Abstract
Because Candida species have innately highly variable antifungal susceptibilities, the availability of a fast and reliable species identification test is very important so that suitable and effective therapeutic measures may be taken. Using three oligonucleotide primers, we established a randomly amplified polymorphic DNA (RAPD) analysis method that enabled direct identification of the most common opportunistic pathogenic Candida species. RAPD analysis revealed a characteristic molecular fingerprint for each Candida species. Differences between the profiles for Candida albicans and C. dubliniensis were evident. RAPD analysis is a relatively easy, reproducible, and reliable technique that can be useful in providing genetic fingerprints for the identification of strains. In addition, a collection of different C. albicans strains was identified by a specific PCR based on multiple secreted aspartic proteinase (SAP) genes and the dipeptidyl aminopeptidase (DAP2) gene. Our findings demonstrate that PCR based upon the SAP and DAP2 sequences is a simple, rapid, clear, and direct technique for the identification and differentiation of C. albicans and C. dubliniensis.
The incidence of candidiasis caused by Candida species continues to increase in proportion to the growing number of immunocompromised, cancer, and postoperative patients. Traditional means of identification of pure cultures of Candida spp. include laborious and slow morphological and assimilation tests that can take several days to identify the isolates in a culture (36), and clinical yeast isolates are sometimes misidentified when automated biochemical systems are used (7). Several PCR methods for the differentiation of some Candida species have been reported (2, 9, 10, 14, 15, 27, 28). Also, many PCR-based methods that use several unique or multicopy molecular targets for the highly sensitive detection of Candida albicans in culture or biological samples have been developed. Only a few methods for the detection and identification of several species by a single and direct PCR (15) or multiplex PCR (3, 12) have been proposed. Unfortunately, those studies did not include a significant number of clinical isolates of each species.
Several studies have proposed that randomly amplified polymorphic DNA (RAPD) analysis could be directly used as an easy and secure tool for the identification of several pathogenic and food-borne microbial species, including Saccharomyces spp. (25), Penicillium spp. (8), and Candida spp. (1, 19, 20, 23, 29, 33, 35). This report describes a stable and repeatable RAPD PCR assay for the direct, easy, and relatively rapid identification of nine commonly isolated pathogenic Candida species of clinical relevance. C. albicans strain identification was confirmed by a very specific PCR based on the secreted aspartic protease (SAP) multigene family and the dipeptidyl aminopeptidase (DAP2) gene that permits the clear differentiation between the phylogenetically closely related species C. albicans and C. dubliniensis.
Candida sp. strains were obtained from four microbiological laboratories from the cities of Mexico City, Guadalajara, Monterrey, and Guanajuato in Mexico. A collection of 36 C. albicans, 47 C. glabrata, 83 C. tropicalis, 38 C. lusitaniae, 12 C. guilliermondii, 2 C. dubliniensis, 12 C. parapsilosis, 6 C. krusei, and 11 C. kefyr strains obtained from different clinical sites were analyzed. In addition, two strains obtained from culture collections (C. albicans ATCC 10231 and ATCC 24433) and donated strains (C. albicans 132A; C. dubliniensis CD36 and CD92; and C. glabrata J931010, 1480.41, LA817, and RA732) were included.
The strains were identified by germ tube formation in serum, formation of chlamydospores on cornmeal agar (Gibco BRL, Gaithersburg, Md.), and the assimilation profiles determined with the ID 32 C kit (BioMerieux SA, Marcy l'Etoile, France).
For DNA extraction, the yeasts were routinely grown on Sabouraud dextrose agar plates at 37°C for 24 to 48 h. A single colony was then subcultured overnight on YPD broth (1% yeast extract, 2% peptone, 2% dextrose) at 37°C with shaking at 200 rpm. DNA was extracted from this culture by an existing protocol (19). DNA concentrations and A260/A280 ratios were determined spectrophotometrically with a spectrophotometer (Lambda 1A; Perkin-Elmer). An A260/A280 ratio of 1.8 to 2.1 was considered acceptable.
The RAPD profiles were obtained with primers (10-mers) OPE-18 (5′-GGACTGCAGA-3′), OPE-04 (5′-GTGACATGCC-3′), and OPA-18 (5′-AGCTGACCGT-3′) (Gibco BRL) (21). RAPD analysis was performed by a previously described method (21) with minor modifications. Briefly, every reaction mixture for RAPD analysis contained 10 ng of genomic DNA; the appropriate primer at 0.4 μM; dATP, dCTP, dGTP, and dTTP each at a concentration of 200 μM; 2 mM MgCl2; and 1.2 U of Taq DNA polymerase in the PCR buffer provided by the manufacturer (Gibco BRL). Amplification consisted of 38 cycles, each of 1 min at 94°C, 1 min at 36°C, and 2 min at 72°C. Five strains of each species except C. dubliniensis were tested on different days starting from the DNA extraction step to confirm the reproducibility of the method.
