Abstract
BACKGROUND
Evidence regarding the effect of postmenopausal estrogen therapy on mood is limited.
METHODS
To determine whether postmenopausal estrogen therapy is associated with fewer depressive symptoms in elderly women, we conducted a cross-sectional study of 6,602 white women ages 71 years or older who were recruited from population-based listings in Baltimore, Md; Minneapolis, Minn; Portland, Ore; and the Monongahela Valley, Pa. Use of estrogen and progestin was determined by interview. Participants completed the Geriatric Depression Scale short form (GDS) and were considered depressed if they reported 6 or more of 15 possible symptoms of depression.
RESULTS
A total of 6.3% (72/1,150) of current estrogen users, 7.2% (142/1,964) of past estrogen users, and 9.0% (313/3,488) of never users reported 6 or more symptoms of depression (P = .004). Current estrogen users had a decreased risk of reporting 6 or more depressive symptoms, compared with not current (past or never) users of estrogen (odds ratio [OR], 0.7; 95% CI, 0.5 to 0.9; P = .01], adjusted for living alone, bilateral oophorectomy, current smoking, physical activity, social network, self-perceived health, cognitive function, functional status, and antidepressant use. However, excluding women who use estrogen or progestin alone, we were unable to find an association between current use of combined estrogen plus progestin therapy and depressive symptoms (adjusted OR, 0.8; 95% CI, 0.5 to 1.4; P = .5).
CONCLUSIONS
This cross-sectional study found that current use of unopposed estrogen was associated with a decreased risk of depressive symptoms in older women. Additional studies are needed to understand the effect of combined estrogen and progestin therapy on the prevalence of depressive symptoms in older women.
Keywords: depression, estrogen replacement therapy, aged, follow-up studies
Women are twice as likely as men to experience depression,1 suggesting that cyclic fluctuations in gonadal hormones may contribute to this problem.2 However, the effect of postmenopausal estrogen therapy on depressive symptoms in older women is unclear.3,4
Several small, randomized, controlled, double-blind trials have examined the effect of short-term (approximately 3 months) estrogen therapy on depression in younger (age <60 years) postmenopausal women, but results have been inconclusive. Four trials found that women treated with estrogen therapy have fewer depressive symptoms than those treated with placebo,5–8 and 8 trials found no difference in depression scores among women receiving estrogen compared with those receiving placebo.9–16 The majority of these trials used unopposed estrogen therapy. Because 2 of the positive studies enrolled recently menopausal women,6,8 it is unclear whether the decrease in depression scores resulted from reduced vasomotor symptoms, or whether direct effects of estrogen were responsible for fewer depressive symptoms. Other studies examining the effect of unopposed estrogen therapy in severely depressed women have been small and have produced inconsistent results.17–21
Very little data exist regarding the mood effects of estrogen in older women. One observational study reported fewer depressive symptoms among elderly women on estrogen therapy, but this study did not adjust for current use of progestins.22 Because unopposed estrogen therapy is strongly associated with endometrial hyperplasia23 and cancer,24 women with a uterus must take a progestin to counteract the endometrial proliferative action of estrogen. Progestins can act as antiestrogens by down-regulating estrogen receptors, so the addition of a progestin may attenuate any benefits of estrogen on depression.4,25 We conducted a cross-sectional analysis to determine the association between estrogen therapy and depressive symptoms in elderly women.
METHODS
Subjects
Between 1986 and 1988, we recruited 9,704 ambulatory, white women at least 65 years of age from population-based listings (such as voter registration lists) in Baltimore, Md; Minneapolis, Minn; Portland, Ore; and the Monongahela Valley, Pa for the Study of Osteoporotic Fractures, a prospective cohort study designed to determine risk factors for osteoporotic fractures.26 At a subsequent visit (1992–1994), 6,602 of these women (76% of survivors) completed at least 10 items on the 15-item Geriatric Depression Scale (GDS). These 6,602 women are the subjects of this analysis. The study was approved by the appropriate Institutional Review Boards, and all subjects provided written informed consent.
Measurements
Use of Estrogen and Progestin.
Participants were instructed to bring all prescription and nonprescription medications with them to the study visit (1992–1994). A staff person reviewed all medications and recorded their names, current doses, and routes of administration. A participant was classified as a current user of estrogen if oral or transdermal estrogen was used in the prior 14 days, and a current user of progestin if an oral progestin was used in the prior 14 days. Use of estrogen creams, injections, or suppositories was not counted as estrogen use; use of megestrol was not counted as progestin use. To identify past use of estrogen, we reviewed questionnaires collected from participants at 5 prior time points between 1986 and 1992. Excluding women who were current users of estrogen at the study visit when depressive symptoms were measured (1992–1994), those who reported past or current use of estrogen on any of the 5 prior questionnaires were classified as past users of estrogen. Those who did not report past or current use of estrogen on any questionnaire were classified as never users.
