Abstract
The role of platelet endothelial cell adhesion molecule-1 (PECAM-1) in endothelial cell–cell interactions and its contribution to cadherin-mediated cell adhesion are poorly understood. Such studies have been difficult because all known endothelial cells express PECAM-1. We have used Madin-Darby canine kidney (MDCK) cells as a model system in which to evaluate the role of PECAM-1 isoforms that differ in their cytoplasmic domains in cell–cell interactions. MDCK cells lack endogenous PECAM-1 but form cell–cell junctions similar to those of endothelial cells, in which PECAM-1 is concentrated. MDCK cells were transfected with two isoforms of murine PECAM-1, Δ15 and Δ14&15, the predominant isoforms expressed in vivo. Expression of the Δ15 isoform resulted in apparent dedifferentiation of MDCK cells concomitant with the loss of adherens junctions, down-regulation of E-cadherin, α- and β-catenin expression, and sustained activation of extracellular regulated kinases. The Δ15 isoform was not concentrated at cell–cell contacts. In contrast, the Δ14&15 isoform localized to sites of cell–cell contact and had no effect on MDCK cell morphology, cadherin/catenin expression, or extracellular regulated kinase activity. Thus, the presence of exon 14 in the cytoplasmic domain of PECAM-1 has dramatic effects on the ability of cells to maintain adherens junctions and an epithelial phenotype. Therefore, changes in the expression of exon 14 containing PECAM-1 isoforms, which we have observed during development, may have profound functional consequences.
INTRODUCTION
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a member of the immunoglobulin gene superfamily. It is highly expressed at sites of endothelial cell–cell contact and is expressed at moderate levels on the surface of platelets and hemopoietic cells. PECAM-1 is involved in leukocyte–endothelium transmigration, modulation of integrin activity on leukocytes and T cells, and angiogenesis (Newman, 1997; Sheibani and Frazier, 1999). Its expression on the surface of endothelial cells and endocardial cells during early embryonic development suggests that PECAM-1 plays a role in the development of the cardiovascular system (Baldwin et al., 1994). However, the role of PECAM-1 in the regulation of endothelial cell adhesive functions and morphogenesis is not understood. Antibodies to PECAM-1 prevent endothelial cell–cell contacts and the formation of monolayers when added to subconfluent cultures (Albelda et al., 1990) but fail to disrupt already confluent monolayers. We have shown that the expression of PECAM-1 in endothelial cells, in which endogenous PECAM-1 expression is lost, results in enhanced morphogenesis in three-dimensional Matrigel cultures (Sheibani et al., 1997). Furthermore, antibodies to PECAM-1 block tubular morphogenesis of human umbilical vein endothelial cells in Matrigel assays (Sheibani et al., 1997) and angiogenesis in mouse corneal assays (DeLisser et al., 1997). Therefore, PECAM-1 appears to play a role in endothelial cell–cell, and perhaps cell–matrix, interactions that are essential during angiogenesis (Sheibani and Frazier, 1999).
PECAM-1 participates in both homophilic and heterophilic interactions. It can bind PECAM-1 (Sun et al., 1996), proteoglycans (DeLisser et al., 1993), αvβ3 integrin (Piali et al., 1995), and CD38 (Deaglio et al., 1998). These interactions are modulated, at least in part, by the cytoplasmic domain of PECAM-1 (Yan et al., 1995). Murine PECAM-1 undergoes alternative splicing, generating eight isoforms that differ only in the length of their cytoplasmic domains (Yan et al., 1995; Sheibani et al., 1999). The isoform that lacks exons 14&15 (Δ14&15), and not “full-length” PECAM-1, is the predominant isoform expressed in the endothelium, followed by the isoform that lacks only exon 15 (Δ15) (Sheibani et al., 1999). The alternative splicing of the cytoplasmic domain may have functional consequences. The alternative splicing of exon 14 in murine PECAM-1 isoforms alters its homophilic binding characteristics when expressed in L-cells, regardless of the presence or absence of other cytoplasmic exons (Yan et al., 1995). Thus, specific interactions between PECAM-1 and intracellular proteins that require the presence of exon 14 may be important in modulating PECAM-1 adhesive functions. We have recently shown that multiple isoforms of PECAM-1 are expressed in vascular beds of different tissues in a developmentally regulated manner (Sheibani et al., 1999), suggesting that different isoforms may differentially modulate the adhesive interactions of endothelial cells during vascular development. For example, in the developing kidney, PECAM-1 isoform(s) that contain exon 14 are expressed early in vascular development and are later replaced by PECAM-1 isoform(s) that lack exon 14 in the maturing blood vessels (Sheibani et al., 1999).
Because all cultured endothelial cells that retain appropriate phenotypic markers express multiple isoforms of endogenous PECAM-1, it has been difficult to study PECAM-1 function in a physiologically relevant cell type. The majority of PECAM-1 structural and functional studies have been performed in nonendothelial cells such as L-cells. These cells were initially selected because they lack cadherin-mediated cell–cell interactions, thus making PECAM-1–mediated interactions easier to detect (Nagafuchi et al., 1994; Wang and Rose, 1997). However, cadherin-mediated cell–cell interactions do occur in endothelial cells and are important for the maintenance of an endothelial permeability barrier. Thus, L-cells may not accurately represent the role of PECAM-1 in endothelial cell adhesion. To investigate the role of PECAM-1 isoforms in the modulation of cellular adhesive functions, we have used Madin-Darby canine kidney (MDCK) cells, an epithelial cell line that, like endothelial cells, forms adherens junctions (Lampugnani et al., 1995; Staddon and Rubin, 1996) but lacks PECAM-1 expression. We demonstrate that PECAM-1 isoforms, with and without exon 14, expressed in MDCK cells can differentially modulate the formation and/or maintenance of adherens junctions by activation of MAPK/extracellular regulated kinase (ERK). Furthermore, the localization of PECAM-1 to sites of cell–cell contact may require cadherin-mediated cell–cell interactions.
