Abstract
The epidemiology and antimicrobial susceptibilities of 111 Campylobacter fetus subsp. fetus strains isolated from 103 patients from 1983 to 2000 in Québec, Canada, were determined. The median number of patients infected annually with this bacteria was seven, with an incidence of 0.1 per 100,000 population. The male-to-female ratio was 1.1 to 1.0. The patients originated from 13 of the 18 Québec socioeconomic regions. The age range of the patients was 6 months to 90 years old, 53% being ≥70 years old and 2% being <20 years old. The isolation site was blood for 69% of the patients, stools for 20%, and other body fluids for 11% of them. Three patients suffered a relapse, with the same strain being isolated from the same site at different times as confirmed by pulse-field gel electrophoresis. All isolates were susceptible to ampicillin, gentamicin, meropenem, and imipenem, with 90% minimal inhibitory concentrations of 4, 1, 0.12, and ≤0.06 μg/ml, respectively. Three percent and two percent of the strains were, respectively, resistant and intermediate to ciprofloxacin. Thirty-four percent of the strains were resistant to tetracycline. There was a nonsignificant increase in resistance to ciprofloxacin (P = 0.27) and to tetracycline (P = 0.65) in recent years. The percentages of intermediate and resistant MICs were, respectively, 12 and 1% for cefotaxime and 71 and 0% for erythromycin. All strains were β-lactamase negative.
Campylobacter fetus subsp. fetus (C. fetus) is a rare but important human pathogen. In contrast to Campylobacter jejuni subsp. jejuni (C. jejuni) and Campylobacter coli (C. coli), it is rarely reported as a cause of primary intestinal infections (23). It is more frequently involved in bloodstream or extraintestinal diseases in patients with serious underlying conditions or in immunocompromised patients (1, 2, 25, 29). The available epidemiological information is based mainly on numerous case reports or small series. Systemic C. fetus infections require prolonged parenteral antibiotic treatment with or without oral therapy (1). Relapsing infection is not uncommon, and some may have a fatal outcome (1, 2).
The objectives of this study were to review the epidemiology of C. fetus isolated in Québec, Canada, from 1983 to 2000, determine the susceptibilities to eight antibiotics, and detect β-lactamase production of 111 C. fetus isolates.
The demographic and clinical information available on the patients infected with C. fetus was reviewed at Hôpital Saint-Luc du CHUM and at the Laboratoire de Santé Publique du Québec, the latter being the provincial laboratory where strains suspected to be C. fetus are sent for confirmation. The information compiled included the date and site of isolation, the geographic origin, and the age and sex of the patients. Information on patients' underlying conditions or treatment could not be obtained.
Susceptibilities to ampicillin, cefotaxime, ciprofloxacin, gentamicin, erythromycin, imipenem, meropenem, and tetracycline were determined using an agar dilution method (34). The antibiotic concentrations ranged from 0.06 to 128 μg/ml. Suspensions of 24-h blood agar cultures were adjusted to a 0.5 McFarland turbidity standard in Mueller-Hinton broth diluted 1:10. A final inoculum of 104 CFU was applied to Mueller-Hinton plates (BBL) with a Cathra 3-mm replicator. The plates were incubated for 48 h at 35°C under microaerophilic conditions (5% O2, 10% CO2, 85% N2) using gas generator envelopes (Difco). Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains. NCCLS susceptibility criteria were used to interpret the results for aerobic organisms, since no such criteria are available for C. fetus (24). A nitrocefin solution method in microwells was used for β-lactamase detection as previously described (17). S. aureus ATCC 29213 and S. aureus ATCC 25923 were used as positive and negative controls, respectively. Three C. jejuni strains in which β-lactamase had previously been isolated and characterized were also used as positive controls (18).
The significance of differences was analyzed by the chi-square test for linear trend using Epi Info, version 6.0, software (Centers for Disease Control and Prevention, Atlanta, Ga.). A P value of 0.05 or less was considered statistically significant.
