Radium Hospital, Oslo, Norway), and calnexin (A. Helenius, Federal Polytechnical School, Zürich, Switzerland). Secondary antibodies were from Amersham Pharmacia Biotech or Dianova (Hamburg, Germany).
Cells
MDCK II cells were stably transfected with the pCB6 plasmid containing the human TfR with (m-hTfR cells) or without (hTfR cells) a single myc tag at its cytoplasmic N terminus. Transfection was carried out by the calcium phosphate method and was followed by selection with G418 (Life Technologies). To avoid loss of expression, cells were not used for more than 8–10 passages. Cells were maintained as described (Bomsel et al., 1989). Unless indicated otherwise, cells were seeded at high confluence onto prewet polycarbonate filters (Corning Costar Europe, Badhoevedorp, The Netherlands) and used after 4 d in culture with daily medium changes.
Labeling Conditions
To label the plasma membrane, the basolateral side of the cells was incubated on ice with 50 μg/ml Tf-HRP in internalization medium (IM) (of G-MEM, 10 mM Hepes pH 7.4, 5 mM glucose) containing 2 mg/ml BSA (IM/BSA). Unbound label was removed by two washes with ice-cold PBS+/BSA (5 mg/ml BSA, 1 mM CaCl2, 1 mM MgCl2). To label recycling endosomes, Tf conjugates (HRP or rhodamine; 50 μg/ml) were prebound on ice and then internalized at 37°C for 10 min in IM/BSA. Residual plasma membrane label was removed by an ice-cold deferoxamine mesylate wash as described (Jing et al., 1990). Sphingomyelin–BODIPY (4,4-difluoro-4-borα-32,42-diazα-s-indacene) (5 μM) was added to both sides of the filters for 30 min at 37°C simultaneously with continuous Tf–rhodamine (25 μg/ml) uptake. To label apical early endosomes, dextran–Oregon Green (10 kDa dextran; 3 mg/ml in IM) or HRP (5 mg/ml in IM) was added to the cells from the apical side for 10 min at 37°C. Noninternalized label was removed by three washes with PBS+/BSA. To label late endosomes, cells were incubated with HRP (5 mg/ml) at 37°C for 15 min and the label was chased for 30 min in IM/BSA. When cells were labeled with HRP or Tf-HRP, enzymatic activity was measured as described (Gruenberg et al., 1989).
Fluorescence
For fluorescence experiments, cells were grown on either glass coverslips or 12-mm Transclear Costar filters. When appropriate, filter-grown cells were labeled with lysine-fixable endocytic tracers before fixation. In this case, ice-cold 3% paraformaldehyde (PFA) was added to the cells and fixation was completed after 20 min at room temperature. For immunofluorescence experiments, cells were fixed with either 3% PFA for 20 min at room temperature or with methyl alcohol for 4 min at −20°C. PFA autofluorescence was quenched with 50 mM NH4Cl, and the cells were permeabilized with 0.05% saponin during incubation with the primary antibody. Fish skin gelatin (0.2%) was used to block unspecific binding. Cholesterol was labeled with filipin (50 μg/ml) after PFA fixation as described (Kobayashi et al., 1999). Antibodies were added to the apical side of whole filters. Filters were cut out from their supports, mounted onto microscope slides in Mowiol (Calbiochem, La Jolla, CA), and observed with a confocal laser scanning microscope as described (Rojo et al., 1997). When cells were labeled with sphingomyelin–BODIPY, the filters were mounted onto microscope slides in ice-cold PBS+ and observed without previous fixation.
Immunoelectron Microscopy
MDCK cells were perforated and labeled exactly as described previously (Ikonen et al., 1996). Briefly, MDCK cells grown on filters were perforated with the use of nitrocellulose filters applied to the apical surface. The opened cells were then labeled with anti-caveolin-1 antibodies (Dupree et al., 1993) followed by 10-nm protein A–gold (University of Utrecht, Utrecht, The Netherlands) and embedded in Epon. Cells were sectioned perpendicular to the filter support. Sections were examined on a JEOL (Tokyo, Japan) 1010 microscope.
Subcellular Fractionation
All experiments were carried out with m-hTfR and hTfR cells in parallel. Cells grown on 75-mm Costar filters were scraped with a rubber policeman, and postnuclear supernatants were prepared as described (Bomsel et al., 1990). The postnuclear supernatants (4 mg/ml, 500 μl) were precleared by centrifugation at 13,000 rpm for 5 min and then incubated with 9E10 culture supernatant (0.05 mg/ml) for 30 min on a rotating wheel at 2 rpm. Unspecific binding was reduced by a 15-min incubation with 0.3 M KCl. Salt washes were omitted when assaying for the presence of EEA1 in the isolated fractions. Endosomal membranes were then sedimented through a 20% sucrose cushion onto a 35% sucrose cushion at 150,000 × g in a swing-out ultracentrifuge rotor. For immunoisolation, the 20/35% sucrose interface was collected and membranes (25 μg) were incubated with anti-mouse Dynabeads (50-μl slurry) in PBS containing 5 mg/ml BSA for 2 h on a rotating wheel at 2 rpm. Fifty percent of the input material for the immunoisolation, referred to as “IN,” and the unbound material after immunoisolation, referred to as “UB,” were sedimented at 150,000 × g for 30 min and resuspended in sample buffer. The beads were washed with PBS containing 5 mg/ml BSA and 0.3 M KCl, and the final bead pellet was taken up in sample buffer (“B”).
pH Measurements
Subconfluent m-hTfR cells, grown on glass coverslips, were labeled either by preincubation with Tf-FITC (50 μg/ml) at 4°C followed by internalization at room temperature for 5 min or by continuous internalization with Tf-FITC (25 μg/ml) for 20 min at 37°C. Endosomal pH was measured by ratio fluorescence imaging as described (Piguet et al., 1999) with the use of a Zeiss Axiovert S100 TV fluorescence microscope and a 100×, 1.3 numerical aperture oil-immersion objective (Carl Zeiss, Feldbach, Switzerland). Coverslips were inserted into a perfusion chamber (Medical Systems, Greenvale, NY) at room temperature in 1 ml of IM and imaged with a 12-bit, cooled charge-coupled device interlined camera (Visitron Systems, Puchheim, Germany) controlled by MetaMorph/Metafluor software (Universal Imaging, West Chester, PA). Images were acquired for 1 s a wave length (λ) = 490 ± 6 nm and for 2 s at λ = 440 ± 6 nm with the use of a DeltaRam monochromator (PTI, Monmouth Junction, NJ), a 510 DRLP dichroic mirror, and a 535 ± 25 nm emission filter (Omega Opticals, Brattleboro, VT). Calibration and image processing were performed as described previously (Demaurex et al., 1998).
Lipid Analysis
Cells were metabolically labeled overnight with [32P]orthophosphate (0.5 mCi/filter; Amersham Pharmacia Biotech) or [14C]acetate (50 μCi/filter; Amersham Pharmacia Biotech), and purified endosomal fractions were prepared. Lipid extraction was carried out as described (Kobayashi et al., 1998b). Phospholipids were separated by two-dimensional chromatography on 10 × 10 cm silica gel 60 HPTLC plates (Merck) in chloroform:methanol:32% ammonia (65:35:5, vol/vol) for the first dimension and chloroform:acetone:methanol:acetic acid:water (50:20:10:12.5:5, vol/vol) for the second dimension. Cholesterol was separated in one dimension by TLC. The migration was carried out in two steps, first in chloroform:methanol:32% ammonia (65:35:5, vol/vol) to a height of 6 cm, then in hexane:diethyl ether:acetic acid (16:4.2, vol/vol) to the top. Lipids were detected by autoradiography and quantitated with a PhosphorImager with the use of the Molecular Imager System (Bio-Rad Laboratories, Glattbrugg, Switzerland).
Electrophoresis
Bidimensional electrophoresis was carried out as described (Pasquali et al., 1997). Cells were labeled metabolically overnight with [35S]Met (0.5 mCi/filter; New England Nuclear Life Science Products, Brussels, Belgium), and purified endosomal fractions were prepared. Samples were taken up directly in 400 μl of isoelectric focusing buffer and loaded in-gel for 8 h at room temperature with the use of 18-cm immobilized pH gradient strips with nonlinear isoelectric point gradients from 3.5 to 10. The first dimension was run for 65 kV/h at 15°C. The second dimension was run on 9–16% SDS-polyacrylamide gels at 6°C. The gels were treated with Entensify (New England Nuclear Life Science Products) and dried, and proteins were revealed by autoradiography.