MATERIALS AND METHODS
Cells and DNA Transfection
MDCK epithelial cells were obtained from the American Type Culture Collection (Rockville, MD) and maintained in α-MEM with 10% heat-inactivated FCS and 10 mM HEPES. For DNA transfection, 5 × 105 cells (stable) or 8 × 105 cells (transient) were plated in a 100-mm tissue culture dish. The next day, cells were rinsed twice with serum-free medium and transfected with expression plasmids containing the cDNA encoding for PECAM-1 isoforms Δ15 or Δ14&15 or empty vector by Lipofectin as described previously (Sheibani et al., 1997). Cells were either harvested 48 h after transfection (transient) or fed with growth medium containing 400 μg/ml G418 to select for stable clones. Stable clones were isolated, expanded, and screened for the expression of PECAM-1 by Western blot and FACScan analysis.
Western Blot Analysis
To screen the clones of stably transfected cells, ∼106 cells were washed with PBS, resuspended in 0.1 ml of 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, and stored at −70°C until all of the clones were available. For other protein analysis, 3 × 105 cells were plated in 100-mm dishes, and 3 d later, cells were fed with either regular growth medium or serum-free medium to starve the cells for 2 additional days. Starved cells were stimulated with regular serum-containing medium for 10 min. Plates were then rinsed twice with cold serum-free medium containing 0.5 mM Na3OV4, lysed in 0.8 ml of lysis buffer (50 mM HEPES, 150 mM NaCl, 0.1 mM EDTA, 1 mM each CaCl2 and MgCl2, 1% NP-40, 0.5% deoxycholate, 100 mM NaF, 3 mM Na3OV4, and a cocktail of protease inhibitors), and transferred to a microfuge tube on ice. Samples were rocked for 30 min at 4°C and centrifuged for 15 min at 14,000 × g, and cleared lysates were transferred to clean tubes. Protein concentrations were determined by the DC protein assay kit (Bio-Rad, Hercules, CA), and aliquots corresponding to equal amounts of protein were mixed with 6× SDS sample buffer containing β-mercaptoethanol, boiled for 3 min, and analyzed by SDS-PAGE with the use of 12% Tris-glycine gels (Novex, San Diego, CA). Proteins were transferred to nitrocellulose and processed as described previously (Sheibani et al., 1998). A polyclonal antibody to murine PECAM-1 extracellular domain (a gift of Dr. B.A. Imhof) that recognizes all PECAM-1 isoforms and a polyclonal antibody to the murine PECAM-1 exon 14 peptide that recognizes only PECAM-1 isoforms that contain exon 14 (Sheibani et al., 1999) were used for blotting. The antibodies to E-cadherin, α-catenin, β-catenin, and γ-catenin were obtained from Transduction Laboratories (Lexington, KY). The antibody to phospho-MAPK was from Promega (Madison, WI), and the antibody to ERK-1 was from Santa Cruz Biotechnology (Santa Cruz, CA). The mAb to vimentin was from Santa Cruz Biotechnology, and the mAb that reacts with an epitope on a wide range of cytokeratins (40–60 kDa) was from DAKO (Carpinteria, CA).
FACScan Analysis
Cells were removed by EDTA (0.04% in PBS with 0.1% BSA) and washed once with Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 7.6, 150 mM NaCl), and ∼106 cells were resuspended in 0.5 ml of TBS with 1% goat serum and incubated on ice for 20 min. Cells were pelleted and resuspended in 0.25 ml of TBS with 1% BSA containing the primary antibody. For PECAM-1, the rat anti-mouse mAb 390 (a gift of Dr. S.B. Albelda) was used at 10 μg/ml. The rat anti-mouse uvomorulin (Sigma Chemical, St. Louis, MO) was used at a 1:500 dilution. After 30 min of incubation with the primary antibody on ice, cells were pelleted, washed twice with 2 ml of TBS with 1% BSA, and resuspended in 0.25 ml of TBS with 1% BSA containing a 1:100 dilution of FITC-conjugated goat anti-rat immunoglobulin G (Pierce, Rockford, IL) for 30 min on ice. Cells were pelleted, washed with TBS plus 1% BSA as described above, and resuspended in 0.5 ml of TBS with 1% BSA. Samples were analyzed on a FACScan (Becton Dickinson, San Jose, CA).
Indirect Immunofluorescence Analysis
Cells (2 × 104) were plated on glass coverslips until they were semiconfluent. Coverslips were rinsed in PBS, and cells were fixed with 3% paraformaldehyde for 15 min at room temperature, washed with TBS, and incubated with primary antibodies to PECAM-1 or uvomorulin in TBS with 1% ovalbumin at concentrations similar to those used for FACScan analysis (see above) for 30 min at 37°C. Coverslips were rinsed with TBS, incubated with FITC-conjugated antibody in TBS with 1% ovalbumin for 30 min at 37°C, washed, and mounted in TBS with 50% glycerol. Cells were viewed on a Nikon (Garden City, NY) phase-epifluorescence microscope with the use of a 40× fluorescence lens and photographed with TMAX 400 black-and-white film.