Depressive Symptoms.
Depressive symptoms were measured using the GDS short form, a validated and reliable, 15-item, self-report, depressive symptoms checklist designed to detect the presence of current depression in the elderly.27 Using a cut point of 6 or more symptoms, the GDS has a sensitivity of 88% and specificity of 62%, as compared with a structured clinical interview for depression.28,29 For the 3% of participants who completed between 10 and 14 GDS items, we estimated the total number of depressive symptoms by dividing the reported number of symptoms by the proportion of items completed.
Other Measurements.
Age, marital status, living status, smoking, physical activity, current use of antidepressant medications, and current perceived health status (excellent/good vs fair/poor/very poor) were determined from a questionnaire administered to all participants at the study visit (1992–1994) and reviewed by an interviewer. Self-reported education, alcohol use, and past hysterectomy and/or oophorectomy were determined in 1986-1988.
Physical activity was estimated in 1992–1994 using a modified Paffenbarger scale that assesses the type and duration of weight-bearing activities in a variety of settings.30 Participants were asked about the frequency with which they performed each of 40 activities during the previous year. The number of times each activity was performed was multiplied by 5.0 for low-intensity (e.g., walking or gardening), 7.5 for medium-intensity (e.g., dancing or tennis), or 10.0 for high-intensity activities (e.g., jogging or skiing). Scores were added to calculate total physical activity (weighted number of times in past year).
We measured social support in 1992–1994 with the Lubben Social Network Scale.31 Participants answered 11 questions regarding contact with spouse, relatives, and friends. Scores for the individual questions were added to create a total social network score. Higher scores indicate better social network. As a measurement of access to medical care, we asked participants, “Do you have a doctor or regular place you go for health care or advice about health care?”
Weight was measured in 1992–1994 using a balance beam scale, and height was measured using a stadiometer.32 Body mass index was calculated as weight in kilograms divided by height in meters squared. Cognitive function was measured in 1992–1994 by a trained examiner using Digit Symbol, a subtest of the Wechsler Adult Intelligence Scale.33 Scores on Digit Symbol reflect the number correct within the timed trial, thus lower scores indicate poorer performance.
We measured functional status in 1992–1994 using a 39-point scale with up to 3 points (some difficulty, much difficulty, unable to do) for each of 13 activities (e.g., dressing, bathing, preparing meals, doing housework, shopping, walking 2 or 3 blocks) based on a modified version of the Stanford Health Assessment Questionnaire.34 Higher scores indicate worse functional status.
Statistical Analysis
We compared differences in characteristics among current, past, and never estrogen users using χ2tests for dichotomous variables and analysis of variance for continuous variables. We used forward stepwise logistic regression to analyze the adjusted risk of reporting 6 or more symptoms of depression associated with current versus not current (including past and never) hormone use, adding those variables associated (at P < .10) with depressive symptoms (GDS ≥ 6) to multivariable models. For all analyses, we report odds ratios (OR) with 95% confidence intervals (95% CI). Analyses were performed using Statistical Analysis Software (SAS, Cary, NC).
RESULTS
A total of 1,150 women (17%) reported current use of estrogen, including 869 who were taking estrogen alone, and 281 who were taking estrogen and progestin. Compared with not current users of estrogen, current estrogen users were younger, more likely to be married, less likely to live alone, and better educated (Table 1). They were more likely to have had a hysterectomy, more likely to have had a bilateral oophorectomy, more physically active, and more likely to have a primary doctor. Current estrogen users had lower body mass index, better cognitive function and better functional status, and were more likely to use antidepressants than not current users. Over 89% of current estrogen users were taking conjugated equine estrogens; over 97% of current progestin users were taking medroxyprogesterone acetate.
Table 1.