Three PCR procedures based on C. albicans sequences were designed: a multiplex PCR for amplification of the SAP1-SAP2-SAP3 and the SAP4-SAP5-SAP6 genes; a specific PCR for independent amplification of the SAP1, SAP2, and SAP3 genes; and a specific PCR for amplification of the DAP2 gene. Sequence data for C. albicans were obtained from GenBank and the Stanford Genome Technology Center website (http://www.sequence.stanford.edu/group/candida). Clustal X (version 1.81) software was used for gene alignment (34), and DNAMAN software (version 3, 1994 to 1997; Lynnon BioSoft) was used for primer design and estimations of the secondary structures and melting temperatures.
To design the multiplex PCR for the SAP1-SAP2-SAP3 and the SAP4-SAP5-SAP6 genes, alignment was performed by using the sequences of the following genes (the GenBank accession numbers are given in parentheses): SAP1 (L12449, L12450, L12451, L12452, and X56867), SAP2 (M83663), SAP3 (L22358), SAP4 (L25338), SAP5 (Z30191), SAP6 (Z30192), SAP7 (Z30193), SAP8 (AF043330), SAP9 (AF043331), and Apr1 (U36754). Two forward primers, SAP123 (5′-CTGATTTATGGGTTCCTGAT-3′; positions 385 to 405 of the C. albicans SAP1 gene) and SAP456 (5′-AAAAGATCACCTTTATTTTTAGA-3′; positions 282 to 304 of the C. albicans SAP4 gene), were chosen for specific amplification of the SAP1-SAP2-SAP3 and SAP4-SAP5-SAP6 DNA fragments, respectively. Only one reverse universal primer, SAP123456 (5′-TCAAAAAGTTATCACCAAGAAT-3′; positions 1188 to 1209 of the C. albicans SAP4 gene), was designed for amplification of SAP1-SAP2-SAP3-SAP4-SAP5-SAP6. By using primers SAP123, SAP456, and SAP123456 in the same reaction, two expected bands of approximately 820 and 1,015 bp were observed on 1.2% agarose electrophoresis gels when C. albicans DNA was used.
By using the alignments of the SAP genes (see above), three PCRs for the independent amplification of the SAP1, SAP2, and SAP3 genes were designed. The forward primer used for amplification of the SAP1, SAP2, and SAP3 genes was SAP123, as indicated above. Specific reverse primers Antisense 1 (5′-TGGCAGCATTGGGAGAGTTG-3′; positions 385 to 404 of the C. albicans SAP1 gene), Antisense 2 (5′-GTTGATCAATTGAAGTAGAATC-3′; positions 400 to 421 of the C. albicans SAP2 gene), and Antisense 3 (5′-CATGTCCCTTGTGAAGTAGT-3′; positions 510 to 529 of the C. albicans SAP3 gene) were chosen for specific amplification of the SAP1, SAP2, and SAP3 genes, respectively. The expected sizes of the fragments amplified from the C. albicans SAP1, SAP2, and SAP3 genes were 390, 258, and 172 bp, respectively.
A specific PCR for the C. albicans DAP2 gene was designed by analyzing the DAP2 sequence. Forward primer DAP2 sense (5′-TCTTTACAACACGATGAGATTG-3′; positions 17,085 to 17,106 of C. albicans contig6-2225) and DAP2 antisense (5′-TCTAAATTGATTTTTTCTGATTTC-3′; positions 16,783 to 16,760 of C. albicans contig6-2225) were chosen for the specific amplification of a 347-bp fragment from C. albicans DNA.
All SAP and DAP2 gene-based PCRs were performed in a buffer containing 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM, each primer at a concentration of 0.4 μM, 10 ng of genomic DNA, and 2 U of Taq polymerase (Gibco BRL). Basically, the PCR conditions except the annealing temperature were the same as those described above. Amplification conditions included a denaturation step for 5 min at 94°C, followed by 35 amplification cycles consisting of 1 min at 94°C, 1 min at 55°C (SAP1-SAP2-SAP3, SAP4-SAP5-SAP6, and DAP2) or 59°C (SAP1, SAP2, and SAP3), and 2 min at 72°C. A final extension step was performed for 5 min at 72°C.
