Abstract
This study estimated the regional and age- and gender-specific seroprevalences of herpes simplex virus type 1 (HSV-1) and HSV-2 in Ontario, Canada. Stored serum specimens from subjects aged 15 to 44 years, including men (n = 979), women not under prenatal care (n = 638), and women under prenatal care (n = 701) submitted for routine viral serology were randomly selected according to regional population size from public health laboratories. HSV-1 and HSV-2 testing was done with the MRL enzyme immunoassay (EIA) (Focus Technologies), and HSV-2 was also tested by the Gull/Meridian EIA. Specimens discordant for HSV-2 antibodies between the two EIAs were resolved by a recombinant immunoblot assay (Focus Technologies). The overall age- and gender-standardized seroprevalences of HSV-1 and HSV-2 were 51.1% (95% confidence interval [CI], 50.1 to 52.1) and 9.1% (95% CI, 8.6 to 9.7), respectively. The seroprevalence of HSV-1 antibodies increased from 26.9 to 54.7% in men between 15 to 16 and 40 to 44 years of age, from 32.0 to 88.7% in women not under prenatal care, and from 55.2 to 69.2% in women under prenatal care. The seroprevalence of HSV-2 increased from 3.8 to 21.3% in men between 15 to 16 and 40 to 44 years of age, from 0 to 18.9% in women not under prenatal care, and from 3.4 to 23.1% in women under prenatal care. HSV-2 results were discordant for 3.3% (76 of 2,318) of specimens. Both types of HSV antibodies appeared to be acquired earlier among women under prenatal care than among men and women not under prenatal care. Antibodies were more prevalent among people in northern Ontario (72.9% of subjects [range, 68.4 to 77.4%] for HSV-1 and 13.7% of subjects [95% CI, 10.2 to 17.2%] for HSV-2) than elsewhere.
Herpes simplex virus type 2 (HSV-2) is a sexually transmitted virus that is the most common cause of genital ulceration (8). Many infections are neither recognized (13) nor diagnosed (11). Transmission is facilitated by the common recurrence of infectious episodes of subclinical viral shedding (25). Undiagnosed and untreated genital herpes in pregnant women can lead to transmission vertically to neonates, causing infant morbidity and mortality (4). The presence of herpes lesions is also known to facilitate the transmission of human immunodeficiency virus (HIV) (26). The seroprevalence of HSV-2 antibodies varies considerably by population. In the U.S. general population, HSV-2 antibody prevalence has been reported to be 21.7% (9), whereas rates in the United Kingdom general population have been reported to be 3 to 5% (24). In Canada, the age-adjusted seroprevalence of HSV-2 was determined to be 17.3% (17) among pregnant women in the general population. In Canada, HSV-2 antibody prevalence was 17.5 to 24% in women and 12.8% in men (14, 21) in two large urban centers, and the prevalence was 18.8% in sexually transmitted disease (STD) clinic attendees in two cities (A. Singh, B. Rowmanoski, and T. Wong, abstr. from 39th Annu. Meet. Infect. Dis. Soc., held in San Francisco, Calif., 25 to 28 October 2001).
HSV-1 normally causes oropharyngeal infection and is usually transmitted in infants and children through oral contact (7). The prevalence of HSV-1 antibodies varies by country and type of population. In 1994 to 1995 in the general population in the United Kingdom, the HSV-1 antibody prevalence was approximately 50% among individuals up to 30 years of age (24). In Canadian surveys using stored sera, the seroprevalence ranged from 52.1 to 65.2% in pregnant women (17) and was 54.8% in men and women attending an STD clinic (A. Singh et al., abstr. from 39th Annu. Meet. Infect. Dis. Soc.). In other adult populations in some European countries and in Africa, Asia, and Latin America, HSV-1 antibody prevalence was greater than 85% (15). While most genital herpes infections are caused by HSV-2, there has been a trend toward increasing proportions of genital herpes attributable to HSV-1 infection (13, 23), likely as a result of increased oral-genital sexual contact (12) and delay in HSV-1 infection acquisition.
