Abstract
OBJECTIVE
The Expert Committee on the Diagnosis and Classification of Diabetes retained the 2-hour glucose concentration on an oral glucose tolerance test of ≥11.1 mmol/L (200 mg/dL) as a criterion to diagnose diabetes. Since glycated hemoglobin levels have emerged as the best measure of long-term glycemia and an important predictor of microvascular and neuropathic complications, we evaluated the distribution of hemoglobin A1C (Hb A1C) levels in individuals who had undergone an oral glucose tolerance test to determine how well 2-hour values could identify those with normal versus increased Hb A1C levels.
DESIGN
A cross-sectional analysis of 2 large data sets was performed. We cross-tabulated 2-hour glucose concentrations on an oral glucose tolerance test separated into 4 intervals (<7.8 mmol/L [140 mg/dL], 7.8–11.0 mmol/L [140–199 mg/dL], 11.1–13.3 mmol/L [200–239 mg/dL], and ≥13.3 mmol/L [240 mg/dL]) with Hb A1C levels separated into 3 intervals (normal; <1% above the upper limit of normal; and greater than or equal to the upper limit of normal + 1%).
RESULTS
Approximately two thirds of patients in both data sets with 2-hour glucose concentrations of 11.1 to 13.3 mmol/L (200–239 mg/dL) had normal Hb A1C levels. In contrast, 60% to 80% of patients in both data sets with 2-hour glucose concentrations ≥13.3 mmol/L (240 mg/dL) had elevated Hb A1C levels.
CONCLUSION
Since Hb A1C levels are the best measures presently available that reflect long-term glycemia, we conclude that the 2-hour glucose concentration criterion on an oral glucose tolerance test for the diagnosis of diabetes should be raised from ≥ 11.1 mmol/L (200 mg/dL) to ≥ 13.3 mmol/L (240 mg/dL) to remain faithful to the concept that diagnostic concentrations of glucose should predict the subsequent development of specific diabetic complications (e.g., retinopathy).
Keywords: oral glucose tolerance test, glycated hemoglobin, Hb A1C, diagnosis of diabetes
Prior to 1979, there were at least 6 different sets of criteria used to diagnose diabetes.1 This meant that a person could have had diabetes by one set of criteria, but not by another. On a population basis, the prevalence of diabetes differed markedly depending on the criteria used.1 In 1979, the National Diabetes Data Group recommended one set of criteria2 which was modified only slightly by the World Health Organization31 year later. These criteria were selected based on the results of 3 prospective studies4–6 in which 1,213 subjects without diabetic retinopathy were given oral glucose tolerance tests and followed for 3 to 8 years, at which time 77 of them had developed this complication.7 Based on the 2-hour values of these individuals, a 2-hour glucose concentration of ≥11.1 mmol/L (200 mg/dL) was established as the oral glucose tolerance test criterion for the diagnosis of diabetes.
Several years ago, the American Diabetes Association convened an Expert Committee to revisit the criteria for diagnosing diabetes.8 The committee lowered the fasting plasma glucose concentration criterion for the diagnosis of diabetes from ≥7.8 mmol/L (140 mg/dL) to ≥7.0 mmol/L (126 mg/dL) but decided to retain the 2-hour value of ≥11.1 mmol/L (200 mg/dL) on the oral glucose tolerance test because a large number of epidemiological studies in the literature used this value to define diabetes and changing it “would be very disruptive.”8
We agree with the logic on which the older criteria for the diagnosis were based, that a level of glycemia should be selected that identifies individuals who are at risk for the subsequent development of the specific complications of diabetes (e.g., retinopathy). In recent years, excessive glycation of a variety of proteins has been widely accepted to be a major pathogenic factor in the microvascular complications, especially advanced glycosylation end products which are the result of further metabolism following the initial glycation reaction.9 Important evidence for this statement is that blocking the formation of advanced glycosylation end products in diabetic animals (without lowering elevated glucose concentrations) markedly retarded microvascular complications.9–12
The glycated protein readily available for clinical testing is glycated hemoglobin, a measure of long-term glycemia associated with the development and progression of retinopathy and microalbuminuria in both type 1 and type 2 diabetic patients.13–16 In a previous publication,17 we showed that in the new cohort of diabetic patients, i.e., those with fasting plasma glucose concentrations of 7.0–7.7 mmol/L (126–139 mg/dL), 60% had normal hemoglobin A1C (Hb A1C) levels, certainly not values that place the individual at risk for the microvascular complications of diabetes. Since the 2-hour value on the oral glucose tolerance test of ≥11.1 mmol/L (200 mg/dL) to make the diagnosis of diabetes was based on relatively few subjects,7 we wondered how many of these individuals might also have normal Hb A1C levels. We therefore sought to determine the relationship between glycated hemoglobin levels and the 2-hour glucose concentration criterion on an oral glucose tolerance test for the diagnosis of diabetes.
