Abstract
A single team has reported isolation of nanobacteria in human and bovine blood products, as well as, more recently, kidney stones. This has raised controversy. To confirm the data, we searched for nanobacteria from 10 aseptically removed upper urinary tract (UUT) stones. We used scanning electronic microscopy (SEM) with four stones and culture of stones on either 3T6 fibroblast monolayers or liquid RPMI medium. Detection of nanobacteria was made with a commercially available monoclonal antibody, 16S ribosomal DNA amplification with specific primers, and transmission electronic microscopy (TEM) of inoculated cells. SEM showed nanoparticles in four of four UUT stones similar to those recently described. TEM of inoculated 3T6 cell monolayers has shown transient intracytoplasmic vacuolar formations containing 200- to 500-nm particles in 3 of 10 cell cultures. Gimenez staining, Hoechst staining, and specific monoclonal immunofluorescence failed to reveal nanobacteria. Finally, we could not grow Nanobacterium sp. microorganisms by the techniques described. Although with SEM, we observed nanoparticles morphologically similar to nanobacteria, we failed to isolate Nanobacterium sp. microorganisms in culture and to prove the bacterial nature of these nanoparticles in stones.
Nanoparticles detected in various hot spring sediments (7, 8) and a Martian meteorite (17) have been postulated to be bacterial microorganisms on the basis of their morphological features. In 1997, a Finnish team reported isolation of a hitherto-undescribed bacterial species sharing morphological characteristics in common with nanoparticles (14), thereby providing some evidence in support of earlier suspicions. These microorganisms were the smallest described bacteria to date, with dimensions of 0.08 to 0.5 μm. Furthermore, these organisms were found to produce a biofilm containing hydroxyl apatite or carbonate, preventing their effective staining (12). They were also isolated from commercial serum used in cell culture (11). Nanobacteria have been detected thereafter in blood and blood products derived from horses, as well as blood from human blood donors. Two strains, one of Nanobacterium sanguineum and the other of Nanobacterium sp., were isolated from kidney stones and human and bovine sera, respectively (14). Phylogenetic analysis based on comparison of 16S ribosomal DNA (rDNA) sequences has placed the nanobacteria isolated from fetal calf serum into the α2 subgroup of Proteobacteria (14), closely related to Thiobacillus, a water contaminant, and Agrobacterium and Rhizobium, which are plant-associated bacteria.
The controversy that surrounds this intriguing, apparently transmissible microorganism (1) particularly stems from the fact that isolation of nanobacteria has been reported by only one team and has not been confirmed by others (D. Y. Chang, T. W. Jarrett, L. R. Kavoussi, and J. B. Nelson, abstract from the 95th Annual Meeting of the American Urological Association, J. Urol. 161:249, 2000). Although a strain has been deposited in the German Collection of Microorganisms (DSM no. 5819-5821), it is not yet available to the scientific community. The aims of our study were to confirm the presence of nanoparticles in upper urinary tract (UUT) stones by morphological evidence with scanning electron microscopy (SEM) and to try to culture nanobacteria in cell cultures inoculated with UUT stones.
MATERIALS AND METHODS
Stones.
Ten UUT stones were aseptically removed from 10 French patients who received 1 g of cefotaxime as antibioprophylaxis. Serum was collected from these patients. All patients gave informed consent. One fragment of each stone was preserved for culture analysis, and the other fragment was used to determine its chemical structure by Fourier-transformed infrared (IR) spectroscopy according to the standard method for clinical use (3).
Immunostaining and SEM.
Stones were manually ground and powdered with a Potter device. For immunostaining, stone fragments were deposited on a slide, air dried, heat fixed at 70°C for 10 min, rehydrated and blocked by soaking them in 2% fat dry milk-phosphate buffered saline (PBS), and then processed as previously described (3, 4, 12) with a commercial monoclonal mouse antibody (NanoBac Oy, Kuopio, Finland). For SEM, fragments collected from four stones were fixed with 2.5% glutaraldehyde (Sigma Chemicals, Saint-Louis, Mo.) for 20 min and then extensively washed with sterile PBS and rinsed in distilled water. Fixed material was dehydrated in a graded series of ethanol and dried in an EMscope CPD 750 critical point CO2 apparatus (Emscope Lab., Ashford, United Kingdom). The samples were placed in a JFC-1100 (JEOL, Tokyo, Japan) sputter coater for coating with gold-palladium. The UUT samples were examined with a JEOL 35CF SEM operated at 15 kV, and micrographs were recorded.