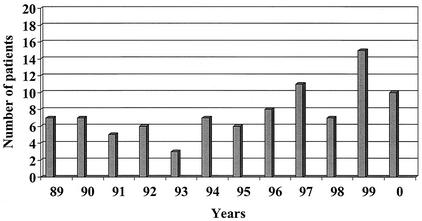
One hundred eleven C. fetus strains were isolated from 103 patients from 1983 to 2000 in Québec, Canada. The Laboratoire de Santé Publique du Québec started keeping the C. fetus isolates in a collection in 1989. Eleven strains from eleven patients were isolated from 1983 to 1988 and represented mainly the Hôpital Saint-Luc collection. From 1989 to 2000, 92 patients (100 strains) were diagnosed as infected with this bacteria, and their annual number varied from 3 to 15 (median 7) (Fig. 1). The male-to-female ratio was 1.1 to 1.0. For seven patients, two isolates were confirmed to be identical by pulsed-field gel electrophoresis (PFGE) (data not shown) and by antimicrobial susceptibility, these were calculated as one strain per patient. For four patients, strains were isolated from different sites during the same time period, and for three patients, strains were isolated from the same site at different times. For another patient, two strains were isolated from two different sites (blood first and then from joint fluids) within a 3-year interval; even though the antimicrobial susceptibilities were identical for both isolates, these were calculated as two different strains, since PFGE has yet to be done.
FIG. 1.
Annual distribution of 92 patients infected with C. fetus from 1989 to 2000.
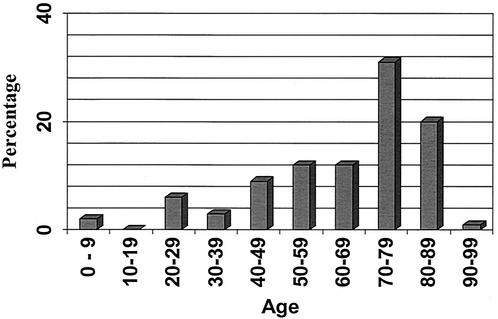
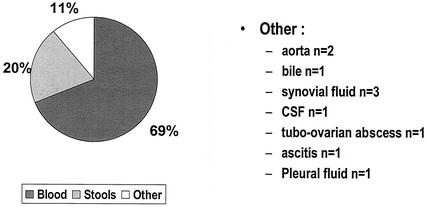
Demographic and clinical information was obtained for 100 patients. The isolates originated from 13 of the 18 Québec socioeconomic regions, and the number tended to be higher in the large urban areas (e.g., Quebec City and the Montreal region). This might be in proportion to the population size or might reflect better laboratory facilities or both. Fifty-three percent of the patients were ≥70 years old, and 2% were <20 years old. The age range of the patients was 6 months to 90 years old (Fig. 2). The isolation site was known for 90 patients as reported in Fig. 3: blood, 69%; stools, 20%; and other body fluids, 11% (aorta, tubo-ovarian pus, cerebrospinal fluid, bile, ascitis, and articular and pleural fluids). Eighteen strains were isolated from stools only.
FIG. 2.
Age distribution of 94 patients infected with C. fetus.
FIG. 3.
Source of isolation for C. fetus in 90 patients.
There were no β-lactamase producers. All isolates were susceptible to ampicillin, gentamicin, meropenem, and imipenem with a 90% minimal inhibitory concentration of 4, 1, 0.12, and ≤0.06 μg/ml, respectively (Table 1). Since 1992, two and three isolates, respectively, had an intermediate and resistant MIC to ciprofloxacin with a nonsignificant increase in resistance in recent years (P = 0.27). The percentages of intermediate and resistant MICs were, respectively, 12 and 1% for cefotaxime and 71 and 0% for erythromycin. The overall tetracycline resistance rate was 34%, with a nonsignificant increase in resistance in recent years (P = 0.65).
TABLE 1.
Susceptibility results for 104 C. fetus strainsa
| Drug | MIC50 | MIC90 | Range | % S | % I | % R |
|---|---|---|---|---|---|---|
| AMP | 2 | 4 | 0.03-8 | 100 | 0 | 0 |
| CTX | 8 | 16 | 1-64 | 87 | 12 | 1 |
| CIP | 0.5 | 1 | ≤0.06-32 | 95 | 2 | 3 |
| GEN | 0.5 | 1 | ≤0.06-2 | 100 | 0 | 0 |
| ERY | 1 | 2 | ≤0.06-4 | 29 | 71 | 0 |
| IPM | ≤0.06 | ≤0.06 | ≤0.06-0.25 | 100 | 0 | 0 |
| MEM | ≤0.06 | 0.12 | ≤0.06-0.50 | 100 | 0 | 0 |
| TET | ≤0.06 | 128 | ≤0.06-128 | 66 | 0 | 34 |
MIC and MIC range are given in micrograms per milliliter. MIC50, MIC at which 50% of the isolates are inhibited. S, susceptible; I, intermediate; R, resistant; AMP, ampicillin; CTX, cefotaxime; CIP, ciprofloxacin; GEN, gentamicin; ERY, erythromycin; IPM, imipenem; MEM, meropenem; TET, tetracycline.