Characteristics of the 6,602 Participants*
| Characteristic | Current Users (n = 1,150) | Past Users (n = 1,964) | Never Users (n = 3,488) | P Value |
|---|---|---|---|---|
| Age, y | 76 ± 4 | 76 ± 4 | 77 ± 5 | <.001 |
| Married, % | 49 | 44 | 36 | <.001 |
| Lives alone, % | 44 | 46 | 53 | <.001 |
| Education, y | 13.2 ± 2.7 | 13.0 ± 2.6 | 12.5 ± 2.8 | <.001 |
| Hysterectomy, % | 65 | 44 | 28 | <.001 |
| Bilateral oophorectomy, % | 34 | 21 | 11 | <.001 |
| Current smoking, % | 5 | 6 | 6 | .75 |
| Current drinks per week | 2.1 ± 3.9 | 2.2 ± 4.2 | 1.8 ± 4.0 | <.001 |
| Physical activity (weighted no. times past year) | 283 ± 248 | 255 ± 268 | 214 ± 243 | <.001 |
| Social network score | 3.2 ± 0.7 | 3.2 ± 0.7 | 3.1 ± 0.7 | <.001 |
| Have a primary doctor, % | 99 | 98 | 97 | <.001 |
| Fair/poor self-perceived health, % | 19 | 18 | 20 | .47 |
| Body mass index (kg/m2) | 25.6 ± 4.2 | 26.4 ± 4.7 | 26.8 ± 4.9 | <.001 |
| Digit Symbol (number correct) | 43 ± 11 | 43 ± 11 | 41 ± 12 | <.001 |
| Functional status score | 2.2 ± 4.4 | 2.2 ± 4.4 | 2.4 ± 4.7 | .07 |
| Current progestin use, % | 24 | 0 | 0 | <.001 |
| Current antidepressant use, % | 11 | 7 | 5 | <.001 |
± values are mean ± standard deviation.
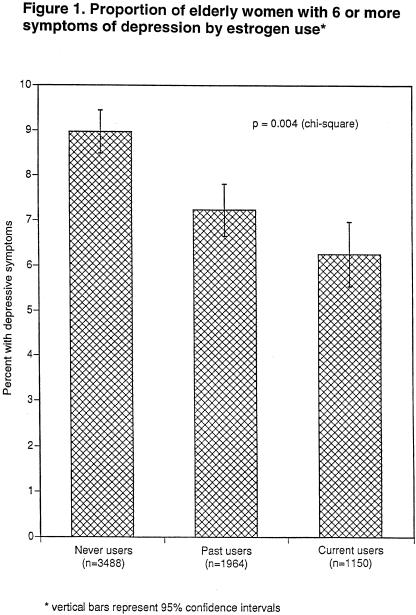
A total of 6.3% of current estrogen users, 7.2% of past estrogen users, and 9.0% of never users reported 6 or more symptoms of depression (GDS ≥ 6) (P = .004)(Fig. 1). Compared with not current (past or never) users of estrogen, current estrogen users had a decreased risk of reporting 6 or more depressive symptoms (OR, 0.7; 95% CI, 0.6 to 0.9; P = .02). This association was unchanged after adjusting for living alone, bilateral oophorectomy, current smoking, physical activity, social network, self-perceived health, cognitive function, functional status, and antidepressant use (OR, 0.7; 95% CI, 0.5 to 0.9; P = .01) (Table 2). Further adjustment for progestin use did not affect this association (OR, 0.6; 95% CI, 0.4 to 0.9; P = .01).
FIGURE 1.
Proportion of elderly women with 6 or more symptoms of depression by estrogen use.
Table 2.
Predictors of Depressive Symptoms in 6,057 Elderly Women
| Predictor | Odds Ratio (95% Confidence Interval) | P value |
|---|---|---|
| Current estrogen use* | 0.7 (0.5 to 0.9) | .01 |
| Lives alone | 0.7 (0.6 to 0.9) | .01 |
| Bilateral oophorectomy | 1.5 (1.2 to 2.0) | .002 |
| Current smoker | 1.7 (1.2 to 2.5) | .004 |
| Greater physical activity (per 255-point increase in score) | 0.8 (0.7 to 1.0) | .01 |
| Greater social network (per 1.0-point increase in score) | 0.4 (0.3 to 0.5) | <.001 |
| Fair/poor self-perceived health | 4.1 (3.3 to 5.2) | <.001 |
| Worse cognitive function (per 12-item decrease in number correct) | 1.3 (1.2 to 1.5) | <.001 |
| Worse functional status (per 4.4-point increase in score) | 1.5 (1.4 to 1.6) | <.001 |
| Current antidepressant use | 1.8 (1.3 to 2.5) | <.001 |
Based on forward stepwise logistic regression model including all variables in Table 1. Variables associated (at P < .10) with a score of ≥ 6 on the Geriatric Depression Scale were retained in the model. Variables not retained in the model were age, marital status, education, hysterectomy, alcohol, having a primary doctor, body mass index, and current progestin use.
Bilateral oophorectomy was associated with a 50% increased risk of reporting 6 or more depressive symptoms among all participants (OR, 1.5; 95% CI, 1.2 to 2.0; P < .001, adjusted for the variables listed in Table 2) and among nonusers of estrogen alone (adjusted OR, 1.5; 95% CI, 1.2 to 2.1; P = .004). The other variables associated with increased depressive symptoms were living with others, current smoking, less physical activity, poorer social network, fair/poor self-perceived health, worse cognitive function, worse functional status, and current antidepressant use (Table 2).