Cell coculture.
Stones were cocultured with 3T6 cells (ATTC CCL 96) in shell vials in Dulbecco-Vogt's modification of Eagle 's medium (DMEM) (Gibco, Paisley, United Kingdom) supplemented with 10% heat-inactivated fetal bovine serum (FBS). Four different batches were tested: Eurobio, batch 941338; Gibco, batches 40G4093I and 40G1891K; and Sigma, batch F2442. 3T6 cells were incubated for 24 h before inoculation of UUT stones. One part of each powdered stone was demineralized by incubation in 10 μl of 1 M HCl for 10 min at room temperature and then neutralized by addition of 10 μl of 1 M NaOH and 2 ml of DMEM-10% FBS. Finally, the suspension was filtered through a 0.22-μm-pore-size filter (Millipore, Saint-Quentin, France). The supernatant from each shell vial was discarded, and then 0.5 ml of the UUT stone suspension was inoculated onto the 3T6 monolayer, and the inoculated vials were centrifuged at 700 × g for 1 h and incubated at 37°C under 5% CO2. The presence of bacteria was monitored weekly by Gimenez staining (9) and Hoechst 33258 staining (Hoechst stain kit; Flow Laboratories, Ayrshire, United Kingdom) as previously described (14). Immunological detection of nanobacteria in cell cultures was attempted with a commercial monoclonal mouse antibody (NanoBac Oy, Kuopio, Finland) and by incorporating the patient's serum into an indirect immunofluorescence assay as previously described (18). Two powdered and demineralized stones were inoculated in parallel in 5 ml of RPMI 1640 with l-glutamine (Gibco) supplemented with 10% FBS in T-25 culture flasks and incubated at 37°C in a 5% CO2 environment for 4 weeks. Flasks were inspected macroscopically and microscopically weekly for biofilm formation and calcification. The presence of bacteria was monitored by Gimenez and Hoechst staining and by PCR-based detection of the universal 16S rRNA gene as previously described (6, 20) every week for 4 weeks. Transmission electron microscopy (TEM) of infected cells was performed at the 4th week. 3T6 cells cultured in DMEM with 10% FBS without inoculation of powdered stones under the same culture conditions were used as negative controls.
RESULTS
SEM.
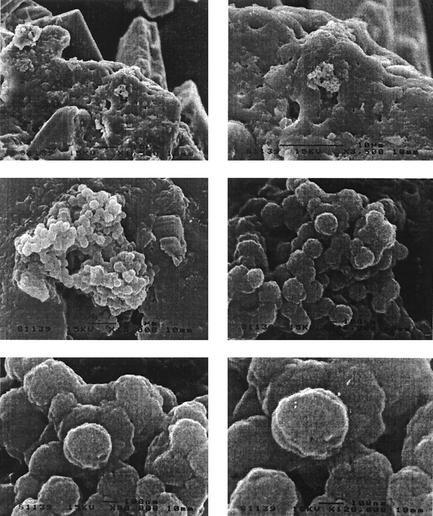
The four UUT stones examined by SEM showed similar characteristics. Spherical coccoid particles were observed, which were grouped in coarse clusters and bound together to a mineral structure (Fig. 1). These spherical units were similar in size and morphology. The size of these particles varied between 200 and 300 nm, and they appeared to have developed in stone cavities. Fourier IR spectroscopy analysis indicated the stones consisted of cystine or oxalate.
FIG. 1.
Details from a fractured UUT stone from SEM analysis showing nanobacterium-like particles. The particles appeared as coccoid structures grouped together in coarse clusters. Their diameter was between 200 and 300 nm. Bar, 1 μm. Magnification, ×15,000.
Cell coculture.