METHODS
We used Hb A1C levels and 2-hour glucose concentrations on an oral glucose tolerance test from 2 data sets; the Meta-Analysis Research Group (MRG) on the Diagnosis of Diabetes Using Glycated Hemoglobin18 and the Third National Health and Nutrition Examination Survey (NHANES III).19,20 In the MRG data set, aggregated from 10 published studies, only subjects whose glycated hemoglobin levels were measured by ion exchange chromatography (Hb A1C) were included. Glucose concentrations in subjects who received a 50 g oral glucose tolerance test, and/or in whom capillary whole blood glucose concentrations were measured, were transformed to represent venous plasma values.19 Using STATA 5.0 (STATA Corp., College Station, Tex) in accordance with the method described by Harris,20,21 subjects in NHANES III were identified who met the following criteria: (1) between 40 and 74 years of age; (2) no known history of diabetes (other than gestational diabetes); (3) fasted overnight appropriately, and (4) fasting, 2-hour post-75 g glucose load and Hb A1C measurements taken according to protocol.
Normal glucose tolerance was defined according to the recommendations of the Expert Committee8(i.e., a fasting plasma glucose concentration <6.1 mmol/L [110 mg/dL] and a 2-hour glucose concentration following a 75 g oral glucose load of <7.8 mmol/L [140 mg/dL]). We defined the normal range for Hb A1C in each data set separately, using values in subjects with normal glucose tolerance. The upper limit of normal of Hb A1C was defined as the mean plus 2 SDs. In the MRG data set, the mean and SD were 5.1% and 0.6%, respectively, defining a normal range of 3.9% to 6.3%. In the NHANES III study, the mean and SD were 5.1% and 0.5%, respectively, which defines the normal range as 4.1% to 6.1%.
For each data set, we cross-tabulated 2-hour glucose concentrations on the oral glucose tolerance test separated into 4 intervals (<7.8 mmol/L [140 mg/dL]; 7.8–11.0 mmol/L [140–199 mg/dL], i.e., the criterion for the diagnosis of impaired glucose tolerance; 11.1–13.3 mmol/L [200–239 mg/dL]; and ≥13.3 mmol/L [240 mg/dL]) with Hb A1C levels separated into 3 intervals (normal, <1% above the upper limit of normal, and ≥ upper limit of normal + 1%). In the MRG data set; there was no weighting; each subject contributed equally. In the NHANES III data set, we weighted the surveyed population in the same manner as the NHANES III investigators19,20 to render Hb A1C distributions representative of the U.S. population in this age group. We separated elevated Hb A1C values into 2 intervals (<1% above the upper limit of normal [slightly elevated] and ≥1% above the upper limit of normal). The lower interval of elevated Hb A1C values was selected because diabetic patients whose glycated hemoglobin levels are <1% above the upper limit of normal have little or no development or progression of retinopathy or microalbuminuria.13–16
We would have liked to define the Hb A1C intervals in terms of SDs, but chose not to for both clinical and technical reasons. The literature which links Hb A1C levels to microvascular complications13–16 classifies degree of diabetic control in terms of absolute Hb A1C levels (e.g., upper limit of normal = 6.0%, mean value in the conventionally treated group = 9.0%, mean value in the intensively treated group = 7.0%) rather than by number of SDs above the mean for a normal population. Absolute values could not be used to define the intervals (e.g., 6.0%–6.9%, 7.0%–7.9%, ≥8.0%) because other species of glycated hemoglobin besides Hb A1C are used in clinical practice (and each has its own normal range), and even within each method, different laboratories have different normal ranges. For instance, in many laboratories, the upper limit of normal for Hb A1C exceeds 6.0%. One might argue that values of Hb A1C < 1% above the upper limit of normal are 3 to 4 SDs above the mean and should not be considered “slightly elevated.” However, in contrast to the average Hb A1C level openface>9% in the U.S. diabetic population,21 the designation of values in this range as “slightly elevated” seems reasonable.