When we excluded women who were taking antidepressants, current estrogen use was still associated with a decreased risk of reporting 6 or more depressive symptoms (OR, 0.6; 95% CI, 0.5 to 0.9; P = .01), adjusted for living alone, bilateral oophorectomy, current smoking, physical activity, social network, self-perceived health, cognitive function, and functional status.
A total of 7.6% of current progestin users, 5.8% of past progestin users, and 8.1% of never users were depressed (P = .3). Current progestin use was not associated with reporting 6 or more depressive symptoms in a univariate model (OR, 1.0; 95% CI, 0.6 to 1.5; P = .8) or after adjusting for potential confounding variables including current estrogen use (OR, 1.2; 95% CI, 0.7 to 2.3; P = .5). Among current estrogen users, the 281 women who were taking estrogen plus progestin did not have an increased risk of depressive symptoms compared with the 869 women who were taking estrogen alone (OR, 1.6; 95% CI, 0.8 to 3.1; P = .2, adjusted for the variables listed in Table 2).
Excluding women who use progestin, either alone (n = 7) or in combination with estrogen (n = 281), current use of unopposed estrogen was associated with a decreased risk of reporting 6 or more depressive symptoms (OR, 0.6; 95% CI, 0.4 to 0.9; P = .009, adjusted for the variables listed in Table 2). However, excluding women who use estrogen alone (n = 869) or progestin alone (n = 7), current use of combined estrogen plus progestin was not associated with depressive symptoms (adjusted OR, 0.8; 95% CI, 0.5 to 1.4; P = .5).
In similar multivariable models, we found a decreased risk of reporting 6 or more depressive symptoms in current estrogen users compared with never users of estrogen (OR, 0.6; 95% CI, 0.4 to 0.8; P = .002) and in past users compared with never users (OR, 0.8; 95% CI, 0.6 to 1.0; P = .06) (Table 3) We also found decreased depressive symptoms in ever users compared with never users of estrogen (OR, 0.7; 95% CI, 0.6 to 0.9; P = .004), but not in current versus past users (OR, 0.8, 95% CI, 0.6 to 1.1; P = .2).
Table 3.
Risk of Depressive Symptoms* Associated with Different Categories of Estrogen Use.
| Odds Ratio (95% Confidence Interval)† | P value | |
|---|---|---|
| Nonuser (past and never) | 1.0 | — |
| Current user | 0.7 (0.5 to 0.9) | .01 |
| Never user | 1.0 | — |
| Past user | 0.8 (0.6 to 1.0) | .06 |
| Current user | 0.6 (0.4 to 0.8) | .002 |
| Past user | 1.0 | — |
| Current user | 0.8 (0.6 to 1.1) | .2 |
| Never user | 1.0 | — |
| Ever user | 0.7 (0.6 to 0.9) | .004 |
Score ≥ 6 on the Geriatric Depression Scale.
From multivariable logistic models adjusted for living alone, bilateral oophorectomy, current smoking, physical activity, social network, self-perceived health, cognitive function, functional status, and antidepressant use.
DISCUSSION
Elderly women who currently use estrogen had fewer depressive symptoms than not current users of estrogen. These findings support the results of a previous observational study that found fewer depressive symptoms among current users of estrogen compared with not current users of estrogen.22 In our study, prior bilateral oophorectomy was associated with depressive symptoms, suggesting that both exogenous and low levels of endogenous estrogens may protect elderly women from incident depression.
Although we had limited power to detect a difference in depressive symptoms between women on combined therapy and not current users, we were unable to demonstrate an association between depressive symptoms and use of combined estrogen and progestin. The possibility that use of a progestin with estrogen counteracts any beneficial effects of estrogen on mood is supported by the results of two small randomized trials. One trial reported more depressive symptoms in women treated with conjugated equine estrogen plus medroxyprogesterone acetate compared with those treated with estrogen alone.35 The other trial found that women randomly assigned to estradiol alone were less depressed than those randomly assigned to estradiol plus levonorgestrel (a progestin), and that women assigned to estradiol plus levonorgestrel were in turn less depressed than those randomly assigned to levonorgestrel alone.5
Depression has been associated with functional deficiency in brain neurotransmitters, such as norepinephrine, serotonin, and dopamine.36 Estrogen may work as an antidepressant by inducing changes in central nervous system receptors for these neurotransmitters.4,37–40 For example, estrogen increases the density of serotonergic binding sites in areas of the brain concerned with the control of mood,41 and may augment serotonergic activity in postmenopausal women.40,42 Estrogen may increase central nervous system levels of norepinephrine and β-endorphin.43,44 Depression and low serum estradiol levels correlate with similar electroencephalogram findings, suggesting that estrogen therapy may reduce depression through direct effects on the brain.45 Estrogens depress the activity of monoamine oxidase (MAO) and decrease plasma MAO levels among depressed patients, suggesting that estrogen may function as an MAO inhibitor.18,46,47 Finally, depression may result from changes in second messenger systems and gene expression, and antidepressant treatments (such as estrogen) may work through intracellular mechanisms that increase the expression of neurotrophic factors necessary for the survival and function of particular neurons.48
We chose to examine the prevalence of depressive symptoms in current compared with not current, rather than never, users of estrogen for the primary analysis because we assumed that any receptor changes associated with estrogen therapy would be transient and that depressive symptoms would return after stopping estrogen. However, we also found a decreased risk of depressive symptoms among past users that approached but did not reach statistical significance (P = .06), suggesting either that the receptor changes associated with estrogen may still be present after estrogen therapy has been discontinued or that selection bias may explain some of the protective effect of estrogen.