Most studies reported sporadic cases of C. fetus infection, and a few reported outbreaks (1, 7). In the province of Québec, from 1989 to 2000, the median number of patients infected annually was seven with an incidence of 0.1 per 100,000 population. PFGE confirmed that three patients had a relapse, with the same C. fetus strain being isolated from the same site at different times. As previously reported, the risk of developing a C. fetus infection is higher for older patients (1, 2). In this study, 50 out of 94 patients, whose age was known, were 70 years old or older. The proportion of these who were suffering from an underlying severe disease is unknown; therefore, the importance of age itself as a risk factor cannot be assessed. Blood cultures are known to be the main source of isolation of C. fetus (1, 2, 8, 10, 14, 20-22). It is also reported as the causative agent of soft tissue, joint infections, meningitis, peritonitis, cholecystitis, salpingitis, septic abortions, endovascular infections, and thrombophlebitis (2, 5, 7, 21, 37). Little information is available in the literature, however, on the relative frequency of recovery of C. fetus from each source, for a large number of strains. In our study, of the 90 patients for which the source of C. fetus was known, for 62 the strain was isolated from blood, for 18 it was isolated from stools, and for 10 it was isolated from other sources as reported. A few cases of diarrheal disease have been reported up to now (13, 15, 23, 26), some in association with septicemia or with another secondary focus. Gastrointestinal C. fetus colonization is hypothesized to be the primary event leading to infection in compromised hosts (1). In our series, 11% of C. fetus strains were isolated from stools only, and this proportion might be underestimated (23). The paucity of reported C. fetus intestinal manifestations might be partly explained by the suboptimal stool culture conditions used by most laboratories (42°C incubation and/or cephalotin containing media), which are aimed principally at recovering C. jejuni or C. coli (23). The use of specific stool culture conditions suitable for C. fetus recovery should be considered when this pathogen is suspected or when investigating diarrheal disease in compromised patients.
We previously reported the susceptibilities of 59 of these C. fetus strains (34). None of the 111 strains produced a β-lactamase; this is in agreement with two previous studies, where no β-lactamase producers were found in 34 strains (9, 30). In our study, gentamicin, imipenem, and meropenem are the antibiotics with the lowest MICs as reported by others, and no resistance has yet been documented (3, 4, 6, 9, 12, 16, 21, 30). All our strains were susceptible to ampicillin, as reported in studies where an inoculum such as ours was used (9, 12, 21, 30). A small number of resistant strains were reported in studies where inocula standardized with a McFarland ranging from 1 to 3 were used; β-lactamase testing was not done on these strains (3, 4, 6). One and twelve percent of our strains were, respectively, resistant and intermediate to cefotaxime. In our previous study, an important discrepancy was seen with cefotaxime, to which 0, 90, and 84% of 30 strains were susceptible using the E test, agar dilution, and disk diffusion methods, respectively (34). In this study, for a high percentage of strains, the MIC of erythromycin was intermediate, as reported by others (4, 6, 9). Three, two, and ninety-five percent of our strains were, respectively, resistant, intermediate, and susceptible to ciprofloxacin, with a nonsignificant increase in resistance in the more recent years. The rate of resistance to tetracycline for our strains was 34%, also with a nonsignificant increase in resistance in the recent years. The isolates were either highly susceptible or highly resistant to tetracycline, which is suggestive of a plasmid-mediated resistance pattern, as reported for C. jejuni and for C. coli (32, 33). Treatment failures associated with the use of expanded-spectrum cephalosporins or erythromycin in systemic C. fetus infections have been described previously (2, 10, 20, 21, 27, 30). The optimal antimicrobial treatment of C. fetus has not been determined (1). Erythromycin is not recommended for serious infections caused by this bacterium (2). Gentamicin, imipenem, chloramphenicol, or meropenem are antibiotics suggested for parenteral treatment of systemic infections (1, 2). Ampicillin is also considered if the C. fetus strain is shown to be susceptible (2). The lower in vitro and bactericidal activities of cefotaxime compared to the ones of ampicillin, gentamicin, and imipenem (21, 30) do not favor this antimicrobial treatment. Even if patients were successfully treated with ciprofloxacin (2, 14), the emergence of fluoroquinolone resistance in C. fetus as reported by us and by others (19, 28, 31, 34, 35, 36) and the rapid emergence of this antimicrobial resistance with or without clinical failure in other Campylobacter species (2, 11, 23, 35) suggest that these antibiotics may have a limited usefulness for the treatment of these infections. To our knowledge, this study represents the largest number of C. fetus cases collected over such a long period of time. Surveillance of epidemiological data and of antimicrobial susceptibility of Campylobacter fetus subsp. fetus is of primary importance, since this bacterium is a major human pathogen and may be an emerging one (1).