RESULTS
Characterization of TfR-expressing MDCK Cell Lines
To characterize endosomes along the TfR recycling pathway in polarized MDCK cells, we decided to use an immunoaffinity purification protocol. We generated stable cell lines expressing human TfR, myc-tagged at the N-terminal cytoplasmic domain (m-hTfR), to allow isolation of endosomes containing m-hTfR with the anti-myc mAb 9E10 on magnetic beads coated with anti-mouse antibodies. As a control, we also prepared stable cell lines expressing untagged human TfR (hTfR), and we carried out all experiments in parallel with cells expressing comparable levels of hTfR or m-hTfR (Figure 1A). We felt that this strategy would provide the most stringent conditions for controlling the specificity of immunoisolation. This proved necessary, because optimization tests revealed that beads without antibodies or beads coated with irrelevant antibodies, which have been commonly used by us and others as negative controls, frequently underestimate nonspecific binding to the beads, which largely depends on the quality of the antibody preparation.
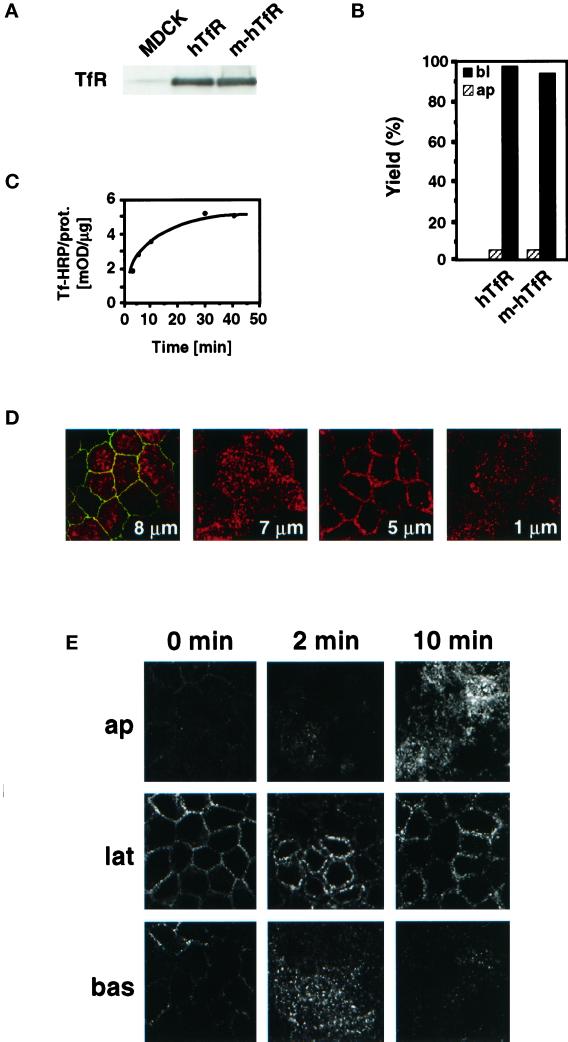
Figure 1.
Characterization of the hTfR-expressing MDCK cell lines. (A) Total cell extracts prepared from untransfected MDCK cells and cells expressing hTfR or m-hTfR were analyzed by SDS-PAGE and Western blotting with the use of antibodies against hTfR. (B) Cell surface distribution of TfR was measured by incubating the apical (ap) or basolateral (bl) side of filter-grown hTfR or m-hTfR cells with Tf-HRP at 4°C. Results are presented as percentages of total cell-associated HRP activity. (C) Continuous Tf-HRP uptake at 37°C was measured in polarized m-hTfR cells as a function of time. Cells were incubated with Tf-HRP. At the indicated times, cell-associated HRP activity was quantified and normalized to total cellular protein. (D) TfR (red) topology in polarized m-hTfR cells was analyzed by immunofluorescence confocal microscopy with the use of antibodies against hTfR. Tight junctions (green) were identified with the use of antibodies against ZO1. The distance between the section plane and the filter is indicated on each panel. (E) Cellular distribution of endocytosed Tf–rhodamine. Tf–rhodamine was bound to the basolateral plasma membrane at 4°C and internalized at 37°C for the indicated times. Confocal microscope sections taken through the apical (ap), lateral (lat), and basal (bas) regions of the cells are shown.
Both cell lines showed the characteristic features of polarized MDCK cells. Like parental cells, cells expressing hTfR or m-hTfR reached a height of ∼12 μm, and their transepithelial resistance was >200 Ω/cm2. The normal polarization of the cells was further confirmed by the typical distribution of ZO1, a marker of tight junctions (Stevenson et al., 1986), which distributed in both cell lines as a ring around the apical portion of the cell (Figure 1D). We next analyzed the cell surface distribution of hTfR and m-hTfR in our cell lines, because endogenous cell surface TfR molecules are known to be restricted to the basolateral plasma membrane domain in MDCK cells (Fuller and Simons, 1986). Binding of human Tf conjugated to HRP (hTf-HRP) to either the apical or the basolateral plasma membrane at 4°C showed that cell surface human receptors were >95% basolateral at steady state (Figure 1B). Under our conditions, parental MDCK II cells did not bind hTf. Exogenous hTfR or m-hTfR was mostly intracellular and could be found within structures that extended throughout the apical and basolateral cytoplasm of fully polarized cells (Figure 1D). No TfR could be detected in the apical pole of the cells above the level of the tight junctions or on the apical plasma membrane. Both m-hTfR (Figure 1C) and hTfR supported hTf-HRP endocytosis, and the kinetics of the process were similar to those reported previously (Harding et al., 1983). Tf–rhodamine endocytosed by cells expressing m-hTfR (Figure 1E) or hTfR distributed first to basolateral endosomes after brief incubation times and then to subapical endosomes, presumably corresponding to the ARC (Apodaca et al., 1994b). These morphological and biochemical observations demonstrate that hTfR and m-hTfR were correctly localized and functional in these cell lines.
Isolation of TfR-positive Endosomes
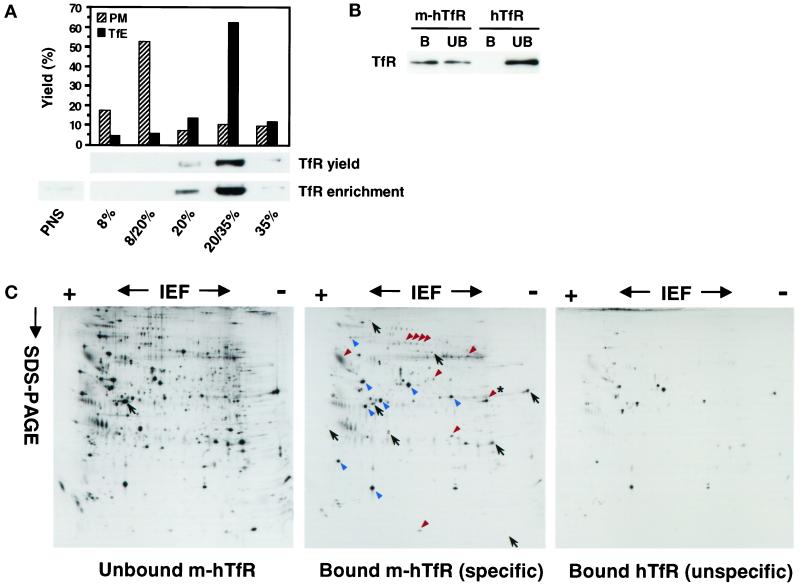
For immunoisolation experiments, postnuclear supernatants (PNSs) were prepared from m-hTfR and hTfR cells and incubated at 4°C with the anti-myc antibody. In a second step, PNSs from these cells were fractionated on a discontinuous sucrose gradient to remove the excess free antibody but also to prepare TfR-enriched fractions for subsequent immunoisolation. The bulk of total cellular TfR (65%) was recovered at the 20/35% interface of the gradient, where the receptor was enriched ≈10-fold (Figure 2A; see also Figure 8B). Because the bulk of the receptor is in endosomes at steady state (Figure 1D), this fraction is likely to contain mostly endosomal TfR. A minor portion of TfR is present on the basolateral cell surface at steady state. Therefore, we also analyzed the distribution of the basolateral plasma membrane on the gradient and found that it was enriched at the 8/20% interface and not at the 20/35% interface (Figure 2A). In a third step, fractions recovered from the 20/35% interface were incubated at 4°C with magnetic beads coated with anti-mouse antibodies and washed. Bead-bound membranes were then recovered with a magnet and analyzed. TfR-containing membranes were immunoisolated with a 50% yield from m-hTfR cells (Figure 2B), and nonspecific binding was negligible, as determined by carrying out the same experiment with hTfR cells (Figure 2B). The total enrichment of the purification is estimated to ≈150-fold with a yield of ≈35% (see Figure 8). The immunoisolated fraction did not contain detectable levels of calnexin, an abundant protein of the endoplasmic reticulum (Trombetta and Helenius, 1998), or p23, an abundant Golgi protein (Rojo et al., 1997). In addition, we did not detect in the fractions the plasma membrane-associated α and β2-adaptins and the trans-Golgi network-associated β1-adaptin. (Kirchhausen, 1999), although the bulk remained membrane associated during isolation. The fraction was also devoid of the small GTPase ARF6 and of annexin II (see Figure 3B and DISCUSSION). Annexin II is very abundant on the cytoplasmic face of the plasma membrane and early endosomes and tightly membrane associated (Harder et al., 1997). Altogether, these observations indicate that the purified fraction was not contaminated to any significant extent with the plasma membrane or with biosynthetic membranes. To analyze the general protein pattern of TfR-containing membranes, cells were metabolically labeled with [35S]Met before the experiment, and then immunoisolated fractions were analyzed by two-dimensional gel electrophoresis and autoradiography (Figure 2C). Only a limited subset of labeled polypeptides copurified with the TfR, confirming that the immunoisolation procedure was highly specific (the protein marked with an asterisk in Figure 2C is discussed below). Isolated proteins (red arrowheads) were highly enriched compared with the unbound material recovered after immunoisolation (black arrows) and were not detected in control samples obtained from hTfR cells (contaminants indicated with blue arrowheads).