Postmenopausal women who take hormone therapy are of higher socioeconomic status, healthier, more likely to receive preventive care, and at lower risk for osteoporosis and coronary heart disease.49–52 These selection factors may have been responsible for the previously observed benefit of estrogen on coronary heart disease that was not confirmed in a large, randomized trial,53 and could contribute to lower depression scores. In addition, women with depressive symptoms may be less likely to receive or comply with estrogen than those who are not depressed. If the protective effect of estrogen therapy on depressive symptoms was entirely due to selection bias, however, we would also expect current users of estrogen plus progestin to have a decreased risk of depressive symptoms. On the contrary, though we cannot exclude the possibility of a type II error, we found no effect of combined estrogen plus progestin on the prevalence of depressive symptoms.
We found that current estrogen users were more likely to report taking antidepressant medications than not current users, suggesting that women taking estrogen were more likely to visit a doctor and thus had greater opportunities to be treated for depression. To address the possibility that greater use of antidepressant medication was responsible for fewer depressive symptoms in the estrogen group, we demonstrated that estrogen was associated with fewer depressive symptoms even among women who were not taking antidepressants. However, because current estrogen users were more likely to take antidepressants, this subset analysis may have excluded more depressed participants from the current users group than from the not current users group, and thus may have been biased toward a benefit from estrogen. Therefore, we also adjusted all of our multivariable models for antidepressant use and still found that estrogen was associated with fewer depressive symptoms.
Although we measured many potential confounding variables, the association between current use of estrogen and greater depressive symptoms may be influenced by unmeasured variables, such as the presence of other chronic medical conditions. A few potential confounding variables, including alcohol use, were assessed 6 years previously, and their values may have changed by the time of our study. Our results may have underestimated the effects of estrogen therapy because some carry-over effect may have occurred in participants who were considered not current users but who had used estrogen more than 14 days prior to assessment. Because we measured depressive symptoms rather than doing a clinical interview for depression, we must conclude that estrogen therapy is associated with fewer depressive symptoms and not necessarily with less clinical depression. However, previous studies have used similar self-report instruments to measure depression,54 and elderly women with depressive symptoms represent a common clinical problem for health care providers.55 Finally, although we are unaware of evidence suggesting that nonwhite women respond differently to estrogen than white women, the association between current use of estrogen and depressive symptoms among elderly white women may differ from that of other patient populations.
CONCLUSION
Depression is a common and readily treatable condition among older women, but few receive appropriate treatment.1 Depressed patients have increased disability, health care utilization, and mortality, as well as reduced productivity and health-related quality of life.56–62 Unopposed estrogen may be an effective therapy for depressive symptoms in elderly women without a uterus, but unopposed estrogen therapy is not an option for women who have a uterus, and combined estrogen and progestin therapy may not improve depressive symptoms. Newer selective estrogen receptor modulators such as raloxifene do not require concurrent use of progestin,63–66 but their effects on depressive symptoms have not been determined. Because of the potential for selection bias in this and other observational studies, any potential benefit of estrogen would require confirmation in a randomized trial before it could be recommended for the prevention or treatment of depressive symptoms.
Acknowledgments
Dr. Whooley is supported by a Research Career Development Award from the Department of Veterans Affairs Health Services Research and Development Service. This study was supported by a grant from the American Federation for Aging Research and by Public Health Service grants AG05394, AG05407, AR35582, AR35583, AR35584, and NS36016.
We are indebted to Li-Yung Lily Lui, MA, MS for her assistance with data analysis.
Investigators in the Study of Osteoporotic Fractures Research Group
University of California, San Francisco (coordinating center): S.R. Cummings, (principal investigator), M.C. Nevitt (co-investigator), D.G. Seeley (project director), D.M. Black (study statistician), H.K. Genant (director, central radiology laboratory), C. Arnaud, D. Bauer, W. Browner, L. Christianson, M. Dockrell, C. Fox, R. Gore, S. Harvey, M. Jaime-Chavez, L. Laidlaw, R. Lipschutz, L. Lui, G. Milani, L. Palermo, R. San Valentin, K. Stone, H. Tabor, D. Tanaka, C. Yeung.