REFERENCES
- 1.Blaser, M. J. 1998. Campylobacter fetus—emerging infection and model system for bacterial pathogenesis at mucosal surfaces. Clin. Infect. Dis. 27:256-258. [DOI] [PubMed] [Google Scholar]
- 2.Blaser, M. J. 2000. Campylobacter jejuni and related species, p. 2276-2285. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingston, Philadelphia, Pa.
- 3.Butzler, J. P., P. Dekeyser, and T. Lafontaine. 1974. Susceptibility of related vibrios and Vibrio fetus to twelve antibiotics. Antimicrob. Agents Chemother. 5:86-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow, A. W., V. Patten, and D. Bednorz. 1978. Susceptibility of Campylobacter fetus to twenty-two antimicrobial agents. Antimicrob. Agents Chemother. 13:416-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dronda, F., I. Garcia-Arata, E. Navas, and L. de Rafael. 1998. Meningitis in adults due to Campylobacter fetus subspecies fetus. Clin. Infect. Dis. 27:906-907. [DOI] [PubMed] [Google Scholar]
- 6.Edmonds, P., C. M. Patton, T. J. Barrett, G. K. Morris, A. G. Steigerwalt, and D. J. Brenner. 1985. Biochemical and genetic characteristics of atypical Campylobacter fetus subsp. fetus strains isolated from humans in the United States. J. Clin. Microbiol. 21:936-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elcuaz, R., A. M. Canas, and B. Lafarga. 1998. Spontaneous peritonitis due to Campylobacter fetus ssp. fetus in a patient with cirrhosis. Clin. Microbiol. Newslett. 20:72-73. [Google Scholar]
- 8.Farrugia, D. C., S. J. Eykyn, and E. G. Smyth. 1994. Campylobacter fetus endocarditis: two case reports and review. Clin. Infect. Dis. 18:443-446. [DOI] [PubMed] [Google Scholar]
- 9.Fliegelman, R. M., R. M. Petrak, L. J. Goodman, J. Segreti, G. M. Trenholme, and R. L. Kaplan. 1985. Comparative in vitro activities of twelve antimicrobial agents against Campylobacter species. Antimicrob. Agents Chemother. 27:429-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francioli, P., J. Herzstein, J.-P. Grob, J.-J. Vallotton, G. Mombelli, and M. P. Glauser. 1985. Campylobacter fetus subspecies fetus bacteremia. Arch. Intern. Med. 145:289-292. [PubMed] [Google Scholar]
- 11.Gaudreau, C., and H. Gilbert. 1998. Antimicrobial resistance of clinical strains of Campylobacter jejuni subsp. jejuni isolated from 1985 to 1997 in Quebec, Canada. Antimicrob. Agents Chemother. 42:2106-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goossens, H., L. V. Coignau, and J. P. Butzler. 1989. In vitro evaluation of combinations against Campylobacter fetus. J. Antimicrob. Chemother. 24:195-201. [DOI] [PubMed] [Google Scholar]
- 13.Harvey, S. M., and J. R. Greenwood. 1983. Probable Campylobacter fetus subsp. fetus gastroenteritis. J. Clin. Microbiol. 18:1278-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichiyama, S., S. Hirai, T. Minami, Y. Nishiyama, Y. Shimizu, K. Shimokata, and M. Ohta. 1998. Campylobacter fetus subspecies fetus cellulitis associated with bacteremia in debilitated hosts. Clin. Infect. Dis. 27:252-255. [DOI] [PubMed] [Google Scholar]
- 15.Klein, B. S., J. M. Vergeront, M. J. Blaser, P. Edmonds, D. J. Brenner, D. Janssen, and J. P. Davis. 1986. Campylobacter infection associated with raw milk. An outbreak of gastroenteritis due to Campylobacter jejuni and thermotolerant Campylobacter fetus subsp. fetus. JAMA 255:361-364. [DOI] [PubMed] [Google Scholar]
- 16.Kwon, S.-Y., D. H. Cho, S. Y. Lee, K. Lee, and Y. Chong. 1994. Antimicrobial susceptibility of Campylobacter fetus subsp. fetus isolated from blood and synovial fluid. Yonsei Med. J. 35:314-319. [DOI] [PubMed] [Google Scholar]
- 17.Lachance, N., C. Gaudreau, F. Lamothe, and F. Turgeon. 1993. Susceptibilities of β-lactamase-positive and -negative strains of Campylobacter coli to β-lactam agents. Antimicrob. Agents Chemother. 37:1174-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachance, N., C. Gaudreau, F. Lamothe, and L. A. Larivière. 1991. Role of β-lactamase of Campylobacter jejuni in resistance to β-lactam agents. Antimicrob. Agents Chemother. 35:813-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier, P. A., D. P. Dooley, J. H. Jorgensen, C. C. Sanders, W. M. Huang, and J. E. Patterson. 1998. Development of quinolone-resistant Campylobacter fetus bacteremia in human immunodeficiency virus-infected patients. J. Infect. Dis. 177:951-954. [DOI] [PubMed] [Google Scholar]