Figure 2.
Purification of TfR-positive endosomes. (A) Lower panel, PNSs prepared from cells expressing m-hTfR were fractionated on a discontinuous sucrose gradient loaded with 8, 20, and 35% sucrose. After centrifugation, fractions were collected and analyzed by SDS-PAGE and Western blotting with the use of anti-TfR antibodies as in Figure 1A. Lanes were loaded with either the same amount of protein (enrichment) or 10% of the volume (yield) of each fraction. Upper panel, To label the plasma membrane (PM), Tf-HRP was bound to the surface of m-hTfR cells at 4°C. To label recycling endosomes (TfE), Tf-HRP was bound to the surface of m-hTfR cells at 4°C and internalized for 10 min at 37°C. PNSs were prepared and fractionated, and the HRP content of the fractions was quantified. (B) Membranes enriched in m-hTfR were prepared with the use of the sucrose gradient, as in A, and further fractionated by immunoisolation with the 9E10 mAb against the myc tag and magnetic beads. As a control for nonspecific binding, the whole purification procedure was carried out in parallel with hTfR cells. Beads were retrieved with a magnet, and then both bound material (B) and unbound material sedimented at 150,000 × g for 30 min (UB) were analyzed for TfR content by Western blotting as in Figure 1A. (C) The purification protocol was carried out as in B with the use of m-hTfR and hTfR cells that had been metabolically labeled with [35S]Met, and samples were analyzed by high-resolution two-dimensional gel electrophoresis followed by autoradiography. The general protein pattern of the immunoisolated fraction [Bound m-hTfR (specific)] was compared with that of the unbound material sedimented at 150,000 × g for 30 min [Unbound m-hTfR] to determine which proteins were specifically immunoisolated (examples are indicated with red arrowheads). The asterisk indicates the position of flotillin-1 in the same gel system. Examples of proteins that were not isolated are indicated with black arrows. Contaminants (blue arrowheads) were determined by comparison with samples from hTfR cells [Bound hTfR (unspecific)]. The position of actin, which is commonly used for orientation in this type of analysis, is indicated on the unbound m-hTfR gel.
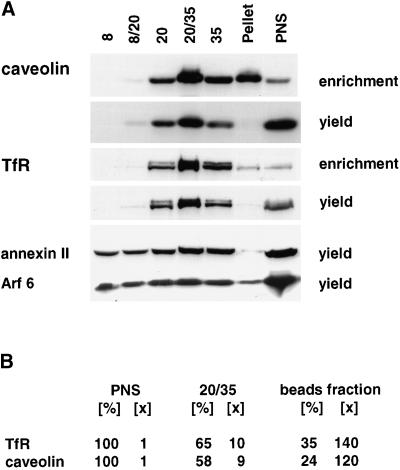
Figure 8.
Caveolin copurifies with the Tf receptor. (A) Cells expressing m-hTfR were fractionated on a discontinuous sucrose gradient loaded with 8, 20, and 35% sucrose, as in Figure 2A. Fractions were collected and analyzed by SDS-PAGE and Western blotting with the use of antibodies against caveolin or TfR as a control. Lanes were loaded either with the same amount of protein (enrichment) or with 10% of the volume (yield) of each fraction. As controls, the figure also shows the distribution of annexin II and Arf6, which were not detected in the immunoisolated fractions. (B) Amounts of caveolin and TfR were quantified by scanning the gels in a typical experiment; yields [%] and enrichment [×] are indicated, both for the 20/30% gradient interface (as in A) and for the immunoisolated fractions (as in Figure 3A).
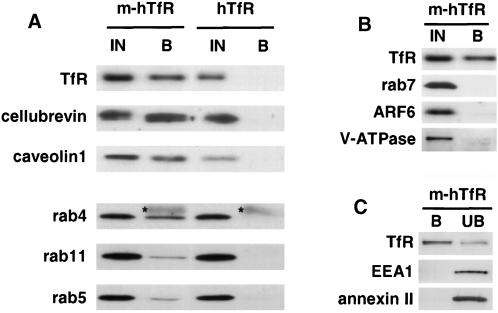
Figure 3.
Protein composition of TfR-positive endosomes. Endosomes containing m-hTfR were purified, with the use of cells expressing hTfR as a control, as in Figure 2, B and C. (A and B) Immunoisolated membranes bound to the beads (B) were analyzed by SDS-PAGE and Western blotting with the use of specific antibodies against the indicated proteins. For comparison, 50% of the total fraction used as starting material for immunoisolation (IN) was also analyzed. Asterisks mark the light chain of the antibody used for immunoisolation. (C) Immunoisolated membranes (B) are compared with unbound material sedimented at 150,000 × g for 30 min (UB) as in Figure 2B. Analysis was as in A and B, with the use of specific antibodies against the indicated proteins.
Characterization of Purified TfR-positive Endosomes
We first analyzed our TfR-positive endosomal fractions for their content in proteins reported to regulate Tf recycling. Figure 3A shows that the v-SNARE cellubrevin, which has been implicated in Tf recycling in nonpolarized cells (Galli et al., 1994) and colocalizes with the TfR by immunofluorescence (McMahon et al., 1993), was isolated with the same efficiency as the TfR itself. The small GTPase rab4 also cofractionated with TfR (Figure 3A). This GTPase has been directly implicated in Tf recycling (van der Sluijs et al., 1992) and colocalizes with the TfR in endosomes, but it is absent from the plasma membrane (Van Der Sluijs et al., 1991). Rab11 is present on early endosomal membranes and the trans-Golgi network and has been proposed to function in Tf recycling (Ullrich et al., 1996). In addition, rab11 was recently shown to be present on apical recycling endosomes in MDCK cells (Casanova et al., 1999). As shown in Figure 3A, rab11 was coisolated with the TfR, although clearly to a lesser extent than rab4, presumably because of its broader cellular distribution. As a control, Figure 3B shows that, as expected, immunoisolated fractions did not contain the late endosomal marker rab7 (Chavrier et al., 1990). Finally, the small GTP-binding protein ARF6 has also been proposed to play a role in TfR trafficking (D'Souza-Schorey et al., 1995), although its precise function is unclear (D'Souza-Schorey et al., 1997; Radhakrishna and Donaldson, 1997; Song et al., 1998; Altschuler et al., 1999). ARF6 is clearly present on the plasma membrane, but its endosomal localization is controversial (Peters et al., 1995; Cavenagh et al., 1996; D'Souza-Schorey et al., 1998). Although tightly membrane-associated (Gu and Gruenberg, 2000), ARF6 was not detected in the fractions (Figure 3B).
Next, we analyzed the distribution of rab5, which is known to localize to the plasma membrane and early endosomes (Chavrier et al., 1990) and to be involved in both internalization and early endosome docking and/or fusion (Gorvel et al., 1991; Bucci et al., 1992). Much like rab11, rab5 did not efficiently cofractionate with TfR during immunoisolation (Figure 3A), although the protein remained membrane-associated. These observations prompted us to test whether our fractions contained the rab5 effector protein EEA1, which regulates early endosome docking and/or fusion (Simonsen et al., 1998). Interestingly, we could not detect EEA1 in immunoisolated fractions enriched in TfR (Figure 3C). Although only peripherally associated with membranes, EEA1 could be sedimented from the supernatant after immunoisolation, indicating that it remained to a large extent membrane-associated (Figure 3C). We then analyzed the distribution of annexin II, which was also implicated in early endosome dynamics and is an abundant component of the plasma membrane and early endosomes, both in nonpolarized baby hamster kidney cells and in polarized MDCK cells (Gruenberg and Emans, 1993; Harder et al., 1997). As shown in Figure 3C, our fractions were also devoid of annexin II, although the protein is tightly membrane-associated (Harder et al., 1997). Thus, we find that TfR copurifies with proteins known to regulate Tf recycling but not with the early endosomal proteins EEA1 and annexin II.