RESULTS
Eight thousand nine hundred fifteen subjects in the MRG data set had Hb A1C levels measured, and 7,248 (81.3%) had normal glucose tolerance. The upper limit of normal of Hb A1C levels in these individuals was 6.3%. The relationship between 2-hour glucose concentrations on an oral glucose tolerance test and Hb A1C levels in this data set is shown in Table 1. In subjects whose 2-hour glucose concentrations were 11.1–13.3 mmol/L (200–239 mg/dL), Hb A1C levels were normal in approximately 60% and slightly elevated in one third. Approximately 5% had Hb A1C levels ≥1% above the upper limit of normal, values much more likely to be associated with the specific diabetic microvascular complications than lower values.13–16 These higher Hb A1C levels were found in only approximately 1% of individuals with impaired glucose tolerance but in over 50% of individuals with 2-hour glucose concentrations ≥13.3 mmol/L (240 mg/dL).
Table 1.
Distribution (%) of Hemoglobin A1C Levels According to the 2-Hour Glucose Concentrations on the Oral Glucose Tolerance Test in the MRG Data Set*
| Hemoglobin A1C, % | |||||
|---|---|---|---|---|---|
| 2-Hr Glucose (mg/dL) | Number of Subjects | % of MRG Data Set | ≤ULN (≤6.3) | <1% Above ULN (6.4–7.2%) | ≥ULN + 1% (≥7.3) |
| <140 | 7,248 | 81.3 | 97.1 | 2.8 | 0.1 |
| 140–199 | 1,109 | 12.4 | 88.4 | 11.1 | 0.8 |
| 200–239 | 209 | 2.4 | 62.2 | 32.1 | 5.7 |
| ≥240 | 349 | 3.9 | 21.8 | 25.8 | 52.4 |
MRG indicates Meta-Analysis Research Group; ULN, upper limit of normal.
In the NHANES III data set, 2,021 subjects (71.3% of the surveyed population and 76.2% of the U.S. population after weighting) had normal glucose tolerance. The upper limit of normal of Hb A1C levels in these individuals was 6.1%. The relationship between 2-hour glucose concentrations on an oral glucose tolerance test and Hb A1C levels in this data set is shown in Table 2. In subjects whose 2-hour glucose concentrations were 11.1–13.3 mmol/L (200–239 mg/dL), Hb A1C levels were normal in nearly 70% and slightly elevated in 30%. Only 1% had Hb A1C levels greater than or equal to the upper limit of normal + 1%. These higher Hb A1C levels were found in 0.1% of patients with impaired glucose tolerance, but in approximately one third of patients with 2-hour glucose concentrations ≥13.3 mmol/L (240 mg/dL).
Table 2.
Distribution (%) of Hemoglobin A1C Levels According to the 2-Hour Glucose Concentrations on the Oral Glucose Tolerance Test in the NHANES III Data Set
| Hemoglobin A1C, % | |||||
|---|---|---|---|---|---|
| 2-Hr Glucose (mg/dL) | Number of Subjects | % of U.S. Population* | ≤ULN (≤6.1) | <1% Above ULN (6.2%–7.0%) | ≥ULN 1 1% (≥7.1%) |
| <140 | 2,021 | 76.2 | 97.2 | 2.7 | 0.1 |
| 140–199 | 554 | 17.1 | 91.4 | 8.5 | 0.1 |
| 200–239 | 111 | 2.8 | 69.4 | 29.5 | 1.1 |
| ≥240 | 150 | 3.9 | 40.9 | 24.7 | 34.4 |
Based on the U.S. population after weighting the surveyed population, which oversampled minorities; NHANES III, Third National Health and Nutrition Examination Survey; ULN, upper limit of normal.
DISCUSSION
Although oral glucose tolerance tests are not recommended by the Expert Committee for routine clinical use, they still remain an approved way to diagnose diabetes if the 2-hour glucose concentrations are ≥11.1 mmol/L (200 mg/dL) and are confirmed.8 The seminal finding of the present study was that approximately two thirds of individuals who would be diagnosed as having diabetes by virtue of a 2-hour glucose concentration of 11.1–13.3 mmol/L (200–239 mg/dL) on an oral glucose tolerance test would have normal Hb A1C levels. This occurred in 2 diverse data sets. The main limitation of the MRG data set is that subject selection was not randomized, i.e., it was not population based. The data were obtained from 4 different populations: subjects with a positive screening test result; subjects self-referred from the general population; subjects referred from high risk populations; and subjects from studies purposefully enriched with persons known to have diabetes.18 Also, there was no information on age/gender/ethnic distributions. On the other hand, NHANES III was a population study, and the similarity of the distributions validates the results from the MRG data set.