Commercial type-specific enzyme-linked immunosorbent assays are available to identify antibodies to both HSV-1 and HSV-2 with good sensitivity (3). To our knowledge, there have been no regionally representative antibody prevalence studies of HSV-1 and HSV-2 among men and nonpregnant women in Canada. This study reports the age-specific seroprevalence of HSV-1 and HSV-2 antibodies among men, women not under prenatal care, and women under prenatal care in a regionally representative sample of stored sera in the Canadian province of Ontario, as determined by commercially available enzyme immunoassays (EIAs), namely, the Gull/Meridian EIA (Meridian Diagnostics, Cincinnati, Ohio) and the MRL EIA (Focus Technologies, Cypress, Calif.).
(This work was presented in part at the International Congress of Sexually Transmitted Infections, 24 to 27 June 2001, Berlin, Germany.)
MATERIALS AND METHODS
Sample selection.
Stored sera, unlinked to patient identities, were taken from public health laboratories in Ontario from March 2000 to January 2001. Specimens used in the study had been submitted to the laboratories from January to December 2000. Only specimens submitted for non-STD screening tests, Epstein-Barr virus, employee health, or prenatal testing were used in the sampling frame to ensure that the sera were from nonhigh-risk, apparently healthy individuals. Specimens were selected from the five health planning regions in Ontario in numbers proportionate to the regional populations.
Specimens stored in the laboratories of all but one region had been entered into a central database housed at Public Health Laboratory Services (Ontario Ministry of Health and Long-Term Care) in Toronto, Ontario. Testing was performed on sera from people in 1-year age groups for people who were 15 to 24 years old and within 5-year age groups for people who were 25 to 29, 30 to 34, 35 to 39, and 40 to 44 years old. The target numbers of specimens within each of the age groups were 100 from men, 50 from women under prenatal care, and 50 from women not under prenatal care. Results are presented for men, women not under prenatal care, and women under prenatal care. Specimen lists were generated by age, gender, and pregnancy status data from the computer, and specimens were randomly selected. All eligible specimen numbers were in a data file, and the random-number selection feature of the statistical package SPSS version 10.0.5 (Chicago, Ill.) was used to select specimens. For some regions, there were insufficient numbers of specimens during the study period for random selection in some age and gender groups; therefore, all specimens available were tested. In one of the health planning regions (Central West), data on specimens had not been entered into the central database during this time and stored sera were not available; therefore, consecutive incoming non-high-risk samples were used. Table 1 shows the population distributions in the five health planning regions and the numbers of specimens obtained from each region.
TABLE 1.
Numbers of samples and proportion of the total number of study samples for each health planning region in Ontario
| Health planning region | No. of samples from:
|
Total no. of samples obtained from region (% of grand total) | % of population living in regiona | ||
|---|---|---|---|---|---|
| Men | Women not under prenatal care | Women under prenatal care | |||
| Central East | 629 | 321 | 348 | 1,298 (56.0) | 45.4 |
| Central West | 66 | 100 | 128 | 294 (12.7) | 19.1 |
| South West | 99 | 80 | 93 | 272 (11.7) | 13.5 |
| East | 174 | 91 | 92 | 357 (15.4) | 13.6 |
| North | 11 | 46 | 40 | 97 (4.2) | 8.5 |
| Total | 979 | 638 | 701 | 2,318 (100) | 100 |
Derived from Canadian census data, Statistics Canada.
Specimens, stored at −20°C, were thawed, aliquoted, and sent refrigerated by courier to the Regional Virology and Chlamydiology Laboratory. The study was approved by the Hamilton Health Sciences Ethics Review Board.
Laboratory methods.
Specimens were tested for HSV-1 and HSV-2 antibodies using the U.S. Food and Drug Administration-approved MRL test (3). A secondary study objective was to compare the MRL test and the Gull/Meridian test (3) for the detection of HSV-2 antibodies. The tests are reliably type specific and demonstrate good sensitivity compared to that of Western blotting (3). Both tests use glycoprotein G antigen (gG-1 or gG-2) bound to enzyme-linked immunosorbent assay plate walls. Specific antibody in the specimens reacts to the incubated wells to allow reaction with the antigen. The enzyme substrate and chromogen are added after the cells are washed, and then the color change is read and cells are quantified.