University of Maryland: J.C. Scott (principal investigator), R. Sherwin (co-investigator), M.C. Hochberg (co-investigator), J. Lewis (project director), E. Peddicord (clinic coordinator), A. Bauer, C. Boehm, G. Cullum, L. Finazzo, M.E. Flaks, T. Ford, D. Harris, B. Hohman, E. Oliner, T. Page, J. Schlossberg, C. Shaffer, A. Trimble, S. Trusty.
University of Minnesota: K. Ensrud (principal investigator), P. Schreiner (co-investigator), C. Bell (project director), E. Mitson (clinic coordinator), C. Bird, D. Blanks, S. Estill, S. Fillhouer, S. Fincham, J. Griffith, J. Hansen, F. Imker-Witte, K. Jacobson, K. Kiel, K. Knauth, N. Nelson, E. Penland-Miller, M. Riley-Alves.
University of Pittsburgh: J.A. Cauley (principal investigator), L.H. Kuller (co-principal investigator), M. Vogt (co-investigator), L. Harper (project director), L. Buck (clinic coordinator), C. Bashada, D. Cusick, G. Engleka, A. Githens, M. Gorecki, K. McCune, D. Medve, M. Nasim, C. Newman, S. Rudovsky, N. Watson.
The Kaiser Permanente Center for Health Research, Portland, Oregon: E. Harris (principal investigator, project director), W.M. Vollmer, E. Orwoll, H. Nelson (co-investigators), K. Crannell (project administrator, clinic coordinator), J. Bender, A. Doherty, K. Easter, M. Erwin, F. Heinith, J. Kann, K. Redden, C. Romero, K. Snider, C. Souvanlasy.
REFERENCES
- 1.Depression Guideline Panel. Depression in Primary Care. Vol 1: Detection and Diagnosis. Clinical Practice Guideline. Washington, DC: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1993. AHCPR 93–0550. [Google Scholar]
- 2.Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798–811. doi: 10.1016/s0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- 3.Jensvold MF, Halbreich U, Hamilton JA. Psychopharmacology and Women: Sex, Gender and Hormones. Washington, DC: American Psychiatric Press; 1996. eds. [Google Scholar]
- 4.Stahl SM. Basic psychopharmacology of antidepressants, part 2: estrogen as an adjunct to antidepressant treatment. J Clin Psychiatry. 1998;4:15–24. [PubMed] [Google Scholar]
- 5.Dennerstein L, Burrows GD, Hyman GJ, Sharpe K. Hormone therapy and affect. Maturitas. 1979;1:247–59. doi: 10.1016/0378-5122(79)90015-x. [DOI] [PubMed] [Google Scholar]
- 6.Sherwin BB, Gelfand MM. Sex steroids and affect in the surgical menopause: a double-blind, cross-over study. Psychoneuroendocrinology. 1985;10:325–35. doi: 10.1016/0306-4530(85)90009-5. [DOI] [PubMed] [Google Scholar]
- 7.Ditkoff EC, Crary WG, Cristo M, Lobo RA. Estrogen improves psychological function in asymptomatic postmenopausal women. Obstet Gynecol. 1991;78:991–5. [PubMed] [Google Scholar]
- 8.Derman RJ, Dawood MY, Stone S. Quality of life during sequential hormone replacement therapy—a placebo-controlled study. Int J Fertil Menopausal Stud. 1995;40:73–8. [PubMed] [Google Scholar]
- 9.George GC, Utian WH, Beaumont PJ, Beardwood CJ. Effect of exogenous oestrogens on minor psychiatric symptoms in postmenopausal women. S Afr Med J. 1973;47:2387–8. [PubMed] [Google Scholar]
- 10.Coppen A, Bishop M, Beard RJ. Effects of piperazine oestrone sulphate on plasma tryptophan, oestrogens, gonadotrophins and psychological functioning in women following hysterectomy. Curr Med Res Opin. 1977;4:29–36. [Google Scholar]
- 11.Campbell S, Whitehead M. Oestrogen therapy and the menopausal syndrome. Clin Obstet Gynaecol. 1977;4:31–47. [PubMed] [Google Scholar]
- 12.Strickler RC, Borth R, Cecutti A, et al. The role of oestrogen replacement in the climacteric syndrome. Psychol Med. 1977;7:631–9. doi: 10.1017/s0033291700006280. [DOI] [PubMed] [Google Scholar]
- 13.Thomson J, Oswald I. Effect of oestrogen on the sleep, mood, and anxiety of menopausal women. Br Med J. 1977;2:1317–9. doi: 10.1136/bmj.2.6098.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coope J. Is oestrogen therapy effective in the treatment of menopausal depression? J R Coll Gen Pract. 1981;31:134–40. [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery JC, Appleby L, Brincat M, et al. Effect of oestrogen and testosterone implants on psychological disorders in the climacteric. Lancet. 1987;1:297–9. doi: 10.1016/s0140-6736(87)92026-5. [DOI] [PubMed] [Google Scholar]