Inhibitor Studies
All of the inhibitors were obtained from Calbiochem (San Diego, CA), and stock solutions were prepared (1000×) as recommended by the supplier. We examined several concentrations of inhibitors, and the optimal concentrations were chosen for the experiments as noted below. These concentrations of inhibitors are similar to those used by many investigators and demonstrated maximal effect and minimal toxicity. Cells (105) were plated in 60-mm dishes, and the next day they were incubated with growth medium containing the indicated concentrations of inhibitors: PD98059 (50 μM; mitogen-activated protein kinase kinase [MEK[ inhibitor), wortmannin (50 nM; phosphatidylinositol 3-kinase [PI-3 kinase] inhibitor), SB203580 (10 μM; p38 inhibitor), LY294002 (20 μM; PI-3 kinase inhibitor), and GF109203x (100 nM; PKC inhibitor). Cells were fed with fresh medium and inhibitors after 2 d. Cells incubated with different inhibitors were examined by phase microscopy and photographed.
Construction of Mutant Δ15 PECAM-1 Isoform
The tyrosine residue in exon 14 of the Δ15 PECAM-1 isoform was mutated to phenylalanine using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) as recommended by the supplier. The oligonucleotide primers containing the desired mutation were 5′-GCCACAGAGACGGTGTTCAGTGAGATCCGG-3′ (sense) and 5′-CCGGATCTCACTGAACACCGTCTCTGTGGC-3′ (antisense). The identity of the mutation was confirmed by DNA sequencing. The mutant Δ15 PECAM-1 isoform was expressed in MDCK cells, and clones expressing similar levels of PECAM-1 compared with wild-type Δ15 PECAM-1 were used for comparison as described above.
RESULTS
Expression of PECAM-1 Isoforms in MDCK Cells
To determine the relationship between PECAM-1 and cadherin-mediated cell–cell adhesion, we used MDCK cells. We chose this epithelial cell line because, like endothelial cells, they form adherens junctions but do not express PECAM-1. Furthermore, the components and organization of adherens junctions in these cells are very similar to those found in endothelial cells and are well characterized (Lampugnani et al., 1995; Staddon and Rubin, 1996). MDCK cells were stably transfected with expression vectors encoding the cDNA for the two predominant murine PECAM-1 isoforms expressed in vivo (Sheibani et al., 1999), Δ14&15 and Δ15, or the empty vector control. It should be noted that “full-length” PECAM-1 is not the most abundant form of PECAM-1 expressed in any tissue or endothelial cell line examined (Sheibani et al., 1999). Approximately 50 G418 resistant clones were isolated from each of the PECAM-1 isoform transfectants and 25 clones were isolated from the empty vector transfected cells. Clones were initially screened by Western analysis of cell lysates (our unpublished results), and several clones from each transfection expressing comparable levels of PECAM-1 were chosen for analysis.
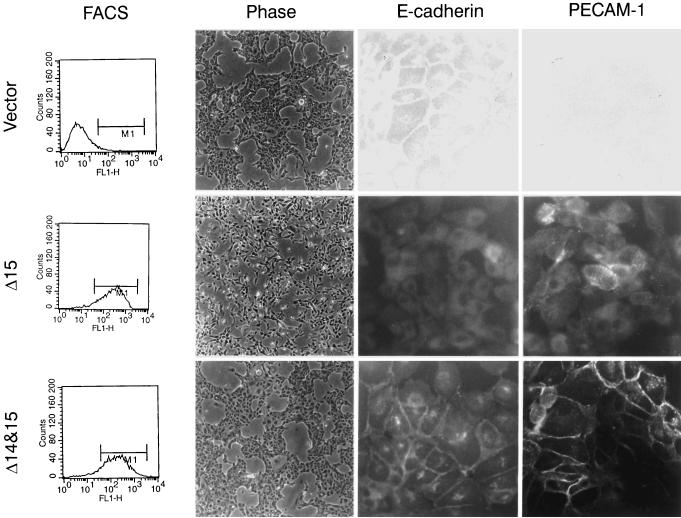
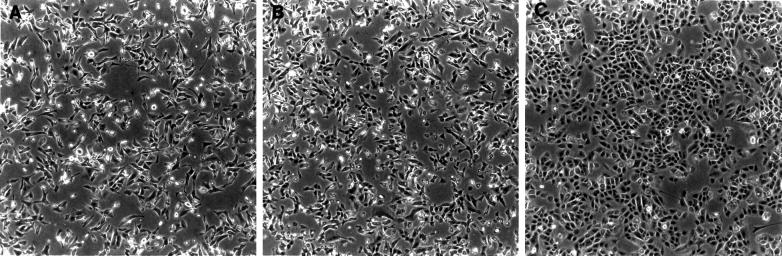
Expression of these two PECAM-1 isoforms had dramatically different effects on the morphology of MDCK cells. Cells transfected with the Δ15 PECAM-1 isoform (Figure 1, middle) lacked the closely packed cobblestone epithelial morphology observed in parental or vector control cells (Figure 1, top) and appeared more disorganized. A similar morphology has been observed in MDCK cells treated with hepatocyte growth factor/scatter factor (HGF/SF) (Royal and Park, 1995; Potempa and Ridley, 1998; Tanimura et al., 1998). This morphology is typical of cells that undergo an epithelial-to-mesenchymal transition and is referred to as a “dedifferentiated” phenotype. These cells exhibit a spindle-shaped fibroblastic morphology and lack contact inhibition as well as monolayer formation. In contrast, the cells transfected with the Δ14&15 PECAM-1 isoform (Figure 1, bottom) exhibited a morphology very similar to that of the parental or vector transfected cells. We (Sheibani and Frazier, 1998) and others (Yan et al., 1995) have demonstrated that the adhesive properties of PECAM-1 depend, to some extent, on the level of PECAM-1 expression. Thus, we have compared the characteristics of clones that express similar levels of PECAM-1, which are also comparable to the levels of PECAM-1 expressed in primary cultures of endothelial cells (Sheibani and Frazier, 1998). MDCK cells that expressed low levels (less than two logs of fluorescence) of Δ15 PECAM-1 did not exhibit the altered morphology or changes in E-cadherin expression (our unpublished results).