During the time when serologic testing was being performed in our lab, the manufacturers of the Gull/Meridian EIA ceased further production of the test and recalled all existing tests (April 2001). However, the discrepancy rate between the two EIA tests may be of interest to those who have previously used the Gull/Meridian test in epidemiologic studies of HSV seroprevalence.
Specimens were classified as positive or negative, according to the manufacturer's package insert instructions. Samples whose Gull/Meridian and MRL EIA results were discordant for HSV-2 were retested by a recombinant immunoblot assay (RIBA; Medical Research Laboratories) according to the manufacturer's package insert instructions. The RIBA was chosen since it is a commercially available test, and in a comparison with the results of Western blotting (2), its results have been shown to have 99% concordance with those of Western blotting for detecting HSV-2 (M. Chernesky, unpublished data).
Statistical methods.
The specimens were randomly selected, and data were entered and analyzed with SPSS version 10.0.5. Seropositivity for HSV-1 was based on the MRL EIA result. For HSV-2, those specimens for which both EIA results were concordant (or, if discordant, confirmed by the RIBA) were deemed to be positive. Age- and gender-specific seroprevalences of HSV-1 and HSV-2 as well as 95% confidence intervals (CI) were calculated by standard methods (10). Seroprevalence estimates are presented according to age group (2-year groupings for individuals 15 to 24 years old and 5-year groupings for individuals 25 to 44 years old). The age- and gender-standardized seroprevalence rates for Ontario and for the different regions of the population were also calculated and directly standardized by using the Statistics Canada Ontario population numbers for the year 2000. The effect of gender on prevalence rates, with age being controlled for, was determined by logistic-regression analysis. Age was entered as a continuous variable up to age 24, with the midpoint being used for the groups of individuals who were 25 to 29, 30 to 34, 35 to 39, and 40 to 44 years old.
RESULTS
A total of 2,318 sera were tested for HSV-1 and HSV-2 antibodies. Table 1 shows the numbers of specimens obtained from men, women not under prenatal care, and women under prenatal care in the five health planning regions in Ontario. The population of Ontario in 2000 was approximately 11.7 million (Statistics Canada, 2000). Sample sizes from the Central West, South West, and North health planning regions for men in particular were lower than desired due to the lack of available specimens, especially from younger people.
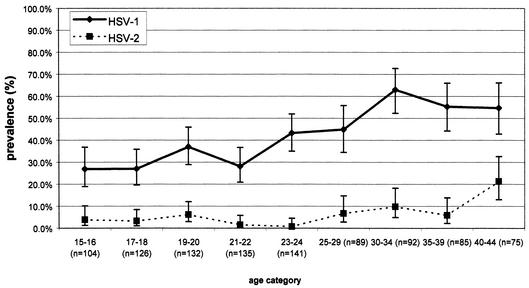
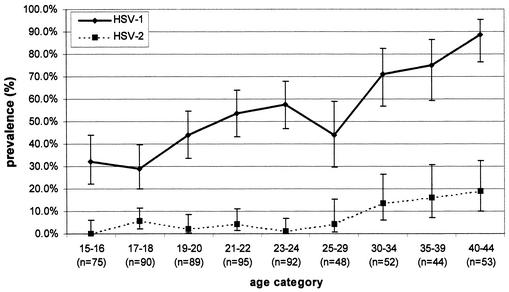
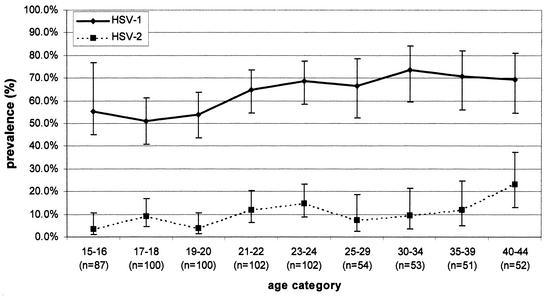
The seroprevalence of HSV-1 in men under 20 was below 30%, while in men aged 30 or over it was above 50% (Fig. 1). In women not under prenatal care (Fig. 2), the seroprevalence of HSV-1 was generally higher than that in men and ranged from 32.0% in 15- to 16-year-olds to 88.7% in 40- to 44-year-olds. In women under prenatal care (Fig. 3), HSV-1 seroprevalence was nearly double (55.2%) that for men and women not under prenatal care at ages 15 to 16, peaking at 64.7 to 73.6% in those over 20 years of age. There were significant increases in the seroprevalence of HSV-1 by age for men, women not under prenatal care, and women under prenatal care (by the chi-square test for trends, the P values were <0.0001, <0.0001, and 0.0002, respectively). The age-standardized seroprevalence rates of HSV-1 were 48.1% (95% CI, 46.5 to 49.7) for men, 63.0% (95% CI, 61.1 to 64.9) for women not under prenatal care, and 66.8% (95% CI, 65.0 to 68.6) for women under prenatal care. The crude (unstandardized) HSV-1 seroprevalences were 40.4% (95% CI, 37.4 to 43.6) for men, 51.9% (95% CI, 47.9 to 55.8) for women not under prenatal care, and 69.2% (95% CI, 65.6 to 72.6) for women under prenatal care. The overall age-standardized seroprevalence for HSV-1 was 51.1% (95% CI, 50.1 to 52.1).