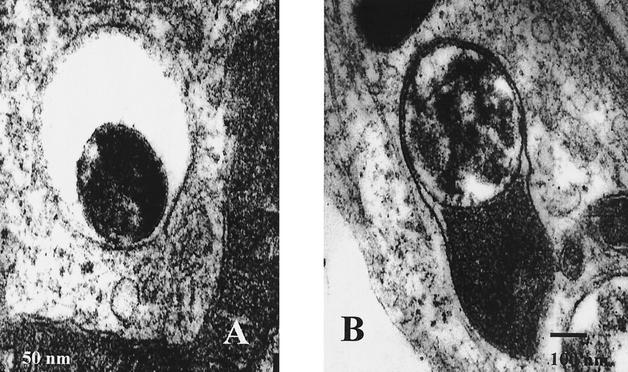
Gimenez staining of inoculated 3T6 cells and on inoculated RPMI 1640 medium failed to reveal nanobacteria after 1 week of culture in any of the 10 stone samples (Table 1). However, after 3 weeks of culture, two to three Gimenez-positive vacuolar inclusions were observed in the cytoplasm of 10 to 20% of 3T6 cells in 9 of 10 inoculated cultures. After 5 weeks, 10 of 10 stone cultures possessed these Gimenez-positive inclusions. Hoechst staining was negative for all cultures and remained negative even after 6 weeks of culture (Table 1). Immunological detection with a commercially available antinanobacterium monoclonal antibody performed with two cultures failed to detect Nanobacterium sp. antigen after either 3 or 5 weeks of culture. Also, immunodetection with the patient's serum failed to detect microorganisms in 10 of 10 inoculated 3T6 cell cultures. TEM showed intracytoplasmic vacuolar formation containing 200- to 500-nm particles in 3 of 10 UUT stone cultures (Table 1). The morphology of nanoparticles was coccoid, and they lay within vesicles in vacuolized 3T6 cells (Fig. 2). No calcification or biofilm formation was observed in the two RPMI T-25 flasks inoculated with stones, and Gimenez and Hoechst staining failed to reveal any microorganisms. PCR incorporating the universal 16S rRNA gene primers failed to produce an amplicon in 10 of 10 inoculated cell cultures and in 2 of 2 inoculated RPMI flasks.
TABLE 1.
Results of cell culture of UUT stones by Gimenez and Hoechst staining, PCR, and TEM
| Stone no. | Result at wk:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
4
|
5
|
6
|
|||||||||||||
| Gimenez | Hoechst | Gimenez | Hoechst | PCR | Gimenez | Hoechst | PCR | Gimenez | Hoechst | PCR | TEM | Gimenez | Hoechst | PCR | Gimenez | Hoechst | PCR | |
| 1 | − | − | − | − | − | + | − | − | + | − | − | + | + | − | − | + | − | − |
| 2 | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + | − | − |
| 3 | − | − | + | − | − | + | − | − | + | − | − | − | + | − | − | + | − | − |
| 4 | − | − | + | − | − | + | − | − | + | − | − | − | + | − | − | + | − | − |
| 5 | − | − | − | − | − | + | − | − | + | − | − | + | + | − | − | + | − | − |
| 6 | − | − | − | − | − | + | − | − | + | − | − | − | + | − | − | + | − | − |
| 7 | − | − | − | − | − | + | − | − | + | − | − | − | + | − | − | + | − | − |
| 8 | − | − | + | − | − | + | − | − | + | − | − | + | + | − | − | + | − | − |
| 9 | − | − | − | − | − | + | − | − | + | − | − | NDa | + | − | − | + | − | − |
| 10 | − | − | + | − | − | + | − | − | + | − | − | ND | + | − | − | + | − | − |
ND, not done.
FIG. 2.
TEM analysis of UUT stone-inoculated 3T6 monolayers. (A) Nanoparticles inside vesicles in 3T6 cells. (B) Capsular form of nanoparticles.