Figure 1.
Characteristics of MDCK cells expressing different PECAM-1 isoforms. MDCK cells were stably transfected with vector control, Δ15 PECAM-1, and Δ14&15 PECAM-1. Clones were isolated and characterized for expression levels of PECAM-1, their morphology, and E-cadherin and PECAM-1 localization. PECAM-1 expression levels were compared by FACScan analysis. The morphologies of cells are shown in phase micrographs (10× objective) of representative clones of transfected cells growing under normal conditions. Note that the Δ15 PECAM-1–expressing cells lack the closely packed epithelial morphology observed in vector control and Δ14&15 PECAM-1 transfected cells. The localization of E-cadherin and PECAM-1 was examined by indirect immunofluorescence (40× objective). Note the lack of E-cadherin and PECAM-1 junctional localization in Δ15 PECAM-1 transfected cell compared with Δ14&15 PECAM-1 transfected cells. The PECAM-1 transfected cells express similar levels of PECAM-1 on their cell surface.
Distribution of E-Cadherin and PECAM-1 Isoforms
The altered morphology or dedifferentiation of Δ15 PECAM-1 transfected MDCK cells suggested that alterations in the organization and/or expression of adherens junction components may have occurred. We examined the expression and localization of E-cadherin in PECAM-1 transfected MDCK cells by FACS and indirect immunofluorescence analysis, respectively. The FACS analysis demonstrated a dramatic decrease in the level of E-cadherin detected on the surface of MDCK cells transfected with the Δ15 isoform compared with vector control, Δ14&15 isoform, and parental cells (our unpublished results). Figure 1 also demonstrates the localization of E-cadherin and PECAM-1 in MDCK cells transfected with the two PECAM-1 isoforms or vector control. A representative clone of each transfectant is shown. The FACS analysis of these clones demonstrates similar levels of each PECAM-1 isoform on the cell surface (Figure 1, left). E-cadherin exhibited a typical junctional localization in Δ14&15 PECAM-1 or vector transfected cells. In contrast, the Δ15 isoform transfected cells lacked detectable junctional E-cadherin localization.
We next examined the localization of the PECAM-1 isoforms in the MDCK cell clones. The Δ14&15 isoform exhibited typical PECAM-1 localization at sites of cell–cell contacts, as has been demonstrated in endothelial cells isolated from a variety of tissues (Albelda et al., 1990; Sheibani et al., 1997). However, the Δ15 isoform exhibited a diffuse cell surface staining that did not localize to sites of cell–cell contact (Figure 1, right). Together, these results in MDCK cells show that PECAM-1 isoforms with alternatively spliced cytoplasmic domains, which differ in the presence or absence of exon 14, organize quite differently. This suggests that different PECAM-1 isoforms can differentially modulate the expression and/or organization of adherens junction components. Furthermore, the junctional localization of PECAM-1 may require the formation of adherens junctions, a characteristic of both epithelial and endothelial cells.
Effects of PECAM-1 Isoform Expression on Adherens Junction Components
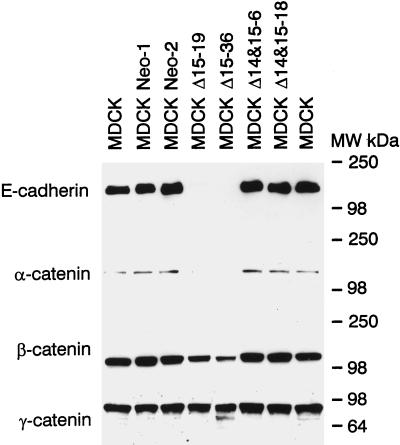
We next determined whether the expression of the adherens junction components E-cadherin and α, β-, and γ-catenin was affected in MDCK cells transfected with Δ15 or Δ14&15 PECAM-1 isoforms. Cell lysates were prepared from parental or two representative clones from vector or PECAM-1 transfected MDCK cells. Figure 2 demonstrates the levels of E-cadherin, α-catenin, β-catenin, and γ-catenin. MDCK cells transfected with the Δ14&15 isoform contained similar levels of these proteins compared with parental or vector transfected cells. In contrast, MDCK cells that expressed the Δ15 isoform exhibited a dramatic decrease in the levels of E-cadherin and α- and β-catenin. The level of γ-catenin was not significantly affected. This decrease in the expression of adherens junction proteins is consistent with the absence of close cell–cell contacts and the dedifferentiated phenotype of MDCK cells expressing the Δ15 isoform.