FIG. 1.
Age-specific seroprevalences of HSV-1 and HSV-2 antibodies among 979 men in the Ontario, Canada, population, determined by the MRL and Gull/Meridian EIAs, with discordant results being resolved by the RIBA.
FIG. 2.
Age-specific seroprevalences of HSV-1 and HSV-2 among 638 women not under prenatal care in the Ontario, Canada, population, determined by the MRL and Gull/Meridian EIAs, with discordant results being resolved by the RIBA.
FIG. 3.
Age-specific seroprevalences of HSV-1 and HSV-2 among 701 women under prenatal care in the Ontario, Canada, population, determined by the MRL and Gull/Meridian EIAs with discordant results being resolved by the RIBA.
Overall, 3.3% (76 of 2,318) of the results of the two EIAs for HSV-2 were discordant, and the RIBA resolved the differences in favor of the MRL test for 61.8% (47 of 76) of the specimens and in favor of the Gull/Meridian test for 38.2% (29 of 76) of the specimens.
Among men, HSV-2 seroprevalence ranged from 0.7 to about 10% among those under the age of 40, increasing to 21.3% among those aged 40 to 44 years (Fig. 1). In women not under prenatal care, seroprevalence ranged between 0 and about 6% for those under the age of 30, increasing to 18.9% among those aged 40 to 44 years (Fig. 2). In contrast to the results for men and women not under prenatal care, HSV-2 seroprevalence appeared to be higher among women under prenatal care under the age of 30. With age being controlled for, the seroprevalences of HSV-1 and HSV-2 were statistically significantly higher for women under prenatal care than for men and women not under prenatal care (P < 0.001 for both viral types). There were significant changes in HSV-2 seroprevalence by age for men, women not under prenatal care, and women under prenatal care (by chi-square tests for trends, P values were 0.001, <0.001, and 0.002, respectively). The overall age-standardized seroprevalence for HSV-2 was 9.1% (95% CI, 8.6 to 9.7). The crude HSV-2 seroprevalences were 5.6% (95% CI, 4.6 to 7.3) for men, 6.0% (95% CI, 4.3 to 8.2) for women not under prenatal care, and 10.0% (95% CI, 7.9 to 12.5) for women under prenatal care. The age-standardized HSV-2 seroprevalences were 8.6% (95% CI, 7.7 to 9.5) for men, 10.5% (95% CI, 9.3 to 11.7) for women not under prenatal care, and 11.9% (95% CI, 10.7 to 13.2) for women under prenatal care.
The age-standardized seroprevalences of antibodies to both HSV-1 and HSV-2 were 6.3% (95% CI, 5.8 to 6.8) overall, 4.8% (95% CI, 4.1 to 5.4) for men, and 8.0% (95% CI, 6.9 to 9.0) for women both under and not under prenatal care.