DISCUSSION
We have tried to demonstrate the presence of nanobacteria in UUT stones by using SEM and culture. Despite previously reported success for these approaches (3, 12, 13), in our hands, only SEM yielded any evidence for the presence of nanoparticles. However, although we observed spherical nanoparticles grouped in clusters binding to the mineral surface and cavities of UUT stones, we saw no clear evidence to support a microbiological nature for these structures. Immunological detection with a commercially available anti-Nanobacterium sp. monoclonal antibody failed to detect nanobacteria as described by Kajander and colleagues (3, 4, 12). Although intracellular structures similar to nanoparticles were transiently observed by TEM in 3T6 cells inoculated with material derived from UUT stones (12, 14), specific immunological and molecular tools failed to confirm these cells contained nanobacteria. Despite the fact that we strictly applied the methods described by Kajander and coworkers, we failed to obtain a nanobacterial culture from FBS and kidney stones. We therefore wonder if there is a culture parameter not mentioned in publications that could explain discrepancy between our results and those previously reported. Alternatively, the formal possibility exists that our specimen series simply did not contain any nanobacteria. However, in an independent study (Chang et al., J. Urol. 161:249, 2000), 16 different renal calculi and 16 human serum samples from patients with nephrolithiasis were been cultured. Detection of nanobacteria was attempted with an enzyme-linked immunosorbent assay (ELISA) and NanoDect-EIA kit (ACELL, Nano Oy, Kuopio, Finland), as well as with 16S rDNA amplification with primers derived from the published Nanobacterium sp. sequences. No evidence for the presence of the specific 16S rDNA sequence or nanobacterial antigen in human kidney stones or serum specimen was found. Although transferable biomineralization has been observed after culture of human serum, human saliva, and dental plaque in DMEM (5), molecular examination of decalcified biofilms failed to detect the nucleic acid or protein that would be expected from growth of a living entity. Alternatively, initiation of biomineralization by macromolecules and transfer by self-propagating microcrystalline apatite was demonstrated. Cell cultures without addition of antibiotics have been performed in our laboratory for the last 17 years. During this period of time, despite using numerous cell lines and different batches of reagents, we have never noticed formation of biofilms on plasticware surfaces. Furthermore, detection of cocultivated microorganisms on cell cultures is routinely performed by PCR with universal 16S rDNA primers (6, 20), and direct immunofluorescence is routinely performed with specific antibodies, and these approaches have never led to the identification of Nanobacterium sp. microorganisms. However, transient Gimenez-positive inclusions are frequently observed in our experience. The structures observed have been routinely considered as artifacts, as are the structures we observed when using TEM on UUT stone material-inoculated 3T6 cells.
In a recent study of patients with polycystic kidney disease (10), the authors found 23 of 31 kidney cystic fluids and 10 of 10 liver cyst fluids positive for Bartonella sp. antibody by immunofluorescence; reactivity between decalcified nanobacteria and hyperimmune serum to Bartonella henselae was observed. We have now performed 2,043 cell cultures of blood and biopsy samples obtained from serologically positive B. henselae and Bartonella quintana patients, using B. henselae and B. quintana polyclonal antibodies to detect positive cell cultures directly on coverslips (16). Not once have cross-reacting nanobacteria been encountered. Likewise, 100% of 22 cow serum samples demonstrated to react with the emerging Bartonella weisii species also reacted with N. sanguineum antigen (2). In three of these cow serum samples, B. weissii was indeed isolated. Based upon the respective 16S rDNA sequences, Bartonella and Nanobacterium spp. are members of the α2 subgroup of Proteobacteria and purportedly share cross-reacting epitopes. Also, Nanobacterium sp. 16S rDNA sequences share 97.8 to 99% similarity with those of Phyllobacterium myrsinacearum, another microorganism identified as a source of contaminating 16S rDNA in PCR studies (5, 19). These data may lead to the hypothesis of laboratory contamination as a possible explanation for discrepancies regarding demonstration of the presence of Nanobacterium sp. Despite our application of strict laboratory rules, we experienced cultured cell contamination by Methylobacterium, which is also a member of the α2 subgroup of Proteobacteria, from three different clinical samples (unpublished data). Culture contamination of clinical samples by the waterborne microorganism Afipia felis (15) has probably been responsible for the hypothesis that A. felis is the etiologic agent of cat scratch disease in humans. These bacterial species share the hospital water supply in the same ecological niche, and thus the hypothesis of contamination by waterborne bacteria cannot be ruled out.
Finally, in trying to confirm the data previously reported for nanobacteria, we have observed spherical particles adherent to UUT stones by using SEM. The morphology and size were compatible with nanobacteria, but we failed to culture these particles. The infectious nature of these particles has to be elucidated, and transmissibility from infected cell culture has to be proven. More investigations have to be done to determine whether the particles observed in UUT stones are bacteria. The availability of the nanobacterial strain for scientific investigators would be an important step in making progress in our research on nanobacteria.
Acknowledgments
We acknowledge the technical assistance of Sandrine Zampa.