Figure 2.
Analysis of adherens junction components in MDCK cells. Cell lysates were prepared from MDCK parental cells and two representative clones of vector control and PECAM-1 isoform transfected cells under normal growth conditions. Equal amounts of protein (30 μg) were analyzed by SDS-PAGE and Western blotting with specific antibodies to E-cadherin, α-catenin, β-catenin, and γ-catenin. Note the dramatic decrease in production of E-cadherin, α-catenin, and β-catenin but not γ-catenin in Δ15 PECAM-1–expressing cells. These experiments were repeated three times with identical results.
Analysis of Intermediate Filaments in PECAM-1 Transfected MDCK Cells
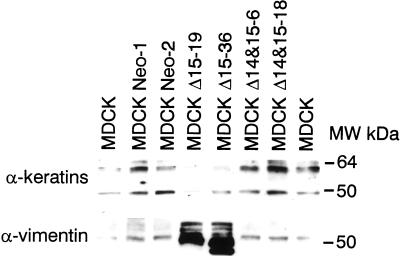
Epithelial cells generally produce intermediate filaments of the cytokeratin type, whereas mesenchymal cells predominantly express vimentin. MDCK cells can express both vimentin and keratin intermediate filaments depending on their differentiation state (Vitranen et al., 1981). We next examined the expression of vimentin and cytokeratins in MDCK cells transfected with the two PECAM-1 isoforms. Figure 3 shows the Western blot analysis of intermediate filament proteins in extracts prepared from these cells. The parental, vector control, and Δ14&15 PECAM-1 transfected MDCK cells expressed a panel of cytokeratins (40–60 kDa), consistent with the epithelial morphology of these cells, but very little or no vimentin. However, this pattern was switched in the Δ15 PECAM-1 transfected MDCK cells, i.e., they expressed very high levels of vimentin and reduced levels of cytokeratins. This is consistent with the mesenchymal phenotype of Δ15 PECAM-1–expressing cells. Such changes have been demonstrated previously in dedifferentiated MDCK cells (Schramek et al., 1997b).
Figure 3.
Loss of cytokeratin expression in dedifferentiated MDCK cells. The levels of cytokeratins and vimentin were examined by SDS-PAGE and Western blotting with specific antibodies as described for Figure 2. Note that the production of cytokeratins is decreased, whereas that of vimentin is increased in Δ15 PECAM-1–expressing MDCK cells.
PECAM-1 Expression Activates MAPK/ERKs
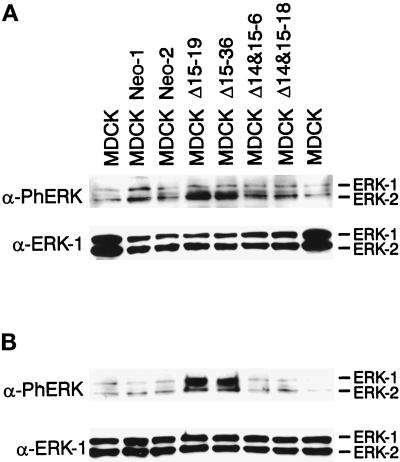
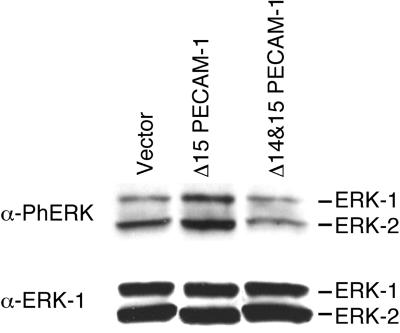
It has been demonstrated previously that the activation of MAPK/ERKs and PI-3 kinase is required for adherens junction disassembly and is essential for the motile response of MDCK cells to HGF/SF (Schramek et al., 1997b; Potempa and Ridley, 1998; Tanimura et al., 1998). Expression of a constitutively active mutant of MEK-1 also induces epithelial dedifferentiation of MDCK cells (Schramek et al., 1997a). Figure 4 shows the enhanced and sustained activation of MAPK/ERKs in Δ15 PECAM-1 transfected cells. Figure 4A demonstrates the steady-state levels of activated MAPK/ERKs in parental, vector, and PECAM-1 transfected cells. Only the MDCK cells transfected with the Δ15 isoform exhibited high levels of active (phosphorylated) MAPK/ERKs, as demonstrated by specific staining with antibody to phospho-MAPK/ERKs. Figure 4B shows the levels of active MAPK/ERKs after serum stimulation. Again, the cells transfected with the Δ15 isoform expressed high levels of active MAPK/ERKs compared with parental, vector, and Δ14&15 isoform transfected MDCK cells. The levels of ERK proteins were not affected under these conditions (Figure 4, A and B, lower panels). Thus, the ability of the Δ15 PECAM-1 isoform to modulate adherens junction assembly correlates with the activation of MAPK/ERKs.
Figure 4.