Overall, individuals infected with HSV-1 were significantly more likely to have HSV-2 than those not infected with HSV-1 (relative risk, 1.7; 95% CI, 1.2 to 2.3); however, the association was significant only for men: among men, the relative risk was 1.9 (95% CI, 1.1 to 3.2), compared to 1.8 (95% CI, 0.9 to 3.4) for women not under prenatal care and 1.0 (95% CI, 0.7 to 1.6) for women under prenatal care.
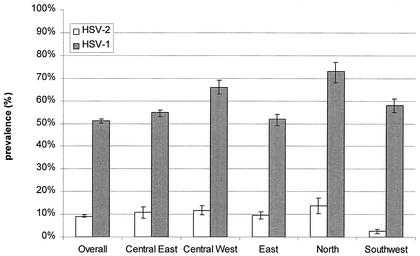
Figure 4 shows the age-standardized overall and regional seroprevalences of HSV-1 and HSV-2. The highest seroprevalences of HSV-1 and HSV-2 were found in the North health planning region (for HSV-1, 72.9% of subjects; 95% CI, 68.4 to 77.4, and for HSV-2, 13.7% of subjects; 95% CI, 10.2 to 17.2). The seroprevalence of HSV-1 was significantly higher in the North region than the overall seroprevalence and the seroprevalences in the Central East, East, and Southwest regions. The seroprevalence of HSV-2 was lowest in the Southwest health planning region (2.6% of subjects; 95% CI, 1.7 to 3.5), significantly lower than in any of the other regions and lower than the overall seroprevalence. The seroprevalence of HSV-2 in the North region was significantly higher than in the Southwest region and higher than the overall seroprevalence.
FIG. 4.
Age-standardized overall and regional prevalences of HSV-1 and HSV-2 in the five health planning regions in the province of Ontario (based on the provincial population in 2000 and on 2,318 specimens).
DISCUSSION
We found that by the age of 15 to 16, up to 55% of the Ontario population had antibodies to HSV-1 and that seroprevalence increased to 89% among individuals in their early forties. In contrast, HSV-2 antibodies were not often found among individuals aged 15 to 16 years (0 to 3.8%), but seroprevalence increased 10-fold to 21% among individuals in their early forties, consistent with the sexually transmitted nature of HSV-2. The age-standardized seroprevalance of HSV-2 among residents of Ontario aged 15 to 44 was 9.1%, considerably lower than the seroprevalence of 21.7% currently estimated for the United States among individuals aged 12 and older (9). This difference may in part be due to the inclusion of older adults in the U.S. survey, according to which seroprevalence ranged from 24.3 to 27.7% among adults 50 years of age and older. Different sampling strategies may also account for the differences. HSV seroprevalence has been reported to be higher among people with a lower socioeconomic status (SES) (9, 16, 21) and among African and Mexican Americans (9). We did not have information for our sample on race or SES. Our sample comprised serum specimens collected from individuals not at high risk for STDs who had been seeking health care, many for screening purposes. Individuals seeking health care or who were being screened for employment purposes may not reflect a random sample of the general population, and our sample may have overrepresented people with a higher SES. While these explanations may account for some of the differences between countries, it is likely that a real difference in levels of seroprevalence exists.
To avoid overestimating the prevalence of HSV-2 in the general population, we excluded specimens that were being tested specifically for STDs other than HSV such as syphilis, chlamydia, and hepatitis, based on the possibility that these individuals could be at higher risk for HSV-2 infection than individuals not being tested for STDs. If this was not the case, exclusion of these specimens may in fact have underestimated the seroprevalence in the general population.
HSV-2 seroprevalence varies considerably worldwide, reaching 90% or more in HIV-infected prostitutes in some developing countries (20). Epidemiologic studies have shown an association between numbers of sexual partners and HSV-2 seroprevalence (9, 15). The age-specific increase in HSV-2 seroprevalence in North American populations, which plateaus with individuals in their thirties (1, 9, 17), likely reflects exposure to different sexual partners in early adulthood (22). HSV-2 antibody prevalence is higher in some groups, such as persons attending STD clinics (11) and homosexual men (15). Epidemiologic studies will continue to be of importance in the monitoring of trends in HSV prevalence and incidence as vaccine development and awareness of the public health impacts of HSV infection, especially its role in facilitating HIV transmission (6), become more recognized.