Acidification Properties of MDCK TfR-containing Recycling Endosomes
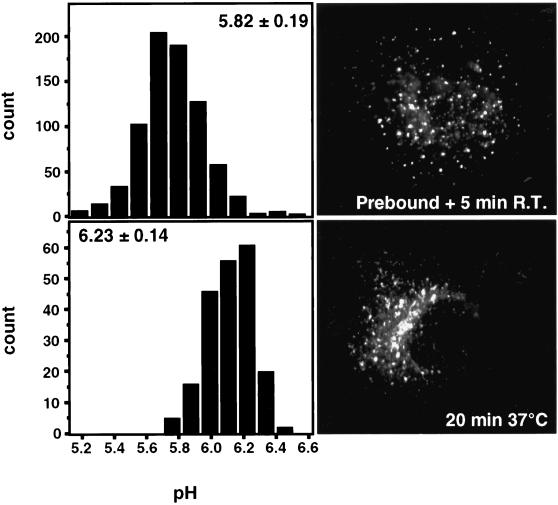
Because endocytosed TfR is expected to transit through early (sorting) endosomes before appearing in recycling endosomes, the absence of EEA1 and annexin II in our fractions was somewhat unexpected. Therefore, we decided to make use of the pH-dependent fluorescence emission of FITC to follow the pathway of Tf-FITC through acidic endosomes by fluorescence ratio imaging at the level of single organelles. Indeed, it is well established that early (sorting) endosomes are acidic, but recycling endosomes were reported to be less acidic in nonpolarized CHO cells (Yamashiro et al., 1984). As shown in Figure 4, Tf-FITC transits soon after internalization through a peripherally located acidic compartment, presumably corresponding to early endosomes (pH = 5.8 ± 0.2). However, transit through this compartment was extremely rapid, because Tf-FITC resided in acidic endosomes only for the time required for image acquisition (i.e., ≈3 min) when internalized at 20°C. Within <2 min at 37°C, corresponding to the situation illustrated in Figure 1E, the bulk of Tf-FITC had already exited these acidic endosomes. As shown in Figure 4, after longer incubation times at 37°C, Tf-FITC distributed to a less acidic compartment (pH = 6.2 ± 0.1), indicating that recycling endosomes in MDCK cells are less acidic than early endosomes. (This pH difference was not due to a direct temperature effect on the fluorescence signal, because all pH measurements were carried out at 20°C). These experiments also show that TfR transit through acidic early endosomes is very rapid and thus that they contain little receptor at steady state. These observations agree well with our findings that TfR does not copurify with the early endosomal proteins EEA1 and annexin II (Figure 3C). TfR may be removed from early endosomes so efficiently that its levels in early endosomes remain too low for these membranes to be isolated under our experimental conditions.
Figure 4.
Recycling endosomes in MDCK cells are less acidic than early endosomes. Tf-FITC was prebound at 4°C to m-hTfR cells and then internalized for 5 min at 20°C (upper panels) or internalized continuously for 20 min at 37°C (lower panels). Typical examples of the respective labels are shown. Because of technical constraints, we used subconfluent cells grown on glass coverslips rather than on filters. The pH of individual organelles was measured by fluorescence ratio imaging of internalized Tf-FITC. The histograms show the pH distribution of 767 and 206 endosomes for the upper and lower panels, respectively, with the mean ± SD given in the left panels.
It is well established that the acidic pH of endosomes depends on the action of the vacuolar (H+)-ATPase (V-ATPase), but the mechanisms regulating vacuolar acidification are not well understood (Stevens and Forgac, 1997). As shown in Figure 3B, V-ATPase could not be detected in the recycling endosome fraction with the use of antibodies against the A subunit, which is part of the peripheral V1 domain, although it remained membrane-associated throughout the purification procedure. This was true whether endosomes were immunoisolated from MDCK cells grown on glass coverslips or on filters (Figure 3B). Altogether, our data show that recycling endosomes are enriched in proteins known to regulate Tf recycling but devoid of EEA1, annexin II, and functional V-ATPase, hence that recycling endosomes differ from early endosomes. The lack of V-ATPase is likely a direct cause of the reduced acidification of recycling endosomes in MDCK cells.
The Apical Endocytic Pathway Intersects the Tf Recycling Pathway
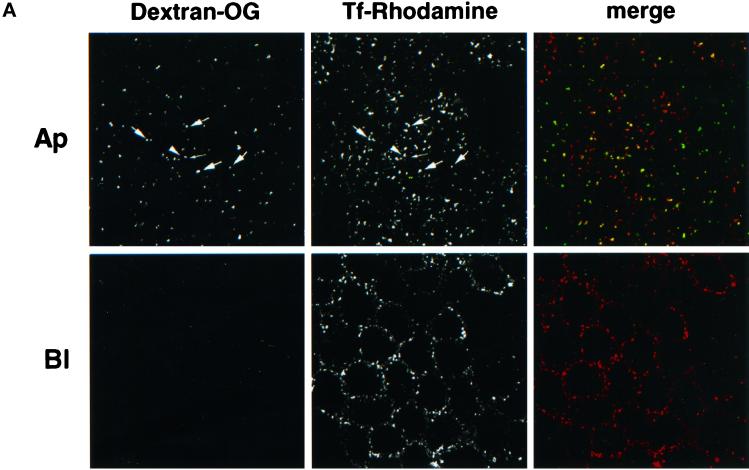
Although mixing of apically and basolaterally internalized fluid phase tracers cannot be observed in early endosomes (Bomsel et al., 1989; Parton et al., 1989), apical and basolateral recycling of membrane-bound tracers as well as transcytosis are believed to occur through a common compartment (Hughson and Hopkins, 1990; Apodaca et al., 1994b; Barroso and Sztul, 1994; Hunziker and Peters, 1998; Fialka et al., 1999). We investigated whether a fluid phase tracer endocytosed from the apical surface was able to reach recycling endosomes containing Tf. As shown in Figure 5A, recycling endosomes labeled with Tf–rhodamine internalized for 30 min from the basolateral surface could be reached by dextran–Oregon Green internalized from the apical surface for 10 min. We then made use of our immunoisolation protocol to quantify bulk transport of an apically endocytosed fluid phase tracer into recycling endosomes. Cells were incubated for 10 min with HRP on the apical side of the monolayer, and then recycling endosomes were immunoisolated as described above. As shown in Figure 5B, significant amounts of HRP cofractionated with TfR, because the yield of latent HRP isolation was ≈20%. In contrast, apically endocytosed HRP, which was chased for 30 min in marker-free medium and therefore reached late endosomes (Bomsel et al., 1989; Parton et al., 1989), did not cofractionate with TfR, as expected. Together, these data confirm that receptor molecules are more efficiently transferred to recycling endosomes than the early endosomal content (Geuze et al., 1983). However, they also indicate that a significant portion of the early endosomal content is transferred to recycling endosomes, consistent with observations that at least 50% of endocytosed fluid phase tracers are regurgitated into the medium (Besterman et al., 1981; Bomsel et al., 1989). In previous studies, it has sometimes been difficult to detect fluid phase markers in recycling endosomes in both polarized cells (Apodaca et al., 1994b; Barroso and Sztul, 1994) and nonpolarized cells (Tooze and Hollinshead, 1991), presumably because dilution of content markers within the thin tubular networks may have rendered detection difficult (Tooze and Hollinshead, 1991; Verges et al., 1999). Finally, and most importantly, these data show that recycling endosomes containing the TfR can be reached by fluid phase tracers endocytosed from the apical surface and hence that the apical endocytic pathway intersects the Tf recycling pathway, in agreement with our recent observations with an in vitro transport assay (Huber et al., 2000).
Figure 5.
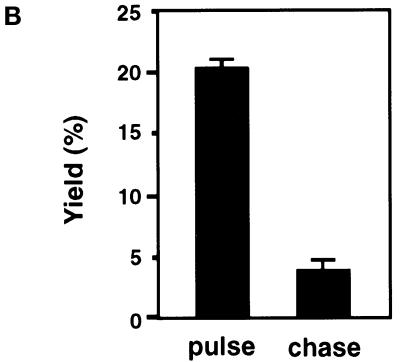
The apical endocytic pathway intersects the Tf recycling pathway. (A) m-hTfR cells were allowed to endocytose Tf–rhodamine from the basolateral medium for 30 min and dextran–Oregon Green (dextran–OG) from the apical medium for the last 10 min, and cells were analyzed by confocal fluorescence microscopy. Confocal sections in the apical (Ap) or basolateral (Bl) region of the cell are shown. Large arrows indicate organelles positive for both Tf–rhodamine and dextran–OG. Small arrows indicate organelles that contain only dextran–OG, and arrowheads indicate organelles that contain only Tf–rhodamine. (B) Cells expressing m-hTfR were allowed to endocytose HRP from the apical medium for 10 min (pulse) or for 15 min followed by a 30-min incubation in marker-free medium (chase). Endosomes containing m-hTfR were immunoisolated as in Figure 2, B and C. Bound material and unbound material sedimented at 150,000 × g for 30 min were analyzed for HRP activity. Results are expressed as percentages of the total HRP activity.