In addition to normal Hb A1C levels in approximately two thirds of people with 2-hour glucose concentrations of 11.1–13.3 mmol/L, less than 5% will have Hb A1C levels ≥1% above the upper limit of normal, values that are associated with much greater development or progression of diabetic retinopathy or microalbuminuria.13–16 Therefore, 95% of these patients will have met the American Diabetes Association's goal Hb A1C value of less than the upper limit of normal + 1%22 and will be treated with diet and exercise rather than pharmacological agents. This is the same treatment that would be offered to patients with impaired glucose tolerance, (i.e., those whose 2-hour glucose concentrations on an oral glucose tolerance test are 7.8–11.0 mmol/L [140–199 mg/dL]).
Therefore, what would be gained by labeling people with a 2-hour glucose concentration on an oral glucose tolerance test of 11.1–13.3 mmol/L (200–239 mg/dL) as having diabetes? Although it is possible that being termed “diabetic” may motivate patients to achieve tighter control, approximately two thirds of these individuals will already have normal Hb A1C levels. Furthermore, given that the average Hb A1C level of people who know that they have diabetes in this country exceeds 9%,21 there is little reason to believe that simply being made aware of the diagnosis will motivate people to achieve near euglycemia. On the other hand, there are potentially negative insurance, employability, psychological, and social costs of carrying the diagnosis of diabetes.23–25 For instance, people with the diagnosis of diabetes are 8 times more likely to be unable to obtain medical insurance because of poor health or illness than people without diabetes.26
Currently, there are 4 ways to evaluate glycemia that could be used to diagnose diabetes: random glucose concentrations, fasting glucose concentrations, oral glucose tolerance tests, and glycated hemoglobin levels (fructosamine levels have not yet been evaluated rigorously as a possible diagnostic tool). Although random glucose concentrations are critically dependent on the time and carbohydrate content of the previous meal, the diagnosis of diabetes is tenable if the value is ≥11.1 mmol/L (200 mg/dL) and the symptoms of uncontrolled diabetes (polyuria, polydipsia) are present.8 The oral glucose tolerance test is a diagnostic test that is not recommended for routine clinical use because of its poor reproducibility and inconvenience.8 Fasting glucose concentrations serve as either a screening or a diagnostic test, depending on the value measured. Fasting plasma glucose concentrations <6.1 mmol/L (110 mg/dL) are normal, 6.1–6.9 mmol/L (110–125 mg/dL) diagnose impaired fasting glucose, and ≥7.0 mmol/L (126 mg/dL) diagnose diabetes (the latter only if confirmed).8 Oral glucose tolerance tests may be ordered for patients with impaired fasting glucose if the physician wishes to determine whether impaired glucose tolerance or diabetes is present by the 2-hour criterion of that diagnostic test. (This diagnosis of diabetes must also be confirmed by a second test.8) Glycated hemoglobin levels are used to monitor glycemia in diabetic patients because these levels are closely associated with the microvascular complications of diabetes.13–16
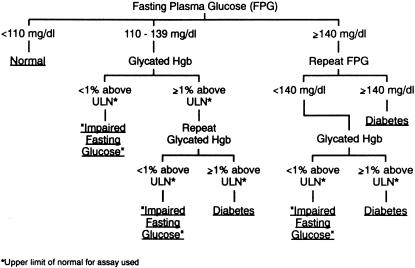
Although glycated hemoglobin levels are not currently recommended for diagnostic use,8 we propose combining them with the screening/diagnostic function of fasting plasma glucose concentrations for clinical decision making (Fig. 1). In our view, the threshold for a valid diagnosis of diabetes must be at a glycemic level that, if not lowered, would lead to the microvascular complications of diabetes. Glycated hemoglobin levels are accurate reflections of this glycemic level because of the importance of excessive glycation in the development and progression of these complications.13–16 Given that 60% of people with fasting plasma glucose concentrations of 7.0–7.7 mmol/L (126–139 mg/dL) have normal glycated hemoglobin levels compared to only 15% to 20% of those who met the older criterion2,3 of ≥7.8 mmol/L (140 mg/dL),17 a glycated hemoglobin level would not be necessary for the diagnosis of diabetes if the latter value were confirmed on another day. A glycated hemoglobin level could help determine whether individuals with fasting plasma glucose concentrations of 6.1–7.7 mmol/L (110–139 mg/dL) should be treated as if they had diabetes or impaired fasting glucose. A glycated hemoglobin level 1-percentage point or more above the upper limit of normal for the assay used was chosen for our diagnostic algorithm for 3 reasons. First, little development or progression of the microvascular complications occur at lower values.13–16 Second, there are potential negative consequences for people carrying the diagnosis of diabetes.23–25 Third, at lower levels of glycated hemoglobin, the glycemic goal of the American Diabetes Association has been met and the treatment with diet and exercise, rather than with pharmacological agents, is the same whether the diagnosis is diabetes or impaired fasting glucose.