HSV-2 antibody prevalence was significantly higher among women under prenatal care than among other women. This may reflect in part the fact that all young pregnant women have been sexually active, compared to fewer of the nonpregnant women. However, the age-standardized prevalence of HSV-2 antibodies of 12% in pregnant women in the present study is lower than the 17.3% in women under prenatal care in the province of British Columbia (15) and lower than the 21% in Sweden (18). While it is difficult to predict the risk of genital viral shedding at the time of delivery in pregnant women with HSV antibodies or those at risk of seroconverting during pregnancy (27), knowledge of the HSV serostatus of pregnant women is important for obstetrical-care providers to enable the implementation of strategies to reduce the risk of transmission to the fetus or newborn. The risk of neonatal herpes infection is much lower in women who have recurrent genital herpes, in contrast to women in whom seroconversion occurs near the time of delivery (4).
In the United States, HSV-2 seroprevalence increased dramatically from 5.6% in 12- to 19-year-olds to 27.8% in 30- to 39-year-olds and remained fairly constant beyond age 39 (9). In the present study, HSV-2 seroprevalence was low in men and women not under prenatal care under the age of 30 years and then rose considerably in individuals who were 40 to 44 years old, whereas the seroprevalence for women under prenatal care was consistently higher at the younger ages, reaching a prevalence similar to that of men and women who were 40 to 44 years old. This finding suggests that the significant differences in levels of prevalence between women under prenatal care and men and other women were largely driven by differences at the younger ages. Our age-stratified sampling method resulted in significant differences between the crude and age-standardized seroprevalence estimates. Crude estimates were significantly lower than age-standardized estimates for both viral types for men and women not under prenatal care, since we oversampled younger individuals in whom the levels of prevalence of both types were lower than those for older individuals. The lack of difference between crude and adjusted rates for women under prenatal care may be due to the higher seroprevalence of HSV among these women at younger ages, resulting in larger overall seroprevalence estimates.
Since a larger-than-normal proportion of genital herpes infections have recently been attributed to HSV-1 (12, 23), these infections have increasing implications for childbirth outcomes. It has been noted that HSV-2 seroprevalence studies likely underestimate the prevalence of genital herpes by about 15% due to genital herpes infections caused by HSV-1 (12). In the present study, HSV-1 appeared to be more prevalent in 15- to 29-year-old women under prenatal care than in men and women not under prenatal care. However, we cannot determine the likelihood of sexual transmission of HSV-1 in our sample due to a lack of information on sexual risk behaviors. Despite a documented increase in the sexual transmission of HSV-1, nonsexual transmission remains the dominant route.
We used the results of the RIBA as the indication for HSV-2 positivity when there was discordance between results of the two EIAs. Although Western blotting has been acknowledged as the “gold standard,” the RIBA has high sensitivity (97 to 100%) compared to that of Western blotting (3). Our rate of discordance between the two EIAs was smaller (3.3%) than in a recent study of blood donors, where results for 9% of the specimens were discordant for HSV-2 by the same EIAs as those used in our study (19).
A limitation of the present study was the low number of specimens from some regions. For example, the North region of Ontario was slightly underrepresented but had the highest seroprevalence of both HSV-1 and HSV-2, significantly higher than the overall seroprevalence estimates. However, weighting the data to reflect the population distribution of the five health planning regions in the province did not substantially alter the seroprevalence estimates. Absolute differences between weighted and unweighted age-specific seroprevalences ranged from 0 to 5.3% for HSV-1 and from 0 to 1.8% for HSV-2. In a small study of Inuit people in northern Canada, the seroprevalences of HSV (non-type specific) were 60% in children younger than 2 years of age and 100% in individuals aged six and older. This high prevalence likely reflects the low SES and crowded living conditions in that population (16). We were unable to determine the proportion of samples in the Northern region that came from individuals of aboriginal origin.
Type-specific serologic testing for antibodies to HSV has several potential uses. Seroprevalence studies provide information on regional distribution and patterns of infection. The present study found an increased seroprevalence of both types of HSV in northern Ontario compared to levels in other regions. This information can assist public health departments in identifying priorities in their STD programs. These tests can also be used for individual patients who wish to know their serostatus, for example, with serodiscordant couples.