Raft Components in Recycling Endosomes
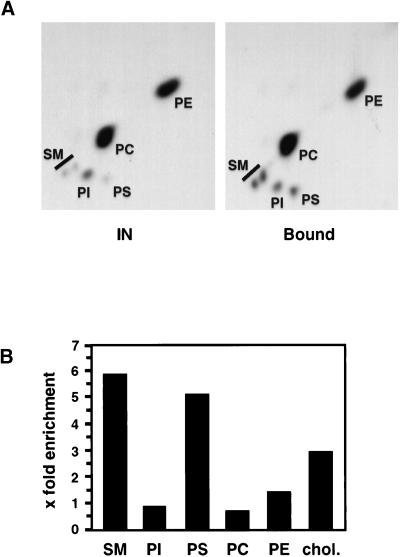
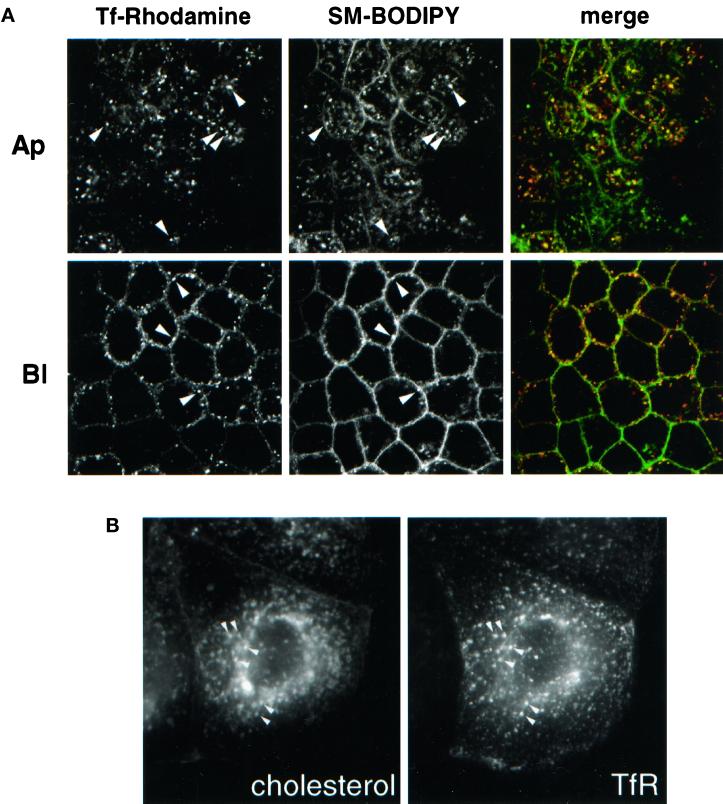
Next, we analyzed the lipid composition of recycling endosomes after metabolic labeling of m-hTfR cells with [32P]orthophosphate or [14C]acetate overnight. Recycling endosomes were prepared by immunoisolation as described above. Lipids were then extracted and analyzed by TLC on silica gel plates. As shown in Figure 6B, cholesterol and sphingomyelin as well as phosphatidylserine were highly enriched in the purified recycling endosome fraction (a typical phospholipid TLC analysis is shown in Figure 6A). To further confirm this result, we incorporated sphingomyelin–BODIPY into the plasma membrane of filter-grown m-hTfR MDCK cells and then incubated the cells at 37°C to follow the subcellular distribution of the lipid (Pagano et al., 1991). As shown in Figure 7A, sphingomyelin–BODIPY colocalized intracellularly with endocytosed Tf–rhodamine in both the apical and basolateral regions of the cells, suggesting that the lipid was present in recycling endosomes. The steady-state distribution of endogenous cholesterol can easily be revealed by light microscopy with the use of filipin as a fluorescent marker (Kobayashi et al., 1999). Much like sphingomyelin–BODIPY, cholesterol colocalized intracellularly with endocytosed Tf–rhodamine (Figure 7B).
Figure 6.
Raft components are enriched in purified recycling endosomes. Endosomes containing m-hTfR were purified, with the use of cells expressing hTfR as a control, as in Figure 2, B and C, from cells that had been metabolically labeled with [32P]orthophosphate (for phospholipids) or [14C]acetate (for cholesterol). Lipids were extracted and analyzed by two-dimensional TLC (phospholipids) or one-dimensional TLC (cholesterol). (A) Two-dimensional TLC of 32P-labeled immunoisolated fractions from cells expressing m-hTfR (Bound) compared with the starting material (IN). The same amounts (dpm) were loaded in each case. Only samples from m-hTfR cells are shown in this typical experiment. (B) Radioactivity was quantified with the PhosphorImager. Indicated values correspond to the enrichment over the starting material (expressed as relative specific activity), normalized to nonspecific binding measured with the use of hTfR cells. SM, sphingomyelin; PI, phosphatidylinositol; PS, phosphatidylserine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; chol., cholesterol.
Figure 7.
Raft components distribute to recycling endosomes. (A) Filter-grown m-hTfR cells were allowed to incorporate sphingomyelin (SM)–BODIPY for 30 min from both sides of the filters. Simultaneously, Tf–rhodamine was internalized from the basolateral side of the filters. Confocal sections in the apical (Ap) or basolateral (Bl) region of live cells are shown. Arrowheads indicate intracellular colocalization between sphingomyelin–BODIPY and Tf–rhodamine in both the apical and basolateral regions of the cells. (B) Cells expressing m-hTfR grown on coverslips were processed for double-label immunofluorescence analysis, with the use of antibodies against hTfR as in Figure 1C, and filipin to reveal the distribution of cholesterol. Arrowheads indicate intracellular colocalization between cholesterol and TfR.
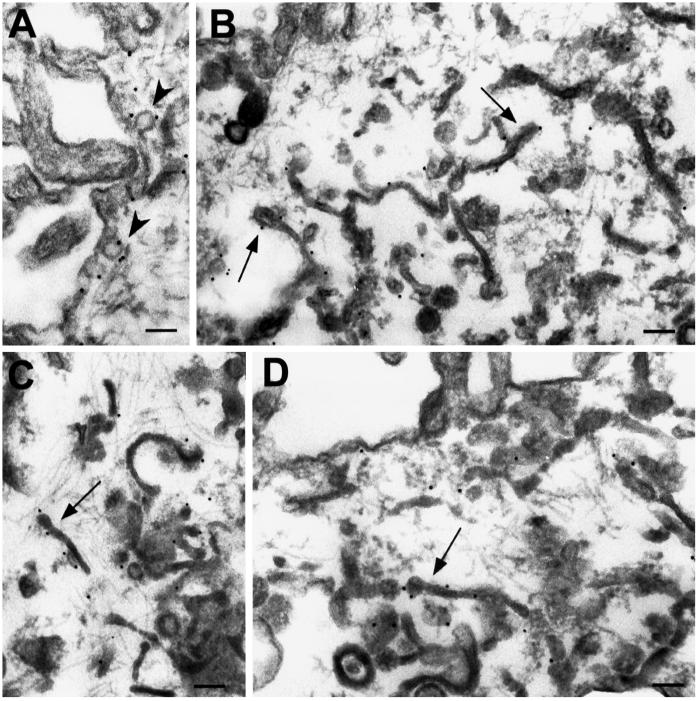
Sphingomyelin and other sphingolipids can transiently associate with cholesterol, presumably forming liquid-ordered phase microdomains in the plane of the membrane (reviewed by Brown and London, 1998), which were termed rafts by Simons and collaborators (Simons and Ikonen, 1997). Accumulating data suggest the involvement of rafts in signal transduction, pathogen infection, and cell motility (Field et al., 1997; Okamoto et al., 1998; Abrami and van Der Goot, 1999; Manes et al., 1999; Viola et al., 1999) as well as in protein sorting in the trans-Golgi network, including in MDCK cells (Simons and Ikonen, 1997). Raft components, although present on both plasma membrane domains, are more abundant on the apical plasma membrane of MDCK cells (van Meer et al., 1987). The presence of raft lipids in recycling endosomes prompted us to test whether these also contained caveolin-1. This cholesterol-binding protein is known to associate with cell surface invaginations termed caveolae, which are composed of lipid rafts, and was also shown to be present intracellularly (Dupree et al., 1993; Kurzchalia and Parton, 1999). We found that caveolin-1 efficiently cofractionated with the TfR, both on the gradient (Figure 8A) and during immunoisolation (Figure 3B), yields and enrichment during purification being comparable for both proteins (Figure 8B). We were unable to localize caveolin-1 on cryosections with available antibodies. However, after preembedding labeling of permeabilized cells, anticaveolin-1 antibodies decorated a network of thin tubules with a morphology similar to that of recycling endosomes (Figure 9). Remarkably, a polypeptide specifically enriched in purified recycling endosomes (Figure 2C, asterisk) was identified by tandem-mass spectrometry with the use of the same two-dimensional gel system (M. Fivaz, F. Vilbois, and G. van Der Goot, personal communication) as the recently described protein flotillin-1, a component of lipid rafts and caveolae (Bickel et al., 1997), which is particularly abundant in kidney cells (Volonte et al., 1999). This polypeptide was significantly enriched, because it could not be detected even in the original PNS (Figure 2C). Altogether, these data demonstrate that raft lipids as well as caveolin-1, and presumably flotillin-1, which are known to associate with membrane microdomains, are specifically enriched in purified recycling endosomes. The recycling endosome membrane thus appears to be heterogeneous in composition, like the plasma membrane (Harder et al., 1998; Abrami and van Der Goot, 1999), and to contain both raft and nonraft components (e.g., TfR).