FIGURE 1.
An approach to the diagnosis of diabetes mellitus and impaired fasting glucose utilizing both fasting plasma glucose concentrations and glycated hemoglobin levels.
Our 2 studies in large populations examining the distribution of Hb A1C levels in the same subjects across intervals of fasting plasma glucose concentrations17 and 2-hour values on an oral glucose tolerance test (Tables 1 and 2)allow a comparison of these 2 glucose indices of glycemia. The distribution of Hb A1C levels was very similar in the intervals juxtaposed in Table 3. Thus, on a population basis using Hb A1C levels as the “gold standard” of glycemia, there is an equivalence between the corresponding fasting and 2-hour intervals shown in Table 3.
Table 3.
Population Equivalence* of Fasting Plasma Glucose Concentrations and 2-Hour Values on an Oral Glucose Tolerance Test as Reflected in Distributions of Hemoglobin A1C Levels
| Fasting Plasma Glucose | 2-Hour Glucose |
|---|---|
| <6.1 mmol/L (<110 mg/dL) | <7.8 mmol/L (<140 mg/dL) |
| 6.1–6.9 mmol/L (110–125 mg/dL) | 7.8–11.0 mmol/L (140–199 mg/dL) |
| 7.0–7.7 mmol/L (126–139 mg/dL) | 11.1–13.3 mmol/L (200–239 mg/dL) |
| ≥7.8 mmol/L (≥140 mg/dL) | ≥13.3 mmol/L (≥240 mg/dL) |
This paper has several limitations, including the absence of clinical data on the study subjects, the use of a 2-hour value on a single oral glucose tolerance test without confirmatory retesting, and the limitations on the studies from which the MRG data were derived.17
In conclusion, if an oral glucose tolerance test is performed for the diagnosis of diabetes, a clinician should consider whether strict adherence to the Expert Committee's 2-hour glucose concentration criterion of 11.1 mmol/L (200 mg/dL) is warranted since most patients having 2-hour glucose concentrations of 11.1–13.3 mmol/L (200–239 mg/dL) have Hb A1C levels that are not associated with subsequent development of specific diabetic complications. We also conclude that diagnostic levels of glucose should predict the subsequent development of specific diabetic complications (e.g., retinopathy).
Acknowledgments
The secretarial skills of Willie Nelson are gratefully acknowledged. We are deeply indebted to all of the investigators who contributed their data to the MRG data set. A complete list of these investigators has been previously published (JAMA. 1996;276:1246-52). These data have been reported in abstract form (Diabetes. 1999;48(suppl 1):87A). Dr. Schriger is supported in part by an unrestricted grant from the MedAmerica Corporation. Dr. Davidson is supported by NIH grant 5U01 DK54047.