The results of this study demonstrate that the serologic evidence of infection with HSV-2 is common in the population, although perhaps not as common as has been reported in the United States. The often subclinical or asymptomatic nature of HSV-2 infection poses a challenge for the identification of genital herpes infections and prevention of transmission. Population-based and age-specific HSV antibody prevalence information will be important for the development of future vaccine programs. Our results suggest that effective vaccination of pre-sexually active adolescents may reduce the acquisition of HSV-2 by women in their childbearing years. In the absence of an effective vaccine, there is debate about the appropriateness and benefit of widespread screening of asymptomatic individuals. Women in the first trimester of pregnancy may benefit the most from screening (5). The settings in which HSV screening would be efficacious and cost-effective should be determined.
Acknowledgments
This study was funded by GlaxoSmithKline Research and Development, London, United Kingdom. Kits for Gull/Meridian testing were provided by the Laboratory Centre for Disease Control, Health Canada, Ottawa, Canada.
We thank the following laboratories for supplying the serum specimens: Public Health Laboratory Services, Toronto, Canada, the Hamilton Regional Public Health Laboratory, the Kingston Regional Public Health Laboratory, the London Regional Public Health Laboratory, the Orillia Regional Public Health Laboratory, the Thunder Bay Regional Public Health Laboratory, and the Windsor Regional Public Health Laboratory.
REFERENCES
- 1.Armstrong, G. L., J. Schillinger, L. Markowitz, A. J. Nahmias, R. E. Johnson, G. M. McQuillan, and M. E. St. Louis. 2001. Incidence of herpes simplex virus type 2 infection in the United States. Am. J. Epidemiol. 153:912-920. [DOI] [PubMed] [Google Scholar]
- 2.Ashley, R. 1995. Herpes simplex virus, p. 375-395. In E. Lennette (ed.), Diagnostic procedures for viral, rickettsial, and chlamydial infections, 7th ed. American Public Health Association, Washington, D.C.
- 3.Ashley, R. L. 2001. Sorting out the new HSV type specific antibody tests. Sex. Transm. Infect. 77:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, Z. A., S. Selke, J. Zeh, J. Kopelman, A. Maslow, R. L. Ashley, D. H. Watts, S. Berry, M. Herd, and L. Corey. 1997. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 337:509-515. [DOI] [PubMed] [Google Scholar]
- 5.Brown, Z. A. 2000. HSV-2 specific serology should be offered routinely to antenatal patients. Rev. Med. Virol. 10:141-144. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, D. W., J. N. Simonsen, L. J. D'Costa, A. R. Ronald, G. M. Maitha, M. Gakinya, M. Cheng, J. O. Ndinya-Achola, P. Piot, and R. C. Brunham. 1989. Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet ii:403-407. [DOI] [PubMed] [Google Scholar]
- 7.Corey, L. 1990. Genital herpes, p. 391-413. In K. K. Holmes et al. (ed.), Sexually transmitted diseases, 2nd ed. McGraw Hill, New York, N.Y.
- 8.Corey, L., and H. H. Handsfield. 2000. Genital herpes and public health: addressing a global problem. JAMA 283:791-794. [DOI] [PubMed] [Google Scholar]
- 9.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1967 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 10.Gardner, M. J., S. B. Gardner, and P. D. Winter. 1991. Confidence interval analysis (CIA) version 1.1. BMJ Publishing Group, London, United Kingdom.