Expression of the Δ15 PECAM-1 isoform in MDCK cells results in the activation of MAPK/ERKs. Cell extracts were prepared from parental or two representative clones of vector or PECAM-1 transfected cells grown under normal conditions (A) or serum starved for 48 h followed by 10 min of serum stimulation (B). Equal amounts of protein (30 μg) were analyzed by SDS-PAGE and Western blotting with either antiphospho-MAPK/ERKs (upper panels) or anti-ERK-1 (lower panels). Note the increased levels of constitutive (A) and serum-stimulated (B) phosphorylated active MAPK/ERKs in the Δ15 PECAM-1–expressing cells. These experiments were repeated three times with identical results.
We next determined whether sustained activation of MAPK/ERKs is necessary for the dedifferentiated phenotype of MDCK cells expressing the Δ15 isoform. MDCK cells expressing the Δ15 isoform were incubated with various inhibitors of signal-transducing kinases, and the effects on the morphology of cells was assessed. Figure 5 demonstrates the morphology of cells after incubation with vehicle (A), 50 nM wortmannin (a PI-3 kinase inhibitor) (B), and 50 μM PD98059 (a MEK inhibitor) (C) for 4 d. Inhibition of MAPK/ERKs activity by PD98059 resulted in the reestablishment of an epithelial morphology in these cells (Figure 5, compare A and C). The wortmannin effects were minimal. Similar results were observed in the presence of LY294002, another inhibitor of PI-3 kinase (our unpublished results). However, prolonged incubation with LY294002 resulted in extensive cell death. SB203580 (a p38 inhibitor) and GF109203x (a PKC inhibitor) had no effect on the morphology of dedifferentiated cells. None of these inhibitors had any effect on the morphology of the vector or the Δ14&15 PECAM-1 transfected MDCK cells, nor did they affect the expression of PECAM-1 and/or components of the adherens junctions in these cells (our unpublished results). The inhibitors were not cytotoxic (except LY294002) at the concentrations used in this study. Thus, sustained activation of MAPK/ERKs is essential for the dedifferentiated phenotype of MDCK cells induced by the expression of Δ15 PECAM-1.
Figure 5.
Inhibition of MAPK/ERKs in Δ15 PECAM-1–expressing cells restores the closely packed epithelial morphology. MDCK cells expressing the Δ15 PECAM-1 isoform were incubated with the vehicle (A), wortmannin (B), or PD98059 (C) in growth medium. The morphology of the cells was monitored microscopically and photographed (10× objective). Note that the closely packed epithelial morphology was observed only when the cells were incubated with PD98059. These experiments were repeated twice with two different batches of the same inhibitors with identical results.
To demonstrate that activation of MAPK/ERKs by expression of the Δ15 PECAM-1 isoform is not due to long-term selection of stable clones in the presence of G418, we assessed the level of active phosphorylated MAPK/ERKs in MDCK cells transiently transfected with vector, Δ15 PECAM-1, or Δ14&15 PECAM-1. Forty-eight hours after transfection, MAPK/ERKs phosphorylation levels were assessed by Western blotting (Figure 6). Expression of the Δ15 PECAM-1 isoform, but not the vector or Δ14&15 PECAM-1, resulted in an enhanced level of phosphorylated (activated) MAPK/ERKs (Figure 6, top), whereas levels of total ERK proteins remained the same. Therefore, expression of the Δ15 PECAM-1 isoform, but not Δ14&15 PECAM-1, in MDCK cells correlates with the activation of MAPK/ERKs. It was difficult to see an effect on the morphology of Δ15 PECAM-1 transfected cells in these experiments because of the short-term and nonuniform nature of transient expression.
Figure 6.
Transient transfection of MDCK cells with the Δ15 PECAM-1 isoform results in activation of MAPK/ERKs. MDCK cells were transfected with empty vector, Δ15 PECAM-1, or Δ14&15 PECAM-1, and 48 h later the cells were lysed and equal amounts of protein (30 μg) were analyzed by SDS-PAGE and Western blotting with either antiphospho-MAPK/ERKs (upper panels) or anti-ERK-1 (lower panels). Note the increased levels of phosphorylated active MAPK/ERKs in the Δ15 PECAM-1 transfected cells. These experiments were repeated three times with two different plasmid DNA preparations with identical results.