REFERENCES
- 1.Abbott, A. 1999. Battle lines drawn between “nanobacteria”researchers. Nature 401:105.. [DOI] [PubMed] [Google Scholar]
- 2.Breitschwerdt, E. B., S. Sontakke, A. Cannedy, S. I. Hancock, and J. M. Bradley. 2001. Infection with Bartonella weissii and detection of Nanobacterium antigens in a North Carolina beef herd. J. Clin. Microbiol. 39:879-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciftçioglu, N., M. Björklund, K. Kuorikoski, K. Bergström, and E. O. Kajander. 1999. Nanobacteria: an infectious cause for kidney stone formation. Kidney Int. 56:1893-1898. [DOI] [PubMed] [Google Scholar]
- 4.Ciftçioglu, N., and E. O. Kajander. 1998. Interaction of nanobacteria with cultured mammalian cells. Physiopathology 4:259-270. [Google Scholar]
- 5.Cisar, J. O., D. Q. Xu, J. Thompson, W. Swaim, L. Hu, and D. J. Kopecko. 2000. An alternative interpretation of nanobacteria-induced biomineralization. Proc. Natl. Acad. Sci. USA 97:11511-11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drancourt, M., C. Bollet, A. Carlioz, R. Martellin, J.-P. Gayral, and D. Raoult. 2000. Evaluation of 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J. Clin. Microbiol. 38:3623-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folk, R. L. 1993. SEM imaging of bacteria and nanobacteria in carbonate sediments and rocks. J. Sediment. Petrol. 63:990-999. [Google Scholar]
- 8.Folk, R. L., and F. L. Lynch. 1997. The possible role of nanobacteria (dwarf bacteria) in clay-mineral diagenesis and the importance of careful sample preparation in high-magnification SEM study. J. Sediment. Res. 67:583-589. [Google Scholar]
- 9.Gimenez, D. F. 1964. Staining rickettsiae in yolk-sac cultures. Stain Technol. 39:135-140. [DOI] [PubMed] [Google Scholar]
- 10.Hjelle, T. J., M. A. Miller-Hjelle, I. R. Poxton, O. Kajander, N. Ciftçioglu, M. L. Jones, R. C. Caughey, R. Brown, P. D. Millikin, and F. S. Darras. 2000. Endotoxin and nanobacteria in polycystic kidney disease. Kidney Int. 57:2360-2374. [DOI] [PubMed] [Google Scholar]
- 11.Kajander, E. O. August 1992. Culture and detection method for sterile-filterable autonomously replicating biological particles. U.S. patent 5,135,851.
- 12.Kajander, E. O., and N. Ciftcioglu. 1998. Nanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc. Natl. Acad. Sci. USA 95:8274-8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajander, E. O., N. Ciftçioglu, M. A. Miller-Hjelle, and J. T. Hjelle. 2001. Nanobacteria: controversial pathogens in nephrolithiasis and polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 10:445-452. [DOI] [PubMed] [Google Scholar]
- 14.Kajander, E. O., I. Kuronen, A. Pelttari, and N. Ciftçioglu. 1997. Nanobacteria from blood, the smallest culturable autonomously replicating agent on Earth, p. 420-428. In Instruments, methods, and missions for the investigation of extraterrestrial microorganisms. SPIE Proceedings Series, vol. 3111. Society of Photo-Optical Instrumentation Engineers, Washington, D.C.
- 15.La Scola, B., and D. Raoult. 1999. Afipia felis in hospital water supply in association with free-living amoebae. Lancet 353:1330.. [DOI] [PubMed] [Google Scholar]
- 16.La Scola, B., and D. Raoult. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37:1899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKay, D. S., E. K. Gibson, Jr., K. L. Thomas-Keprta, H. Vali, C. S. Romanek, S. J. Clemett, X. Chillier, C. R. Maechling, and R. N. Zare. 1996. Search for past life on Mars: possible relic biogenic activity in Martian meteorite ALH84001. Science 273:924-930. [DOI] [PubMed] [Google Scholar]
- 18.Raoult, D., M.-L. Birg, B. La Scola, P.-E. Fournier, M. Enea, H. Lépidi, V. Roux, J.-C. Piette, F. Vandenesch, D. Vital-Durand, and T. Marrie. 2000. Cultivation of the bacillus of Whipple's disease. N. Engl. J. Med. 342:620-625. [DOI] [PubMed] [Google Scholar]
- 19.Tanner, M. A., B. M. Goebel, M. A. Dojka, and N. R. Pace. 1998. Specific ribosomal DNA sequences from diverse environmental settings correlate with experimental contaminants. Appl. Environ. Microbiol. 64:3110-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]