For all PCR-based procedures, amplification was done with a DNA thermal cycler (9600; Perkin-Elmer), the final volume of the reaction mixture was 25 μl, and samples were overlaid with 20 μl of mineral oil (U.S. Biochemicals, Cleveland, Ohio) prior to PCR amplification. A sample (5 to 10 μl) of each PCR product was analyzed by electrophoresis in 1.2% (wt/vol) agarose (Gibco BRL) gel slabs (14 cm by 10 cm by 6 mm) with Tris-acetate buffer (1× TAE; 0.04 M Tris-acetate [pH 8.4], 1 mM EDTA) at 80 V for 2 to 3 h. When products of similar sizes were obtained, electrophoresis was performed with high-performance agarose (Agarose 1000; Boehringer Mannheim Corp., Indianapolis, Ind.). The gels were soaked in ethidium bromide solution (0.5 μg/ml), and the DNA was visualized in a transilluminator under UV light. The DNA molecular size marker was derived from bacteriophage lambda DNA digested with EcoRI and HindIII (Sigma Chemical Co., St. Louis, Mo.). Also, a 100-bp DNA ladder (Gibco BRL) was used as a molecular size marker.
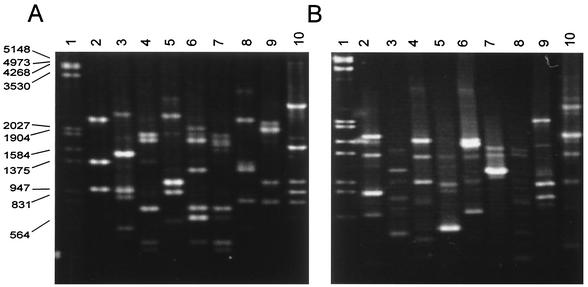
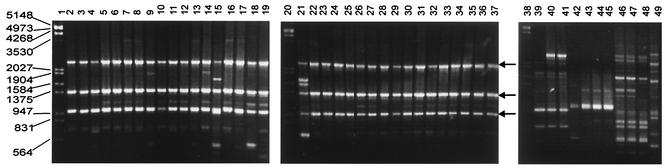
The RAPD patterns obtained for the different strains of Candida species with oligonucleotides OPE-18 and OPA-18 were species specific (Fig. 1). C. albicans, C. tropicalis, C. glabrata, C. parapsilosis, and C. guilliermondii had profiles with limited polymorphisms. The RAPD patterns for the C. albicans strain collection were obtained by using oligonucleotides OPE-18 (Fig. 2), OPE-04, and OPA-18 independently. With oligonucleotide OPE-18, the RAPD patterns of the collection of Candida sp. strains showed the principal monomorphic bands considered for identification (Fig. 2 and 3). Basically, the same pattern was observed within species; and species-specific monomorphic bands were obtained for C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. kefyr, C. lusitaniae, and C. guilliermondii (Table 1). Three repetitions of RAPD analysis showed the same fingerprints, and nonconsistent bands were considered.
FIG. 1.
RAPD patterns for Candida species obtained with primers OPE-18 (A) and OPA-18 (B). Lane 1, molecular size marker (in base pairs); lanes 2 to 10, C. albicans ATCC 10231, C. dubliniensis CD36, C. tropicalis, C. glabrata, C. parapsilosis, C. kefyr, C. krusei, C. lusitaniae, and C. guilliermondii, respectively.
FIG. 2.
RAPD patterns for C. albicans strains obtained with primer OPE-18. Arrows indicate the monomorphic bands useful for identification. Lanes 1, 20, and 38, molecular size markers (in base pairs); lanes 2 to 37, C. albicans ATCC 10231, CAL19, CAL20, CAL21, CAL22, CAL23, CAL24, CAL25, CAL26, CAL27, CAL28, CAL30, CAL31, CAL32, CAL33, CAL34, CAL35, CAL36, CAL37, CAL38, CAL39, CAL40, CAL41, CAL43, CAL1, CAL3, CAL7, CAL8, CAL9, CAL10, CAL11, CAL12, CAL13, CAL15, and CAL16, respectively; lane 39, Saccharomyces cerevisiae DBY; lane 40, Saccharomyces pasturianus; lane 41, Saccharomyces carlsbergensis, lane 42; Schizosaccharomyces pombe 972 h−; lane 43, S. pombe 975; lane 44, S. pombe LV7 h−; lane 45, S. pombe 4D; lane 46, Kluyveromyces lactis wm27; lane 47, Kluyveromyces lactis wm37; lane 48, Kluyveromyces lactis 2/1; lane 49, Yarrowia lipolytica SA-1.