Inhibition of MAPK/ERKs Restores E-Cadherin Expression and Junctional Localization of PECAM-1 in Dedifferentiated Cells
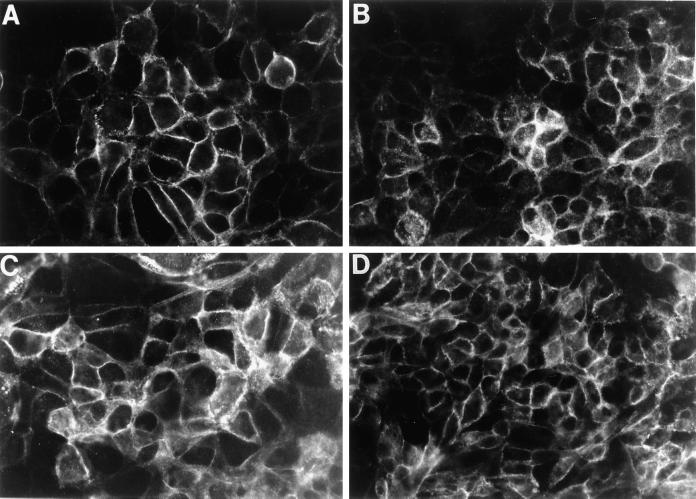
The data presented thus far suggest that sustained activation of MAPK/ERKs is necessary to maintain the dedifferentiated phenotype of MDCK cells expressing the Δ15 PECAM-1 isoform (Figure 5). Incubation of these cells with the specific inhibitor of MAPK/ERKs (PD98059) resulted in reestablishment of a closely packed epithelial morphology. We next asked whether incubation of these cells with PD98059 also restores E-cadherin expression. PD98059 does restore E-cadherin expression, which localizes to sites of cell–cell contact (Figure 7, A and B). Interestingly, upon restoration of the E-cadherin–containing cell–cell contacts, Δ15 PECAM-1 exhibited junctional localization in these cells (Figure 7, C and D). The E-cadherin and PECAM-1 expression patterns were similar to those shown for Δ14&15 PECAM-1–expressing MDCK cells in Figure 1. Therefore, these results indicate that junctional localization of PECAM-1 is dependent on the expression of E-cadherin and the formation of adherens junctions. This is further supported by our previous observation that the expression of PECAM-1 in thrombospondin-transfected bEND cells, which lack endogenous PECAM-1 and are unable to form adherens junctions, fails to localize to sites of cell–cell contact regardless of the isoform expressed (Sheibani et al., 1997). This is consistent with alterations in the expression and localization of adherens junction components we have observed in these cells (our unpublished results).
Figure 7.
Inhibition of MAPK/ERKs in Δ15 PECAM-1–expressing cells restores E-cadherin expression and junctional localization of PECAM-1. MDCK cells (2 × 104) expressing the Δ15 PECAM-1 isoform were plated on glass coverslips and incubated with medium containing PD98059 (50 μM) for 4 d. Cells were fixed and stained with antibodies to E-cadherin (A and B) or PECAM-1 (C and D) as described in MATERIALS AND METHODS. The localization of E-cadherin and PECAM-1 was examined by indirect immunofluorescence (40× objective). Two different clones of MDCK Δ15 PECAM-1 were used (A and C, clone 19; B and D, clone 36). Note the reexpression and localization of E-cadherin at sites of cell–cell contact (A and B). Δ15 PECAM-1 isoform now also exhibits a junctional localization. Cells incubated with the vehicle alone for the duration of the experiments showed no effect on morphology and/or the expression of E-cadherin and PECAM-1 (our unpublished results).
Mutation of a Single Amino Acid in Exon 14 of Δ15 PECAM-1 Blocks the Dedifferentiation of MDCK Cells
Our data suggest that the presence of exon 14 in Δ15 PECAM-1 is responsible for the activation of MAPK/ERKs and the dedifferentiation of MDCK cells. Exon 14 has been proposed to be an important modulator of PECAM-1 adhesive properties. Famiglietti et al. (1997) demonstrated that lack of exon 14, or mutation of the tyrosine residue in exon 14, of PECAM-1 is sufficient to promote homotypic interactions in L-cells. The tyrosine in exon 14 forms an immunoreceptor tyrosine-based inhibitory motif (ITIM) (Newman, 1999) that acts as a docking site for SH2-containing phosphatases and perhaps other signaling molecules. To determine if the presence of tyrosine 686 in exon 14 is essential for the ability of Δ15 PECAM-1 to result in dedifferentiation of MDCK cells, we mutated the tyrosine residue to a phenylalanine (Y→F). The mutant Y→F Δ15 PECAM-1 isoform was expressed in MDCK cells, and multiple clones expressing levels of PECAM-1 similar to those of Δ15 or Δ14&15 PECAM-1 transfected cells were analyzed for morphological and phenotypic changes as described above. The MDCK cells that expressed the Y→F Δ15 PECAM-1 isoform behaved similarly to the MDCK cells that expressed the Δ14&15 PECAM-1 isoform (our unpublished results). Therefore, the presence of the tyrosine residue in exon 14 appears to be essential for the ability of Δ15 PECAM-1 to activate MAPK/ERKs and cause dedifferentiation of MDCK cells concomitant with the loss of adherens junctions.
DISCUSSION
PECAM-1 mRNA undergoes alternative splicing to generate eight different isoforms that differ only in their cytoplasmic domains (Yan et al., 1995; Sheibani et al., 1999). We have recently demonstrated that multiple isoforms of PECAM-1 are expressed in vascular beds of different tissues in a developmentally regulated pattern (Sheibani et al., 1999), suggesting that different functional properties of PECAM-1 provided by different cytoplasmic domain isoforms may be required during vascular development. Expression of these isoforms in L-cells (a nonendothelial cell line) suggested that exon 14 is a major regulator of PECAM-1 adhesive function because PECAM-1 isoforms that contained exon 14 participated in “heterotypic” interactions, whereas those that lacked exon 14 participated in “homotypic” interactions (Yan et al., 1995), regardless of the presence or absence of other exons. In the present studies, we have used epithelial MDCK cells, which, like endothelial cells, form cadherin-mediated adherens junctions. Thus, MDCK cells may be a more relevant cell model system in which to study these interactions than L-cells, which normally are incapable of forming cadherin-mediated adherens junctions (Nagafuchi et al., 1994; Wang and Rose, 1997). PECAM-1 isoforms with and without exon 14 were expressed in MDCK cells to evaluate the adhesive properties of these PECAM-1 isoforms and determine their effects on cadherin-mediated cell junctions.