Figure 9.
Immunoelectron microscopic localization of caveolin-1 in MDCK cells. Filter-grown MDCK cells were perforated with the use of nitrocellulose to remove parts of the apical surface. Cells were labeled for caveolin-1, fixed, and processed for Epon embedding. (A) Image shows an area of the lateral membrane with gold labeling associated with caveolae (arrowheads). (B–D) Images show areas underlying the apical plasma membrane. Labeling for caveolin-1 is associated with extensive tubular structures (arrows). Bars, 100 nm.
DISCUSSION
In this paper, we show that recycling endosomes in polarized MDCK cells are enriched in proteins that regulate the TfR cycle but do not contain molecules involved in the dynamics of early endosomes. These observations suggest that different molecular mechanisms regulate membrane traffic in sorting and recycling endosomes. We also show that recycling endosomes in MDCK cells are less acidic than early endosomes, because they lack functional V-ATPase. Consistent with observations that the apical endocytic pathway intersects the basolateral Tf recycling pathway, we show that recycling endosomes are enriched in raft components, which are known to be abundant at the apical plasma membrane. Together, these data support the notion that apically and basolaterally endocytosed molecules meet in a common recycling endosome and suggest that selective partitioning into membrane microdomains may contribute to protein sorting.
Molecular Composition
Purified recycling endosomes are enriched in proteins known to regulate Tf cycling, including the v-SNARE cellubrevin and the small GTPases rab4 and to some extent rab11, in agreement with Verges et al. (1999). In contrast, these fractions strikingly lack the early endosomal proteins EEA1 and annexin II. Our finding that EEA1 is absent from recycling endosomes is consistent with its characteristic highly punctate and scattered pattern observed by immunofluorescence (Mu et al., 1995), including in CHO cells (Gu et al., 1997), in which recycling endosomes are clustered in the perinuclear region (Yamashiro and Maxfield, 1984; Marsh et al., 1995). Altogether, these observations suggest that EEA1 is restricted to early endosomes, hence that its function as a rab5 effector in docking and/or fusion (Simonsen et al., 1998; Christoforidis et al., 1999) is also limited to these membranes, in agreement with Trischler et al. (1999). Because EEA1 binds to phosphatidylinositol 3-phosphate rich membranes through its FYVE finger domain, in addition to interacting with membrane-bound GTP-rab5 (Stenmark et al., 1996; Simonsen et al., 1998), one may speculate that phosphatidylinositol 3-phosphate itself is restricted to early endosomes. In contrast to EEA1, rab5 can be detected in recycling endosomes, although at low abundance, consistent with partial colocalization of rab5 with TfR (Chavrier et al., 1990). This somewhat broader distribution of rab5 compared with EEA1 agrees with the view that rab5 may exert its functions through more than one effector (Stenmark et al., 1995; Gournier et al., 1998; Simonsen et al., 1998).
Our observation that annexin II is not found on recycling endosomes agrees with the distribution of the protein to early endosomal elements with a characteristic tubulo-vesicular and cisternal appearance, which are labeled by fluid phase markers within 5–10 min (Emans et al., 1993; Harder et al., 1997). The precise function of the protein is unclear, although previous studies suggested that it is involved in endosomal membrane dynamics (Gruenberg and Emans, 1993; Harder and Gerke, 1993). Together, our observations suggest that EEA1 and annexin II act at the level of early endosomes exclusively, in contrast to cellubrevin and rab4, which regulate Tf recycling. Hence, different molecular mechanisms are likely to regulate membrane traffic in recycling and early endosomes.
Topology and Organization
We find that transit of endocytosed Tf through early endosomes is extremely rapid, because Tf exits acidic endosomes in <3 min at 37°C. Then, endocytosed Tf first appears in recycling endosomes in the basolateral cytoplasm before reaching a compartment located in the supranuclear apical portion of the cell, presumably corresponding to the ARC (Hughson and Hopkins, 1990; Apodaca et al., 1994b; Barroso and Sztul, 1994). Both populations should contain large amounts of TfR at steady state and therefore are probably equally well represented in our biochemical analysis, lacking EEA1, annexin II, and functional V-ATPase, as opposed to early endosomes. Thus, recycling endosomes appear to distribute both along the basolateral membrane and close to the centrioles, which are located underneath the apical membrane in MDCK cells (Buendia et al., 1990). This dual distribution appears to contrast with the exclusive pericentriolar localization of recycling endosomes in nonpolarized CHO cells (Yamashiro et al., 1984). However, a similar dual distribution can be observed in neurons, in which the distribution of TfR is polarized (Parton et al., 1992). In these cells, the intracellular localization of TfR is not restricted to the vicinity of centrioles, because tubular TfR-containing endosomes are abundant in the dendrites. Recent studies suggest that, in MDCK cells, the majority (>65%) of recycling to the basolateral surface occurs from basolateral early endosomes, whereas segregation of basolateral receptors from receptors intended for transcytosis takes place in apical recycling endosomes (Sheff et al., 1999). Based on these and our observations, we propose that, after rapid transit through basolateral early endosomes, recycling of receptors back to the cell surface begins in proximal elements of the recycling pathway in the basolateral cytoplasm and continues in more distal elements in the apical cytoplasm, perhaps reflecting the rapid and slow recycling routes in nonpolarized cells (Mayor et al., 1993; Presley et al., 1993; Gruenberg and Maxfield, 1995).
Acidification Properties
Our observations that recycling endosomes are less acidic than early endosomes might help to explain the barrier function of epithelial cells, because recycling endosomes intersect apical and basolateral uptake routes. Some receptor–ligand complexes may escape dissociation in early endosomes, transit being very rapid. If recycling endosomes were acidic, these complexes might dissociate and, like any solute, free ligand molecules would be regurgitated in a nonpolarized manner to both sides of the monolayer. Our observations, however, suggest that uncoupling is unlikely to occur in recycling endosomes and hence that receptor-bound ligand molecules that escaped from early endosomes can be recycled and undergo a second round of internalization. Reduced acidification may thus contribute to limit the delivery of endocytosed ligands to the wrong side of the epithelium.
The acidic pH of endosomes depends on the action of the V-ATPase (Stevens and Forgac, 1997). Several mechanisms have been proposed to regulate the activity of this pump, including reversible dissociation of the V1 and V0 domains (Kane, 1995), disulfide bond formation at the catalytic site (Feng and Forgac, 1994), control of pump density (Sturgill-Koszycki et al., 1994a,b), and counteraction by other pumps (Cain et al., 1989; Fuchs et al., 1989). Our data show that the limited acidification of recycling endosomes in MDCK cells is due to the absence of the pump, or at least the V1 domain, suggesting that pH may be regulated via V-ATPase exclusion from recycling endosomes or by regulated V0–V1 dissociation. Intriguingly, we find that the pH of recycling endosomes is nevertheless slightly acidic, perhaps resulting from the transfer of acidic content from early endosomes or from the action of residual V-ATPase molecules below the detection threshold.
Raft Components
We find that the raft lipids cholesterol and sphingomyelin are enriched in recycling endosomes. In marked contrast, late endosomes contain little free cholesterol and sphingomyelin but high amounts of the unconventional lipid lysobisphosphatidic acid (Kobayashi et al., 1998b, 1999). Thus, raft lipids appear not to distribute stochastically within all endosomal membranes, raising the question of how they are sorted away from the pathway leading to degradation. Raft components may be internalized via clathrin-coated vesicles but there may also be, at least in some cell types, raft-specific pathways for endocytosis (Parton, 1996). Caveolae, which form cell surface invaginations containing the cholesterol-binding protein caveolin, and lipid rafts apparently can be internalized, at least in some cell types (Parton, 1996; Schnitzer et al., 1996a,b). Our findings that raft components are enriched in recycling endosomes suggest that internalization of caveolae, whether occurring through a specialized pathway or not, leads to endosomes.