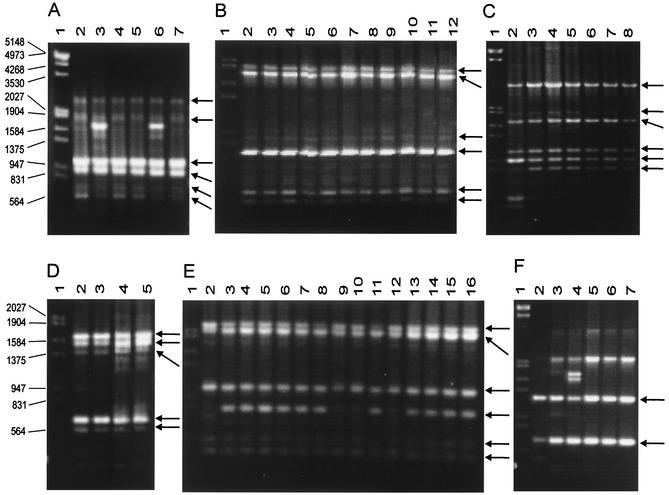
FIG. 3.
RAPD patterns for C. glabrata, C. tropicalis, C. guilliermondii, C. kefyr, C. lusitaniae, and C. parapsilosis strains obtained with primer OPE-18. Arrows indicate the monomorphic bands for each species. Lanes 1, molecular size markers (in base pairs). (A) Lanes 2 to 7, C. glabrata CGL29, CGL1, CGL3, CGL2, CGL47, and CGL42, respectively; (B) lanes 2 to 12, C. tropicalis CTR64, CTR65, CTR66, CTR67, CTR68, CTR69, CTR70, CTR71, CTR72, CTR73, and CTR74, respectively; (C) lanes 2 to 8, C. guilliermondii CGU6, CGU7, CGU8, CGU9, CGU10, CGU11, and CGU12, respectively; (D) lanes 2 to 5, C. kefyr CKE10, CKE11, CKE1, and CKE2, respectively; (E) lanes 2 to 16, C. lusitaniae CLU8, CLU25, CLU26, CLU27, CLU28, CLU29, CLU30, CLU31, CLU32, CLU33, CLU34, CLU35, CLU36, CLU37, and CLU38, respectively; (F) lanes 2 to 7, C. parapsilosis CPA4, CPA8, CPA9, CPA10, CPA11, and CPA12, respectively.
TABLE 1.
RAPD diagnostic monomorphic bands for identification of Candida spp.
| Primer | Sizes (bp) of RAPD monomorphic bands
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| C. albicans | C. dubliniensis | C. tropicalis | C. glabrata | C. parapsilosis | C. kefyr | C. krusei | C. lusitaniae | C. guilliermondii | |
| OPE-18 | 2,418, 1,374, 970 | 1,811, 1,504, 948, 882, 508 | 1,986, 1,846, 1,702, 815, 500, 406 | 2,394, 1,835, 1,046, 917, 686, 555 | 1,232, 659 | 1,731, 1,615, 1,492, 722, 610 | 3,591, 2,403, 1,258, 830 | 2,295, 2,081, 1,104, 800, 594, 484 | 3,061, 1,859, 1,671, 1,107, 939, 822 |
| OPE-04 | 2,861, 1,504, 1,284, 815 | 2,529, 1,504, 1,195, 1,083 | 2,974, 1,530, 1,322, 1,220, 1,013, 917, 608, 508 | 1,874, 1,439, 946, 881 | 1,373, 1,240, 1,001, 696, 545 | 2,127, 1,937, 1,796, 1,445, 1,225, 929 | 1,811, 1,268, 1,134, 1,028, 878, 774, 679, 450 | 1,878, 1,692, 1,559, 1,301, 1,038 | 3,805, 3,105, 2,685, 2,239, 1,865, 1,679, 1,312, 1,155, 946, 655, 581 |
| OPA-18 | 1,677, 1,329, 801 | 2,473, 2,029, 1,772, 1,504, 1,400, 1,307, 1,083, 882 | 3,399, 2,615, 1,581, 1,302, 1,066 | 2,002, 1,340, 945, 467 | 1,620, 1,512, 1,306, 969, 600 | 2,180, 1,901, 1,372, 1,108 | 1,443, 1,330, 969, 889, 734, 470 | 2,028, 1,053, 946 | 3,595, 3,165, 2,805, 2,505, 2,325, 1,737, 1,603, 1,375, 985 |
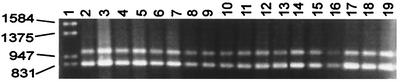
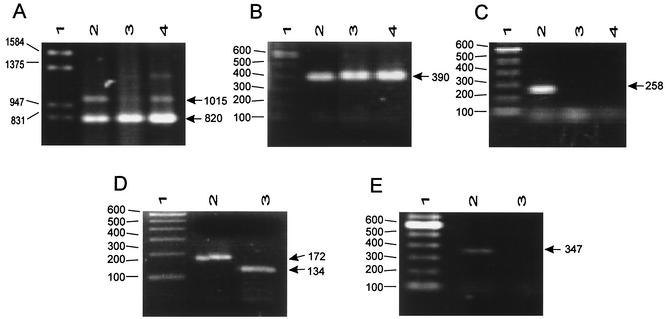
To be able to document more precisely the differences between C. albicans and C. dubiniensis found by RAPD analysis, we performed PCR procedures by amplifying the genes that encode some specific and nonspecific proteases of these phylogenetically related yeasts, which for a long time were not recognized as two different species. The PCR procedures based on the SAP genes and the DAP2 gene designed in this study were able to amplify DNA only from C. albicans and C. dubliniensis. By using multiplex PCRs for the SAP1-SAP2-SAP3 and the SAP4-SAP5-SAP6 genes and a specific SAP1 gene-based protocol, we were able to amplify PCR products from the DNAs extracted from a collection of C. albicans