- 16.Saletu B, Brandstatter N, Metka M, et al. Double-blind, placebo-controlled, hormonal, syndromal and EEG mapping studies with transdermal oestradiol therapy in menopausal depression. Psychopharmacology. 1995;122:321–9. doi: 10.1007/BF02246261. [DOI] [PubMed] [Google Scholar]
- 17.Prange AJ. Estrogen may well affect response to antidepressant. JAMA. 1972;219:143–4. [Google Scholar]
- 18.Klaiber EL, Broverman DM, Vogel W, Kobayashi Y. Estrogen therapy for severe persistent depressions in women. Arch Gen Psychiatry. 1979;36:550–4. doi: 10.1001/archpsyc.1979.01780050060006. [DOI] [PubMed] [Google Scholar]
- 19.Shapira B, Oppenheim G, Zohar J, Segal M, Malach D, Belmaker RH. Lack of efficacy of estrogen supplementation to imipramine in resistant female depressives. Biol Psychiatry. 1985;20:576–9. doi: 10.1016/0006-3223(85)90031-9. [DOI] [PubMed] [Google Scholar]
- 20.Zohar J, Shapira B, Oppenheim G, Ayd FJ, Belmaker RH. Addition of estrogen to imipramine in female-resistant depressives. Psychopharmacol Bull. 1985;21:705–6. [PubMed] [Google Scholar]
- 21.Oppenheim G, Zohar J, Shapiro B, et al. The role of estrogen in treating resistant depression. In: Zohar J, Belmaker RH, et al., editors. Treating Resistant Depression. Boston, Mass: PMA Publishing; 1987. pp. 357–63. In: [Google Scholar]
- 22.Palinkas LA, Barrett-Connor E. Estrogen use and depressive symptoms in postmenopausal women. Obstet Gynecol. 1992;80:30–6. [PubMed] [Google Scholar]
- 23.The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 24.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304–13. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- 25.Sherwin BB. Affective changes with estrogen and androgen replacement therapy in surgically menopausal women. J Affect Disord. 1988;14:177–87. doi: 10.1016/0165-0327(88)90061-4. [DOI] [PubMed] [Google Scholar]
- 26.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–8. [PubMed] [Google Scholar]
- 27.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–11. [PubMed] [Google Scholar]
- 28.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist. 1986;5:165–73. [Google Scholar]
- 29.Gerety MB, Williams J, Jr, Mulrow CD, et al. Performance of case-finding tools for depression in the nursing home: influence of clinical and functional characteristics and selection of optimal threshold scores. J Am Geriatr Soc. 1994;42:1103–9. doi: 10.1111/j.1532-5415.1994.tb06217.x. [DOI] [PubMed] [Google Scholar]
- 30.Paffenbarger RSJ, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 31.Lubben JE. Assessing social networks among elderly populations. Fam Comm Health. 1988;11:42–52. [Google Scholar]
- 32.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, Ill: Human Kinetics Books; 1988. [Google Scholar]
- 33.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corporation; 1988. [Google Scholar]
- 34.Pincus T, Summey JA, Soraci S, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 35.Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–43. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- 36.Arpels JC. The female brain hypoestrogenic continuum from the premenstrual syndrome to menopause. A hypothesis and review of supporting data. J Reprod Med. 1996;41:633–9. [PubMed] [Google Scholar]
- 37.Guicheney P, Leger D, Barrat J, et al. Platelet serotonin content and plasma tryptophan in peri- and postmenopausal women: variations with plasma oestrogen levels and depressive symptoms. Eur J Clin Invest. 1988;18:297–304. doi: 10.1111/j.1365-2362.1988.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 38.Klaiber EL, Broverman DM, Vogel W, Kobayashi Y, Moriarty D. Effects of estrogen therapy on plasma MAO activity and EEG driving responses of depressed women. Am J Psychiatry. 1972;128:1492–8. doi: 10.1176/ajp.128.12.1492. [DOI] [PubMed] [Google Scholar]
- 39.Price WA, Giannini AJ. Antidepressant effects of estrogen. J Clin Psychiatry. 1985;46:506. [PubMed] [Google Scholar]
- 40.Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44:839–50. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 41.Fink G, Sumner BE, Rosie R, Grace O, Quinn JP. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16:325–44. doi: 10.1007/BF02088099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halbreich U, Rojansky N, Palter S, Tworek H, Hissin P, Wang K. Estrogen augments serotonergic activity in postmenopausal women. Biol Psychiatry. 1995;37:434–41. doi: 10.1016/0006-3223(94)00181-2. [DOI] [PubMed] [Google Scholar]