REFERENCES
- 1.Valleron AJ, Eschwege E, Rosselin GE. Agreement and discrepancy in the evaluation of normal and diabetic oral glucose tolerance test. Diabetes. 1975;24:585–93. doi: 10.2337/diab.24.6.585. [DOI] [PubMed] [Google Scholar]
- 2.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. World Health Organization Expert Committee on Diabetes Mellitus: Second Report. Geneva, Switzerland: World Health Organization; 1980. Technical Report 646. [PubMed] [Google Scholar]
- 4.Jarrett RJ, Keen H. Hyperglycaemia and diabetes mellitus. Lancet. 1976;2:1009–12. doi: 10.1016/s0140-6736(76)90844-8. [DOI] [PubMed] [Google Scholar]
- 5.Sayegh HA, Jarrett RJ. Oral glucose-tolerance tests and the diagnosis of diabetes: results of a prospective study based on the Whitehall survey. Lancet. 1979;2:431–3. doi: 10.1016/s0140-6736(79)91489-2. [DOI] [PubMed] [Google Scholar]
- 6.Pettitt DJ, Knowler WC, Lisse JR, Bennett PH. Development of retinopathy and proteinuria in relation to plasma-glucose concentrations in Pima Indians. Lancet. 1980;2:1050–2. doi: 10.1016/s0140-6736(80)92274-6. [DOI] [PubMed] [Google Scholar]
- 7.Davidson MB, Peters AL, Schriger DL. An alternative approach to the diagnosis of diabetes with a review of the literature. Diabetes Care. 1995;18:1065–71. doi: 10.2337/diacare.18.7.1065. [DOI] [PubMed] [Google Scholar]
- 8.Expert Committee. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 9.Bucala R, Cerami A, Vlassara H. Advanced glycosylation end products in diabetic complications. Diabetes Rev. 1995;3:258–68. [Google Scholar]
- 10.Cohen MP, Sharma K, Jin Y, et al. Prevention of diabetic nephropathy in db/db mice with glycated albumin antagonists. J Clin Invest. 1995;95:2338–45. doi: 10.1172/JCI117926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements RS, Jr, Robsion WG, Jr, Cohen MP. Antiglycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J Diabetes Complications. 1998;12:28–33. doi: 10.1016/s1056-8727(97)00051-2. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura S, Makita Z, Ishikawa S, et al. Progression of nephropathy in spontaneous diabetic rats is prevented by OPB-9195, a novel inhibitor of advanced glycation. Diabetes. 1997;46:895–9. doi: 10.2337/diab.46.5.895. [DOI] [PubMed] [Google Scholar]
- 13.The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (Hgb A1C) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–83. [PubMed] [Google Scholar]
- 14.Krolewski AS, Laffel LMB, Krolewski M, Quinn M, Warram JH. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;332:1251–5. doi: 10.1056/NEJM199505113321902. [DOI] [PubMed] [Google Scholar]
- 15.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y, Atsumi Y, Matsuoka K, Onuma T, Tohjima T, Kawamori R. Role of glycemic control and blood pressure in the development and progression of nephropathy in elderly Japanese NIDDM patients. Diabetes Care. 1998;21:116–20. doi: 10.2337/diacare.21.1.116. [DOI] [PubMed] [Google Scholar]
- 17.Davidson MB, Schriger DL, Peters AL, Lorber B. Relationship between fasting plasma glucose and glycosylated hemoglobin: potential for false-positive diagnoses of type 2 diabetes using new diagnostic criteria. JAMA. 1999;281:1203–10. doi: 10.1001/jama.281.13.1203. [DOI] [PubMed] [Google Scholar]
- 18.Peters AL, Davidson MB, Schriger DL, Hasselblad V. A clinical approach for the diagnosis of diabetes mellitus. JAMA. 1996;276:1246–52. [PubMed] [Google Scholar]
- 19.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Comparison of diabetes diagnostic categories in the U.S. population according to 1997 American Diabetes Association and 1980–1985 World Health Organization diagnostic criteria. Diabetes Care. 1997;20:1859–62. doi: 10.2337/diacare.20.12.1859. [DOI] [PubMed] [Google Scholar]
- 20.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 21.Davidson MB. Diabetes care in health maintenance organization and fee-for-service settings. Dis Manage Health Outcomes. 1997;2:189–97. [Google Scholar]
- 22.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 1999;22(suppl 1):S32–41. [PubMed] [Google Scholar]
- 23.Tattersall RB, Jackson GL. Social and emotional complications of diabetes. In: Keen H, Jarret J, editors. Complications of Diabetes. Chicago, Ill: Yearbook Medical Publishers, Inc; 1982. p. 271. [Google Scholar]
- 24.Knowler WC. Screening for NIDDM: opportunities for detection, treatment and prevention. Diabetes Care. 1994;17:445–50. doi: 10.2337/diacare.17.5.445. [DOI] [PubMed] [Google Scholar]
- 25.Harris MI, Cowie CC, Eastman R. Health-insurance coverage for adults with diabetes in the U.S. population. Diabetes Care. 1994;17:585–91. doi: 10.2337/diacare.17.6.585. [DOI] [PubMed] [Google Scholar]