strains and C. dubliniensis (Fig. 4 and 5A and B). The SAP2- and DAP2-based PCR procedures amplified from C. albicans DNA products of 258 bp (Fig. 5C) and 347 bp (Fig. 5E), respectively, but no DNA fragment could be obtained from C. dubliniensis. When the SAP3-based PCR was performed, products of 172 and approximately 134 bp from C. albicans and C. dubliniensis, respectively, could be distinguished by using high-performance agarose (Fig. 5D). In all PCR procedures a minimum of 100 yeast cells suspended in water and blood was required for a positive reaction (data not shown).
FIG. 4.
Expected amplification products of approximately 820 and 1,015 bp from DNA extracted from C. albicans strains for identification of C. albicans strains by multiplex PCR for the SAP1-SAP2-SAP3 and the SAP4-SAP5-SAP6 genes. Lane 1, molecular size marker (in base pairs); lanes 2 to 19, C. albicans ATCC 10231, 132A, CAL19, CAL20, CAL21, CAL22, CAL23, CAL24, CAL25, CAL26, CAL27, CAL28, CAL30, CAL31, CAL32, CAL33, CAL34, and CAL35, respectively.
FIG. 5.
Identification and differentiation of C. albicans and C. dubliniensis by PCR-based protocols. (A) Multiplex PCR for the SAP1-SAP2-SAP3 and the SAP4-SAP5-SAP6 genes; (B) PCR for the SAP1 gene; (C) PCR for the SAP2 gene; (D) PCR for the SAP3 gene; (E) PCR for the DAP2 gene. Arrows show the expected amplification products. Lanes 1, molecular size marker (in base pairs); lanes 2, C. albicans ATCC 10231; lanes 3, C. dubliniensis CD36; lanes 4, C. dubliniensis CD92.
The data presented here describe the species-specific RAPD patterns of Candida spp. obtained with three primers. The SAP multigene family-based PCR procedures and the DAP2 gene-based PCR procedure were also performed to distinguish between C. albicans and C. dubliniensis and to confirm the identities of C. albicans isolates.
The “gold standard” for definitive yeast identification requires assimilation and fermentation tests, which can take several days for identification (36). Several PCR methods for the differentiation among some Candida species have been reported. The drawbacks to these approaches are the additional experimental procedures required, including hybridization with species-specific probes (9), restriction fragment length polymorphism analysis (37), enzyme immunoassay (EIA) (10, 11, 28), 5′ exonuclease assay with fluorescent DNA probes (13, 27), and sequencing or a second PCR with species-specific primers (2, 17), to ensure the identity of each species after DNA amplification. The PCR-EIA method is more expensive and complex than RAPD analysis. Although it may have a better potential for automation, better reproducibility, and a better sensitivity of detection, precise identification of fungi by the PCR-EIA method requires a battery of genus- and species-specific probes to identify species (10, 11, 28), whereas species identification by RAPD analysis requires only PCR with a single primer, agarose electrophoresis, UV transillumination, and band comparison analysis.