- 20.Montero, A., X. Corbella, J. A. Lopez, M. Santin, and I.-H. Ballon. 1997. Campylobacter fetus-associated aneurysms: report of a case involving the popliteal artery and review of the literature. Clin. Infect. Dis. 24:1019-1020. [DOI] [PubMed] [Google Scholar]
- 21.Morooka, T., and T. Oda. 1989. In vitro evaluation of antibiotics for treatment of meningitis caused by Campylobacter fetus subspecies fetus. Pediatr. Infect. Dis. J. 8:653-654. [DOI] [PubMed] [Google Scholar]
- 22.Morrison, V. A., B. K. Lloyd, J. K. S. Chia, and C. U. Tuazon. 1990. Cardiovascular and bacteremic manifestations of Campylobacter fetus infection: case report and review. Rev. Infect. Dis. 12:387-392. [DOI] [PubMed] [Google Scholar]
- 23.Nachamkin, I. 1999. Campylobacter and Arcobacter, p. 716-726. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 24.National Committee for Clinical Laboratory Standards. 2001. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, 5th ed. NCCLS publication no. M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Penner, J. L. 1998. The genus Campylobacter: a decade of progress. Clin. Microbiol. Rev. 1:157-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rennie, R. P., D. Strong, D. E. Taylor, S. M. Salama, C. Davidson, and H. Tabor. 1994. Campylobacter fetus diarrhea in a Hutterite colony: epidemiological observations and typing of the causative agent. J. Clin. Microbiol. 32:721-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Righter, J., W. A. Wells, G. D. Hart, and D. J. McNeely. 1983. Relapsing septicemia caused by Campylobacter fetus subsp. fetus. Can. Med. Assoc. J. 128:686-689. [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez, R., V. Fernández-Baca, M. D. Díaz, P. Muñoz, M. Rodríguez-Créixems, and E. Bouza. 1994. Evolution of susceptibilities of Campylobacter spp. to quinolones and macrolides. Antimicrob. Agents Chemother. 38:1879-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skirrow, M. B., D. M. Jones, E. Sutcliffe, and J. Benjamin. 1993. Campylobacter bacteraemia in England and Wales, 1981-91. Epidemiol. Infect. 110:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spelhaug, D. R., M. J. R. Gilchrist, and J. A. Washington II. 1981. Bactericidal activity of antibiotics against Campylobacter fetus subspecies intestinalis. J. Infect. Dis. 143:500. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, D. E., and A. S.-S. Chau. 1997. Cloning and nucleotide sequence of the gyrA gene from Campylobacter fetus subsp. fetus ATCC 27374 and characterization of ciprofloxacin-resistant laboratory and clinical isolates. Antimicrob. Agents Chemother. 41:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor, D. E., and P. Courvalin. 1988. Mechanisms of antibiotic resistance in Campylobacter species. Antimicrob. Agents Chemother. 32:1107-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor, D. E., R. S. Garner, and B. J. Allan. 1983. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 24:930-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tremblay, C., and C. Gaudreau. 1998. Antimicrobial susceptibility testing of 59 strains of Campylobacter fetus subsp. fetus. Antimicrob. Agents Chemother. 42:1847-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trieber, C. A., and D. E. Taylor. 2000. Mechanisms of antibiotic resistance in Campylobacter, p. 441-454. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 36.Watine, J., J. Martorell, T. Bruna, J. L. Gineston, J. L. Poirier, and G. Lamblin. 1995. In vivo pefloxacin-resistant Campylobacter fetus responsible for gastro-intestinal infection and bacteremia associated with arthritis of the hips. Yonsei Med. J. 36:202-205. [DOI] [PubMed] [Google Scholar]
- 37.Yao, J. D. C., H. M. C. Ng, and I. Campbell. 1993. Prosthetic hip joint infection due to Campylobacter fetus. J. Clin. Microbiol. 31:3323-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]