- 43.Oppenheim G. Estrogen in the treatment of depression: neuropharmacological mechanisms. Biol Psychiatry. 1983;18:721–5. [PubMed] [Google Scholar]
- 44.Kumar MS, Chen CL, Muther TF. Changes in the pituitary and hypothalamic content of methionine-enkephalin during the estrous cycle of rats. Life Sci. 1979;25:1687–96. doi: 10.1016/0024-3205(79)90410-7. [DOI] [PubMed] [Google Scholar]
- 45.Saletu B, Brandstatter N, Metka M, et al. Hormonal, syndromal and EEG mapping studies in menopausal syndrome patients with and without depression as compared with controls. Maturitas. 1996;23:91–105. doi: 10.1016/0378-5122(95)00946-9. [DOI] [PubMed] [Google Scholar]
- 46.Coope J. Hormonal and non-hormonal interventions for menopausal symptoms. Maturitas. 1996;23:159–68. doi: 10.1016/0378-5122(95)00971-x. [DOI] [PubMed] [Google Scholar]
- 47.McEwen BS, Biegon A, Davis PG, et al. Steroid hormones: humoral signals which alter brain cell properties and functions. Recent Prog Horm Res. 1982;38:41–92. doi: 10.1016/b978-0-12-571138-8.50007-x. [DOI] [PubMed] [Google Scholar]
- 48.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 49.Cauley JA, Cummings SR, Black DM, Mascioli SR, Seeley DG. Prevalence and determinants of estrogen replacement therapy in elderly women. Am J Obstet Gynecol. 1990;163:1438–44. doi: 10.1016/0002-9378(90)90602-4. [DOI] [PubMed] [Google Scholar]
- 50.Barrett-Connor E. Postmenopausal estrogen and prevention bias. Ann Intern Med. 1991;115:455–6. doi: 10.7326/0003-4819-115-6-455. [DOI] [PubMed] [Google Scholar]
- 51.Sturgeon SR, Schairer C, Brinton LA, Pearson T, Hoover RN. Evidence of a healthy estrogen user survivor effect. Epidemiology. 1995;6:227–31. doi: 10.1097/00001648-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol. 1996;143:971–8. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 53.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 54.Zweifel JE, O'Brien WH. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology. 1997;22:189–212. doi: 10.1016/s0306-4530(96)00034-0. [DOI] [PubMed] [Google Scholar]
- 55.Lebowitz BD, Pearson JL, Schneider LS, et al. Diagnosis and treatment of depression in late life. Consensus statement update. JAMA. 1997;278:1186–90. [PubMed] [Google Scholar]
- 56.Johnson J, Weissman MM, Klerman GL. Service utilization and social morbidity associated with depressive symptoms in the community. JAMA. 1992;267:1478–83. [PubMed] [Google Scholar]
- 57.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA. 1989;262:914–9. [PubMed] [Google Scholar]
- 58.Broadhead WE, Blazer DG, George LK, Tse CK. Depression, disability days, and days lost from work in a prospective epidemiologic survey. JAMA. 1990;264:2524–8. [PubMed] [Google Scholar]
- 59.Simon GE. Psychiatric disorder and functional somatic symptoms as predictors of health care use. Psychiatr Med. 1992;10:49–59. [PubMed] [Google Scholar]
- 60.Katon W, Berg AO, Robins AJ, Risse S. Depression—medical utilization and somatization. West J Med. 1986;144:564–8. [PMC free article] [PubMed] [Google Scholar]
- 61.Spitzer RL, Kroenke K, Linzer M, et al. Health-related quality of life in primary care patients with mental disorders. Results from the PRIME-MD 1000 Study. JAMA. 1995;274:1511–7. [PubMed] [Google Scholar]
- 62.Whooley MA, Browner WS. Association between depressive symptoms and mortality in older women. Arch Int Med. 1998;158:2129–35. doi: 10.1001/archinte.158.19.2129. [DOI] [PubMed] [Google Scholar]
- 63.Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45–52. doi: 10.3181/00379727-217-44204. [DOI] [PubMed] [Google Scholar]
- 64.Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–7. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 65.Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women's health. J Women's Health. 1997;6:523–31. doi: 10.1089/jwh.1997.6.523. [DOI] [PubMed] [Google Scholar]
- 66.Mitlak BH, Cohen FJ. In search of optimal long-term female hormone replacement: the potential of selective estrogen receptor modulators. Horm Res. 1997;48:155–63. doi: 10.1159/000185507. [DOI] [PubMed] [Google Scholar]