A few PCR procedures for the direct identification of Candida species have been reported. These approaches only require electrophoresis on an agarose gel and ethidium bromide staining to detect PCR products. However, some of these studies are limited because of the small number of medically important species included (2, 15, 22), the small number of strains or isolates of each species probed (15, 22), the need for the use of several species-specific primers (2, 14, 15, 16, 22), or the requirement of a multiplex PCR (3, 12, 15, 16, 22) or because they did not assay whether the methods are able to distinguish between C. albicans and C. dubliniensis (2, 3, 15).
Two similar studies proposed a direct multiplex PCR based on amplification of fungal fragments of different sizes (internal transcribed spacers ITS1 and ITS2 and the 5.8S ribosomal DNA region) to identify several fungal and Candida species (3, 12). However, those studies were not blinded, did not include strain collections to assess intraspecific variations, and did not distinguish between C. dubliniensis and C. albicans. Another recently published paper describes a topoisomerase II gene-based PCR that can identify several medically important Candida species including C. dubliniensis; however, this approach must be performed with several mixtures of primers or by a nested PCR approach (16).
A simpler procedure for the identification of nine Candida spp. could be performed by the RAPD fingerprinting assay described here. The procedure required only electrophoresis on an agarose gel and ethidium bromide staining to detect PCR products. Highly consistent, clear, and repetitive RAPD profiles were obtained with each of three different primers and collections of clinical strains isolated in different cities and on different dates. The limiting factor of this technology is that RAPD analysis cannot be adapted to the identification of Candida spp. in clinical samples like other PCR methods designed to amplify specific genes can. Moreover, in order to maintain the reproducibility of the characteristic RAPD patterns, constant amounts of DNA must be used, e.g., 10 ng in this study, whereas other PCR methods can be optimized to directly detect smaller amounts of the target DNA present in clinical samples.
The results of this study reinforce the value of previously described RAPD analysis procedures for the identification of Candida spp. (19, 20, 23, 29, 33, 35). Our study and those of others reported previously (17, 19, 21, 23, 24, 29, 33) have shown that RAPD methods performed with different oligonucleotides basically generated consistent patterns, with several shared fragments unique to each species. RAPD profiles are highly consistent due to the low degree of diversity and primary clonal nature of populations of several pathogenic yeasts, including C. albicans (32) and C. glabrata (21). Unfortunately, a few reports have described the intraspecific diversity and reproductive capabilities of other Candida spp. All these data suggest that the major monomorphic bands obtained by RAPD analysis are useful for the differentiation of pathogenic Candida species.
Several of the PCR procedures reported previously were unable to distinguish between C. albicans and C. dubliniensis (12, 14). For example, in our laboratory, a PCR based on the previously described CHS1 gene (15) produced no discernible differences in the sizes of the PCR products between these two species (data not shown). Recently, several reports described relatively simple phenotypic (4, 10, 13, 18, 26), genotypic, or PCR-based methods that can be used to distinguish between these species by PCR fingerprinting (5, 24), multiplex PCR (22), or other direct PCR procedures (6, 16, 18). In the present study, three additional PCR procedures based on the SAP and DAP2 genes have been proposed as additional strategies for the specific identification of C. albicans and as a means of distinguishing C. albicans from C. dubliniensis. The RAPD analysis procedure with three independent primers described here emphasizes the evident differences in RAPD profiles between C. albicans and C. dubliniensis described previously (30, 31).
Many studies have described molecular diagnostic and identification procedures for Candida spp. of medical importance, but an extensive comparative study to evaluate the sensitivities, reproducibilities, and costs of several methods with isolates from various origins does not exist. The assays described here allow the relatively rapid identification of Candida species and offer alternatives to conventional morphologically and physiologically based identification procedures and their associated problems.
Acknowledgments
We are grateful to the following individuals for donating strains: Derek J. Sullivan, University of Dublin; David R. Soll, University of Iowa; Luis Rivera, Universidad de Guanajuato; Fernando Velarde, Hospital Civil de Guadalajara; and Octavio Novoa, Hospital Durango de México.
C.B.-M. is a fellow from CONACyT and PIFI, I.P.N. L.V.-T. and C.H.-R. received support from COFAA and EDD, I.P.N. Sequence data for Candida albicans were obtained from the Stanford Genome Technology Center website (http://www.sequence.standford.edu/group/candida). Sequencing of C. albicans was accomplished with the support of NIDR and the Burroughs Wellcome Fund. This work was supported by grants from CONACyT (grants 26437-N and CGPI200430), I.P.N., Mexico City, Mexico.