- 11.Koutsky, L. A., C. E. Stevens, K. K. Holmes, R. L. Ashley, N. B. Kiviat, C. W. Critchlow, and L. Corey. 1997. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N. Engl. J. Med. 326:1533-1539. [DOI] [PubMed] [Google Scholar]
- 12.Lafferty, W. E., L. Downey, C. Celum, and A. Wald. 2000. Herpes simplex virus type 1 as a cause of genital herpes: impact on surveillance and prevention. J. Infect. Dis. 181: 1454-1457. [DOI] [PubMed] [Google Scholar]
- 13.Langenberg, A. G. M., L. Corey, R. L. Ashley, W. P. Leong, and S. E. Straus. 1999. A prospective study of new infections with herpes simplex virus type 1 and type 2. N. Engl. J. Med. 341:1432-1438. [DOI] [PubMed] [Google Scholar]
- 14.McDonald, A. D., M. C. Williams, R. West, and J. Stewart. 1974. Neutralizing antibodies to herpesvirus types 1 and 2 in Montreal women. Am. J. Epidemiol. 100:124-129. [DOI] [PubMed] [Google Scholar]
- 15.Nahmias, A. J., F. K. Lee, and S. Beckman-Nahmias. 1990. Sero-epidemiological and sociological patterns of herpes simplex virus in the world. Scand. J. Infect. Dis. 69:19-36. [PubMed] [Google Scholar]
- 16.Nicolle, L. E., G. Y. Minuk, B. Postl, N. Ling, D. L. Madden, and J. H. Hoofnagle. 1986. Cross-sectional seroepidemiologic study of the prevalence of cytomegalovirus and herpes simplex virus infection in a Canadian Inuit (Eskimo) community. Scand. J. Infect. Dis. 18:19-23. [DOI] [PubMed] [Google Scholar]
- 17.Patrick, D. M., M. Dawar, D. A. Cook, M. Krajden, H. C. Ng, and M. L. Rekart. 2001. Antenatal seroprevalence of herpes simplex virus type 2 (HSV-2) in Canadian women: HSV-2 prevalence increases throughout the reproductive years. Sex. Transm. Dis. 28:424-428. [DOI] [PubMed] [Google Scholar]
- 18.Persson, K., A. Mansson, E. Jonsson, and E. Nordenfelt. 1995. Decline of herpes simplex virus type 2 and Chlamydia trachomatis infections from 1970 to 1993 indicated by a similar change in antibody pattern. Scand. J. Infect. Dis. 27:195-199. [DOI] [PubMed] [Google Scholar]
- 19.Ribes, J. A., M. Hayes, A. Smith, J. L. Winters, and D. J Baker. 2001. Comparative performance of herpes simplex virus type 2-specific serologic assays from Meridian Diagnostics and MRL Diagnostics. J. Clin. Microbiol. 39:3740-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmogoyi, M., A. Wald, and L. Corey. 1998. Herpes simplex virus-2 infection. An emerging disease? Infect. Dis. Clin. N. Am. 12:47-61. [DOI] [PubMed] [Google Scholar]
- 21.Stavraky, K. M., W. E. Rawls, J. Chiavetta, A. P. Donner, and J. M. Wanklin. 1983. Sexual and socioeconomic factors affecting the risk of past infections with herpes simplex virus type 2. Am. J. Epidemiol. 118:109-121. [DOI] [PubMed] [Google Scholar]
- 22.Steben, M., and S. L. Sacks. 1997. Genital herpes: the epidemiology and control of a common sexually transmitted disease. Can. J. Hum. Sex. 6:127-134. [Google Scholar]
- 23.Tayal, S. C., and R. S. Pattman. 1994. High prevalence of herpes simplex virus type 1 in female anogenital herpes simplex in Newcastle upon Tyne 1983-92. Int. J. STD AIDS 5:359-361. [DOI] [PubMed] [Google Scholar]
- 24.Vyse, A. J., N. J. Gay, M. J. Slomka, R. Gropal, T. Gibbs, P. Morgan-Capner, and D. W. Brown. 2000. The burden of infection with HSV-1 and HSV-2 in England and Wales: implications for the changing epidemiology of genital herpes. Sex. Transm. Infect. 76:183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wald, A., J. Zeh, S. Selke, R. L. Ashley, and L. Corey. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 333:770-775. [DOI] [PubMed] [Google Scholar]
- 26.Wald, A., and K. Link. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive person: a meta-analysis. J. Infect. Dis. 185: 45-52. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson, D., S. Barton, and F. Cowan. 2000. HSV-2 specific serology should not be offered routinely to antenatal patients. Rev. Med. Virol. 10:145-153. [DOI] [PubMed] [Google Scholar]