It is attractive to speculate that the selective incorporation of raft components in recycling endosomes contributes to regulate protein/lipid sorting and trafficking. Lipids and proteins, which tend to partition within raft domains, may be preferentially incorporated within the recycling and/or transcytotic pathways. Indeed, once internalized, fluorescent derivatives of sphingolipids are rapidly returned to the cell surface much like recycling receptors in nonpolarized cells (for review, see Kobayashi et al., 1998a). Moreover, glycosylphosphatidyl inositol-anchored proteins, which are associated with rafts on the cell surface, follow the same pathway as TfR after endocytosis into nonpolarized cells, albeit with slower kinetics, and their retention in endosomes depends on membrane cholesterol (Mayor et al., 1998). A similar mechanism may extend to transcytosing proteins, because in small intestinal explants, dimeric IgA internalized and transcytosed via the polymeric IgR was found to associate with a detergent-insoluble membrane fraction (Hansen et al., 1999). Mechanisms regulating the incorporation of raft components into the recycling pathway remain to be determined. It has been proposed that preferential packing of sphingolipids and cholesterol is facilitated by the long, saturated hydrocarbon chains of sphingolipids but also by intermolecular hydrogen bonds, especially involving the carbohydrate head groups of glycosphingolipids (Simons and Ikonen, 1997), although this issue is controversial (Ostermeyer et al., 1999). One may envision that alkalinization of recycling endosomes could contribute to facilitate head group interactions, mimicking the extracellular environment, and thus the incorporation of raft components into the pathway.
Finally, proteins and lipids present in recycling endosomes must be sorted and then recycled or transcytosed to the opposite membrane domain. In the biosynthetic pathway, it was shown that sorting of apically destined molecules in the trans-Golgi network depends, at least in part, on N-linked carbohydrates (Scheiffele et al., 1995; Gut et al., 1998; Benting et al., 1999). Alternatively, apical sorting was proposed to depend on selective incorporation of apically targeted proteins into raft lipid microdomains (van Meer and Simons, 1988; Lafont et al., 1998, 1999). Based on our observations, we propose that a similar lipid-based sorting mechanism may operate in recycling endosomes to ensure that the proper polarized composition of each membrane domain is maintained.
ACKNOWLEDGMENTS
We thank Marie-Hélène Beuchat for expert technical assistance. We also thank Gisou van Der Goot, Karl Matter, and Irene Fialka for critical reading of the manuscript. We are particularly grateful to Toshihide Kobayashi and Nathalie Mayran for their help with some experiments. This work was supported by grant 961235 from the National Health and Medical Research Council of Australia (to R.G.P.), by grant 31-37296.93 from the Swiss National Science Foundation (to J.G.), by grant KFS240-12-1995 from the Swiss Cancer Research Foundation (to J.G. and L.A.H.), by grant RG 355/94 from the International Human Frontier Science Program (to J.G. and R.G.P.), and by grants 3100-050581.97 (to W.H.) and 31-46859.96 (to N.D.) from the Swiss National Science Foundation.
REFERENCES
- Abrami L, van Der Goot FG. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J Cell Biol. 1999;147:175–184. doi: 10.1083/jcb.147.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler Y, Liu S, Katz L, Tang K, Hardy S, Brodsky F, Apodaca G, Mostov K. ADP-ribosylation factor 6 and endocytosis at the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1999;147:7–12. doi: 10.1083/jcb.147.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Enrich C, Mostov KE. The calmodulin antagonist, W-13, alters transcytosis, recycling, and the morphology of the endocytic pathway in Madin-Darby canine kidney cells. J Biol Chem. 1994a;269:19005–19013. [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994b;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso M, Sztul ES. Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome. J Cell Biol. 1994;124:83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benting JH, Rietveld AG, Simons K. N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells. J Cell Biol. 1999;146:313–320. doi: 10.1083/jcb.146.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman JM, Airhart JA, Woodworth RC, Low RB. Exocytosis of pinocytosed fluid in cultured cells: kinetic evidence for rapid turnover and compartmentation. J Cell Biol. 1981;91:716–727. doi: 10.1083/jcb.91.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF. Flotillin and epidermal surface antigen define a new family of caveolae- associated integral membrane proteins. J Biol Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Norcott JP, Cutler DF. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail. J Biol Chem. 1998;273:2729–2737. doi: 10.1074/jbc.273.5.2729. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Prydz K, Parton RG, Gruenberg J, Simons K. Endocytosis in filter-grown Madin-Darby canine kidney cells. J Cell Biol. 1989;109:3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Buendia B, Bre MH, Griffiths G, Karsenti E. Cytoskeletal control of centrioles movement during the establishment of polarity in Madin-Darby canine kidney cells. J Cell Biol. 1990;110:1123–1135. doi: 10.1083/jcb.110.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CC, Sipe DM, Murphy RF. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc Natl Acad Sci USA. 1989;86:544–548. doi: 10.1073/pnas.86.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenagh MM, Whitney JA, Carroll K, Zhang C, Boman AL, Rosenwald AG, Mellman I, Kahn RA. Intracellular distribution of Arf proteins in mammalian cells: Arf6 is uniquely localized to the plasma membrane. J Biol Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbe S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- Demaurex N, Furuya W, D'Souza S, Bonifacino JS, Grinstein S. Mechanism of acidification of the trans-Golgi network (TGN): in situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J Biol Chem. 1998;273:2044–2051. doi: 10.1074/jbc.273.4.2044. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Boshans RL, McDonough M, Stahl PD, Van Aelst L. A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Li G, Colombo MI, Stahl PD. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C, van Donselaar E, Hsu VW, Yang C, Stahl PD, Peters PJ. ARF6 targets recycling vesicles to the plasma membrane: insights from an ultrastructural investigation. J Cell Biol. 1998;140:603–616. doi: 10.1083/jcb.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree P, Parton RG, Raposo G, Kurzchalia TV, Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N, Gorvel J-P, Walter C, Gerke V, Griffiths G, Gruenberg J. Annexin II is a major component of fusogenic endosomal vesicles. J Cell Biol. 1993;120:1357–1370. doi: 10.1083/jcb.120.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Forgac M. Inhibition of vacuolar H(+)-ATPase by disulfide bond formation between cysteine 254 and cysteine 532 in subunit A. J Biol Chem. 1994;269:13224–13230. [PubMed] [Google Scholar]
- Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialka I, Steinlein P, Ahorn H, Bock G, Burbelo PD, Haberfellner M, Lottspeich F, Paiha K, Pasquali C, Huber LA. Identification of syntenin as a protein of the apical early endocytic compartment in Madin-Darby canine kidney cells. J Biol Chem. 1999;274:26233–26239. doi: 10.1074/jbc.274.37.26233. [DOI] [PubMed] [Google Scholar]
- Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J Biol Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- Franco M, Peters PJ, Boretto J, van Donselaar E, Neri A, D'Souza-Schorey C, Chavrier P. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 1999;18:1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R, Schmid S, Mellman I. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc Natl Acad Sci USA. 1989;86:539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller SD, Simons K. Transferrin receptor polarity and recycling accuracy in “tight” and “leaky” strains of Madin-Darby canine kidney cells. J Cell Biol. 1986;103:1767–1779. doi: 10.1083/jcb.103.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli T, Chilcote T, Mundigl O, Binz T, Niemann H, De Camilli P. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J Cell Biol. 1994;125:1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze HJ, Slot JW, Strous GJ, Lodish HF, Schwartz AL. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Gibson A, Futter CE, Maxwell S, Allchin EH, Shipman M, Kraehenbuhl JP, Domingo D, Odorizzi G, Trowbridge IS, Hopkins CR. Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized M.D.C.K. cells. J Cell Biol. 1998;143:81–94. doi: 10.1083/jcb.143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gournier H, Stenmark H, Rybin V, Lippe R, Zerial M. Two distinct effectors of the small GTPase Rab5 cooperate in endocytic membrane fusion. EMBO J. 1998;17:1930–1940. doi: 10.1093/emboj/17.7.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Setiadi H, McEver RP, Kelly RB. The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J Cell Biol. 1994;124:435–448. doi: 10.1083/jcb.124.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Emans N. Annexins in membrane traffic. Trends Cell Biol. 1993;3:224–227. doi: 10.1016/0962-8924(93)90116-i. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Griffiths G, Howell KE. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Gu F, Aniento F, Parton RG, Gruenberg J. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol. 1997;139:1183–1195. doi: 10.1083/jcb.139.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Gruenberg J. ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J Biol Chem. 2000;275:8154–8160. doi: 10.1074/jbc.275.11.8154. [DOI] [PubMed] [Google Scholar]