REFERENCES
- 1.Andrighetto, C., E. Psomas, N. Tzanetakis, G. Suzzi, and A. Lombardi. 2000. Randomly amplified polymorphic DNA (RAPD) PCR for the identification of yeasts isolated from dairy products. Lett. Appl. Microbiol. 30:5-9. [DOI] [PubMed] [Google Scholar]
- 2.Burgener-Kairuz, P., J.-P. Zuber, P. Jaunin, T. G. Buchman, J. Bille, and M. Rossier. 1994. Rapid detection and identification of Candida albicans and Torulopsis (Candida) glabrata in clinical specimens by species-specific nested PCR amplification of a cytochrome P- 450 lanosterol-α-demethylase (L1A1) gene fragment. J. Clin. Microbiol. 32:1902-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, H. C., S. N. Leaw, A. H. Huang, T. L. Wu, and T. C. Chang. 2001. Rapid identification of yeasts in positive blood cultures by a multiplex PCR method. J. Clin. Microbiol. 39:3466-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman, D., D. Sullivan, B. Harrington, K. Haynes, M. Henman, D. Shanley, D. Bennett, G. Moran, C. McCreary, and L. O'Neill. 1997. Molecular and phenotypic analysis of C. dubliniensis: a recently identified species linked with oral candidosis in HIV-infected and AIDS patients. Oral Dis. 3(Suppl. 1):S96-S101. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Guerra, T. M., E. Mellado, M. Cuenca-Estrella, F. Laguna, and J. L. Rodríguez Tudela. 1999. Molecular characterization by PCR-fingerprinting of C. dubliniensis strains isolated from two HIV-positive patients in Spain. Diagn. Microbiol. Infect. Dis. 35:113-119. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly, S. M., D. J. Sullivan, D. B. Shanley, and D. C. Coleman. 1999. Phylogenetic analysis and rapid identification of Candida dubliniensis based on analysis of ACT1 intron and exon sequences. Microbiology 145:1871-1882. [DOI] [PubMed] [Google Scholar]
- 7.Dooley, D. P., M. L. Beckius, and B. S. Jeffrey. 1994. Misidentification of clinical yeast isolates by using the updated Vitek Yeast Biochemical Card. J. Clin. Microbiol. 32:2889-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupont, J., S. Magnin, A. Marti, and M. Brousse. 1999. Molecular tools for identification of Penicillium starter cultures used in the food industry. Int. J. Food Microbiol. 49:109-118. [DOI] [PubMed] [Google Scholar]
- 9.Einsele, H., H. Hebart, G. Roller, J. Löffler, I. Rothenhöfer, C. A. Müller, R. A. Bowden, J. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elie, C. M., T. J. Lott, E. Reiss, and C. J. Morrison. 1998. Rapid identification of Candida species with species-specific DNA probes. J. Clin. Microbiol. 36:3260-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita, S., B. A. Lasker, T. J. Lott, E. Reiss, and C. J. Morrison. 1995. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J. Clin. Microbiol. 33:962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita, S.-I., Y. Senda, S. Nakaguchi, and T. Hashimoto. 2001. Multiplex PCR using internal transcribed spacer 1 and 2 regions for rapid detection and identification of yeasts strains. J. Clin. Microbiol. 39:3617-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guiver, M., K. Levi, and B. A. Oppenheim. 2001. Rapid identification of Candida species by TaqMan PCR. J. Clin. Pathol. 54:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes, K. A., and T. J. Westerneng. 1996. Rapid identification of Candida albicans, C. glabrata, C. parapsilosis and C. krusei by species-specific PCR of large subunit ribosomal DNA. J. Med. Microbiol. 44:390-396. [DOI] [PubMed] [Google Scholar]
- 15.Jordan, J. A. 1994. PCR identification of four medically important Candida species by using a single primer pair. J. Clin. Microbiol. 32:2962-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanbe, T., T. Horii, T. Arishima, M. Ozeki, and A. Kikuchi. 2002. PCR-based identification of pathogenic Candida species using primer mixes specific to Candida DNA topoisomerase II genes. Yeast 19:973-989. [DOI] [PubMed] [Google Scholar]
- 17.King, D., J. Rhine-Chalberg, M. A. Pfaller, S. A. Moser, and W. G. Merz. 1995. Comparison of four DNA-based methods for strain delineation of Candida lusitaniae. J. Clin. Microbiol. 33:1467-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurzai, O., W. J. Heinz. D. J. Sullivan, D. C. Coleman, M. Frosch, and F. A. Muhlschlegel. 1999. Rapid PCR test for discriminating between Candida albicans and Candida dubliniensis isolates using primers derived from the pH-regulated PHR1 and PHR2 genes of C. albicans. J. Clin. Microbiol. 37:1587-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann, P. F., D. Lin, and B. A. Lasker. 1992. Genotypic identification and characterization of species and strains within the genus Candida by using random amplified polymorphic DNA. J. Clin. Microbiol. 30:3249-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, D., S. Coloe, S. L. Jones, R. Baird, and J. Pedersen. 1996. Genetic speciation of Candida isolates by arbitrary primed polymerase chain reaction. FEMS Microbiol. Lett. 145:23-26. [DOI] [PubMed] [Google Scholar]