- Gut A, Kappeler F, Hyka N, Balda MS, Hauri HP, Matter K. Carbohydrate-mediated Golgi to cell surface transport and apical targeting of membrane proteins. EMBO J. 1998;17:1919–1929. doi: 10.1093/emboj/17.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen GH, Niels-Christiansen LL, Immerdal L, Hunziker W, Kenny AJ, Danielsen EM. Transcytosis of immunoglobulin A in the mouse enterocyte occurs through glycolipid raft- and rab17-containing compartments. Gastroenterology. 1999;116:610–622. doi: 10.1016/s0016-5085(99)70183-6. [DOI] [PubMed] [Google Scholar]
- Harder T, Gerke V. The subcellular distribution of early endosomes is affected by the annexin II2p11(2) complex. J Cell Biol. 1993;123:1119–1132. doi: 10.1083/jcb.123.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Kellner R, Parton RG, Gruenberg J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol Biol Cell. 1997;8:533–545. doi: 10.1091/mbc.8.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Fialka I, Paiha K, Hunzinker W, Sacks DB, Bähler M, Way M, Gagescu R, Gruenberg J. Both calmodulin and the unconventional myosin myr4 regulate membrane trafficking along the recycling pathway of MDCK cells. Traffic. 2000;1:494–503. doi: 10.1034/j.1600-0854.2000.010607.x. [DOI] [PubMed] [Google Scholar]
- Hughson EJ, Hopkins CR. Endocytic pathways in polarized Caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol. 1990;110:337–348. doi: 10.1083/jcb.110.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Peters PJ. Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J Biol Chem. 1998;273:15734–15741. doi: 10.1074/jbc.273.25.15734. [DOI] [PubMed] [Google Scholar]
- Ikonen E, Parton RG, Lafont F, Simons K. Analysis of the role of p200-containing vesicles in post-Golgi traffic. Mol Biol Cell. 1996;7:961–974. doi: 10.1091/mbc.7.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing SQ, Spencer T, Miller K, Hopkins C, Trowbridge IS. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990;110:283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane PM. Disassembly and reassembly of the yeast vacuolar H(+)-ATPase in vivo. J Biol Chem. 1995;270:17025–17032. [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Gu F, Gruenberg J. Lipids, lipid domains and lipid-protein interactions in endocytic membrane traffic. Semin Cell Dev Biol. 1998a;9:517–526. doi: 10.1006/scdb.1998.0257. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998b;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999;11:424–431. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- Lafont F, Lecat S, Verkade P, Simons K. Annexin XIIIb associates with lipid microdomains to function in apical delivery. J Cell Biol. 1998;142:1413–1427. doi: 10.1083/jcb.142.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung SM, Chen D, DasGupta BR, Whiteheart SW, Apodaca G. SNAP-23 requirement for transferrin recycling in streptolysin-O-permeabilized Madin-Darby canine kidney cells. J Biol Chem. 1998;273:17732–17741. doi: 10.1074/jbc.273.28.17732. [DOI] [PubMed] [Google Scholar]
- Leung SM, Rojas R, Maples C, Flynn C, Ruiz WG, Jou TS, Apodaca G. Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase rhoA. Mol Biol Cell. 1999;10:4369–4384. doi: 10.1091/mbc.10.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S, Mira E, Gomez-Mouton C, Lacalle RA, Keller P, Labrador JP, Martinez AC. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 1999;18:6211–6220. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EW, Leopold PL, Jones NL, Maxfield FR. Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J Cell Biol. 1995;129:1509–1522. doi: 10.1083/jcb.129.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Sabharanjak S, Maxfield FR. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 1998;17:4626–4638. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Sudhof TC. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Cardone MH. Regulation of protein traffic in polarized epithelial cells. Bioessays. 1995;17:129–138. doi: 10.1002/bies.950170208. [DOI] [PubMed] [Google Scholar]
- Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. E.E.A1, an early endosome-associated protein: E.E.A1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Murphy C, Saffrich R, Grummt M, Gournier H, Rybin V, Rubino M, Auvinen P, Lutcke A, Parton RG, Zerial M. Endosome dynamics regulated by a Rho protein. Nature. 1996;384:427–432. doi: 10.1038/384427a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane J. Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Ostermeyer AG, Beckrich BT, Ivarson KA, Grove KE, Brown DA. Glycosphingolipids are not essential for formation of detergent- resistant membrane rafts in melanoma cells: methyl-beta-cyclodextrin does not affect cell surface transport of a GPI-anchored protein. J Biol Chem. 1999;274:34459–34466. doi: 10.1074/jbc.274.48.34459. [DOI] [PubMed] [Google Scholar]
- Pagano RE, Martin OC, Kang HC, Haugland RP. A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J Cell Biol. 1991;113:1267–1279. doi: 10.1083/jcb.113.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG. Endocytosis in polarized cells. Semin Cell Biol. 1991;2:387–395. [PubMed] [Google Scholar]
- Parton RG. Caveolae and caveolins. Curr Opin Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- Parton RG, Prydz K, Bomsel M, Simons K, Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J Cell Biol. 1989;109:3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali C, Fialka I, Huber LA. Preparative two-dimensional gel electrophoresis of membrane proteins. Electrophoresis. 1997;18:2573–2581. doi: 10.1002/elps.1150181413. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Hsu VW, Ooi CE, Finazzi D, Teal SB, Oorschot V, Donaldson JG, Klausner RD. Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier JL, Trono D. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley JF, Mayor S, Dunn KW, Johnson LS, McGraw TE, Maxfield FR. The End2 mutation in CHO cells slows the exit of transferrin receptors from the recycling compartment but bulk membrane recycling is unaffected. J Cell Biol. 1993;122:1231–1241. doi: 10.1083/jcb.122.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Aniento F, Gruenberg J. NSF is required for transport from early to late endosomes. J Cell Sci. 1997;110:2079–2087. doi: 10.1242/jcs.110.17.2079. [DOI] [PubMed] [Google Scholar]
- Rojo M, Pepperkok R, Emery G, Kellner R, Stang E, Parton RG, Gruenberg J. Involvement of the transmembrane protein p23 in biosynthetic protein transport. J Cell Biol. 1997;139:1119–1135. doi: 10.1083/jcb.139.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K. N-Glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science. 1996a;274:239–242. doi: 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science. 1996b;274:1069. doi: 10.1126/science.274.5285.239. (errata). [DOI] [PubMed] [Google Scholar]
- Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Song J, Khachikian Z, Radhakrishna H, Donaldson JG. Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J Cell Sci. 1998;111:2257–2267. doi: 10.1242/jcs.111.15.2257. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Aasland R, Toh BH, D'Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J Biol Chem. 1996;271:24048–24054. doi: 10.1074/jbc.271.39.24048. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Stevens TH, Forgac M. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu Rev Cell Dev Biol. 1997;13:779–808. doi: 10.1146/annurev.cellbio.13.1.779. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994a;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994b;263:1359. doi: 10.1126/science.8303277. (errata). [DOI] [PubMed] [Google Scholar]
- Subtil A, Delepierre M, Dautry-Varsat A. An alpha-helical signal in the cytosolic domain of the interleukin 2 receptor beta chain mediates sorting towards degradation after endocytosis. J Cell Biol. 1997;136:583–595. doi: 10.1083/jcb.136.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M. Tubular early endosomal networks in AtT20 and other cells. J Cell Biol. 1991;115:635–653. doi: 10.1083/jcb.115.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trischler M, Stoorvogel W, Ullrich O. Biochemical analysis of distinct Rab5- and Rab11-positive endosomes along the transferrin pathway. J Cell Sci. 1999;112:4773–4783. doi: 10.1242/jcs.112.24.4773. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Helenius A. Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol. 1998;8:587–592. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- Van Der Sluijs P, Hull M, Zahraoui A, Tavitian A, Goud B, Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci USA. 1991;88:6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Simons K. Lipid polarity and sorting in epithelial cells. J Cell Biochem. 1988;36:51–58. doi: 10.1002/jcb.240360106. [DOI] [PubMed] [Google Scholar]
- van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol. 1987;105:1623–1635. doi: 10.1083/jcb.105.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verges M, Havel RJ, Mostov KE. A tubular endosomal fraction from rat liver: biochemical evidence of receptor sorting by default. Proc Natl Acad Sci USA. 1999;96:10146–10151. doi: 10.1073/pnas.96.18.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo: characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem. 1999;274:12702–12709. doi: 10.1074/jbc.274.18.12702. [DOI] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Yamashiro DJ, Maxfield FR. Acidification of endocytic compartments and the intracellular pathways of ligands and receptors. J Cell Biochem. 1984;26:231–246. doi: 10.1002/jcb.240260404. [DOI] [PubMed] [Google Scholar]
- Yamashiro DJ, Maxfield FR. Acidification of morphologically distinct endosomes in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987;105:2723–2733. doi: 10.1083/jcb.105.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Zeng J, et al. Identification of a putative effector protein for rab11 that participates in transferrin recycling. Proc Natl Acad Sci USA. 1999;96:2840–2845. doi: 10.1073/pnas.96.6.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]