- 21.Lockhart, S. R., S. Joly, C. Pujol, J. D. Sobel, M. A. Pfaller, and D. R. Soll. 1997. Development and verification of fingerprinting probes for Candida glabrata. Microbiology 143:3733-3746. [DOI] [PubMed] [Google Scholar]
- 22.Mannarelli, B. M., and C. P. Kurtzman. 1998. Rapid identification of Candida albicans and other human pathogenic yeasts by using short oligonucleotides in a PCR. J. Clin. Microbiol. 36:1634-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melo, A. S., L. P. de Almeida, A. L. Colombo, and M. R. Briones. 1998. Evolutionary distances and identification of Candida species in clinical isolates by randomly amplified polymorphic DNA (RAPD). Mycopathologia 142:57-66. [DOI] [PubMed] [Google Scholar]
- 24.Meyer, W., K. Maszewska, and T. C. Sorrell. 2001. PCR fingerprint: a convenient molecular tool to distinguish between C. dubliniensis and C. albicans. Med. Mycol. 39:185-193. [DOI] [PubMed] [Google Scholar]
- 25.Molnár, O., R. Messner, H. Prillinger, U. Stahl, and E. Slavikova. 1995. Genotypic identification of Saccharomyces species using random amplified polymorphic DNA analysis. Syst. Appl. Microbiol. 18:136-145. [Google Scholar]
- 26.Park, S., M. Wong, S. A. Marras, E. W. Cross, T. E. Kiehn, V. Tyagi, and D. S. Perlin. 2000. Rapid identification of C. dubliniensis using a species-specific molecular beacon. J. Clin. Microbiol. 38:2829-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin, J. H., F. S. Nolte, B. P. Holloway, and C. J. Morrison. 1999. Rapid identification of up to three Candida species in a single reaction tube by a 5′ exonuclease assay using fluorescent DNA probes. J. Clin. Microbiol. 37:165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin, J. H., F. S. Nolte, and C. J. Morrison. 1997. Rapid identification of Candida species in blood cultures by a clinically useful PCR method. J. Clin. Microbiol. 35:1454-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steffan, P., J. A. Vázquez, D. Biokov, C. Xu, J. D. Sobell, and R. A. Akins. 1997. Identification of Candida species by randomly amplified polymorphic DNA fingerprinting of colony lysates. J. Clin. Microbiol. 35:2031-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan, D. J., T. J. Westerneng, K. A. Haynes, D. E. Bennet, and D. C. Coleman. 1995. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 141:1507-1521. [DOI] [PubMed] [Google Scholar]
- 31.Tamura, M., K. Watanabe, T. Imai, Y. Mikami, and K. Nishimura. 2000. New PCR primer pairs specific for Candida dubliniensis and detection of the fungi from the Candida albicans clinical isolates in Japan. Clin. Lab. 46:33-40. [PubMed] [Google Scholar]
- 32.Taylor, J. W., D. M. Geiser, A. Burt, and V. Koufopanou. 1999. The evolutionary biology and population genetics underlying fungal strains typing. Clin. Microbiol. Rev. 12:126-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thanos, M., G. Schönian, W. Meyer, C. Schweynoch, Y. Gräser, T. G. Mitchell, W. Presber, and H.-J. Tietz. 1996. Rapid identification of Candida species by DNA fingerprinting with PCR. J. Clin. Microbiol. 34:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tietz, H.-J., A. Küssner, M. Thanos, M. Pinto de Andrade, W. Presber, and G. Schönian. 1995. Phenotypic and genotypic characterization of unusual vaginal isolates of Candida albicans from Africa. J. Clin. Microbiol. 33:2462-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren, N. G., and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184-1199. In P. R. Murray, E. J. Baron, M. A. Pfaller, P. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology,7th ed. American Society for Microbiology, Washington, D. C.
- 37.Williams, D. W., M. J. Wilson, M. A. O. Lewis, and A. J. C. Potts. 1995. Identification of Candida species by PCR and restriction fragment length polymorphic analysis of intergenic spacer regions of ribosomal DNA. J. Clin. Microbiol. 33:2476-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]