We chose to express Δ15 and Δ14&15 PECAM-1 isoforms rather than full-length and Δ14 PECAM-1 because these two isoforms lacking exon 15 are the most predominant isoforms in mouse tissues as well as in cultured endothelial cells (Piedboeuf et al., 1998; Sheibani et al., 1999). Expression of the Δ14&15 isoform in MDCK cells had no effect on cadherin-mediated cell–cell interactions, and PECAM-1 exhibited a junctional localization seen in many endothelial cells in culture (Albelda et al., 1990; Sheibani et al., 1997). In contrast, expression of the Δ15 isoform in MDCK cells had a dramatic effect on their morphology and phenotype. The cells lost the closely packed epithelial morphology observed in vector or Δ14&15 PECAM-1 transfected cells and had a more elongated fibroblastic morphology without any close cell–cell apposition. Indeed, these cells exhibited a dedifferentiated or mesenchymal phenotype. This same sort of epithelial-to-mesenchymal transition has been observed when MDCK cells are incubated with HGF/SF (Royal and Park, 1995; Potempa and Ridley, 1998; Tanimura et al., 1998). The Δ15 PECAM-1 transfected cells lost expression of cytokeratins and turned on expression of vimentin, consistent with a transition from an epithelial to a mesenchymal phenotype. Furthermore, FACS and immunofluorescence staining of these cells demonstrated the absence of cell surface and junctional E-cadherin. Further analysis of these cells indicated a dramatic decrease in the expression of E-cadherin and associated catenins in Δ15 isoform transfected cells compared with Δ14&15 isoform or vector control cells (Figure 2). Despite high levels of cell surface expression (Figure 1, middle), the Δ15 isoform could not promote cell–cell adhesion in MDCK cells and failed to demonstrate junctional localization. These results indicate that PECAM-1 isoforms, which differ only in a single exon (exon 14) encoding 19 amino acids, can differentially affect the assembly of adherens junctions. To our knowledge, this is the first report indicating a role for PECAM-1 in the modulation of cadherin-mediated cell–cell interactions.
When MDCK cells are incubated with HGF/SF, they lose the adherens junction proteins E-cadherin and β-catenin from intercellular junctions. This is dependent on sustained activation of MAPK/ERKs and possibly PI-3 kinase (Schramek et al., 1997b; Khwaja et al., 1998). The enhanced permeability of endothelial cell monolayers in response to vascular endothelial growth factor, which occurs through disorganization of junctions (loss of VE-cadherin and occludin), is also dependent on the activation of MAPK/ERKs (Kevil et al., 1998). MDCK cells that express the Δ15 PECAM-1 isoform exhibited high levels of phosphorylated MAPK/ERKs under both normal growth conditions (basal) or when cells were stimulated with serum. Incubation of these cells with PD98059 (a MEK inhibitor), which prevents phosphorylation and activation of MAPK/ERKs in vitro and in vivo (Alessi et al., 1995), resulted in the reestablishment of an epithelial morphology, as seen previously in HGF/SF dedifferentiated MDCK cells (Royal and Park, 1995; Potempa and Ridley, 1998; Tanimura et al., 1998). Incubation of Δ14&15 PECAM-1 or vector transfected MDCK cells with PD98059 had no effect on the phenotype and/or morphology of these cells. The PI-3 kinase inhibitors (wortmannin and LY294002) were not effective in reestablishing the closely packed cell colonies in Δ15 PECAM-1–expressing MDCK cells. Inhibitors of p38 MAPK (SB203580) or PKC (GF109203x) also had no effect.
We demonstrate that expression of the Δ15 PECAM-1 isoform in MDCK cells results in activation of MAPK/ERKs whose sustained activity is required for the dedifferentiated phenotype of MDCK cells and the down-regulation of E-cadherin expression. The Δ14&15 PECAM-1 isoform, which fails to activate MAPK/ERKs, had no effect on cadherin-mediated cell–cell interactions. However, when MAPK/ERKs activity was inhibited by PD98059, even the Δ15 PECAM-1 isoform localized to sites of cell–cell contact. This result suggests a rather more passive role for PECAM-1 organization at sites of cell–cell contact. That is, PECAM-1 will localize at cell–cell junctions if they are formed. These results are consistent with a recent report that all PECAM-1 isoforms localize to sites of cell–cell contact regardless of their cytoplasmic domain when expressed in REN (“endothelium-like”) cells that form close cell–cell contacts (Sun et al., 2000). However, the integrity of adherens junctions and their components were not addressed in this study, nor was the signaling role of the PECAM-1 cytoplasmic domain. Thus, the functional roles of the PECAM-1 cytoplasmic domains in modulation of adherens junctions and junctional localization of PECAM-1 isoforms were overlooked.
How does PECAM-1 activate MAPK/ERKs? PECAM-1 has recently been demonstrated to become tyrosine phosphorylated in its cytoplasmic domain upon treatment of endothelial cells with various stimuli (reviewed by Newman, 1999). Adhesion of endothelial cells to fibronectin-coated surfaces rapidly stimulates PECAM-1 tyrosine phosphorylation (Lu et al., 1996). Tyrosine phosphorylation of PECAM-1 results in its association with SHP-2 (Jackson et al., 1997; Masuda et al., 1997), a ubiquitously expressed tyrosine phosphatase with two tandem SH2 domains. These SH2 domains not only target SHP-2 to tyrosine-phosphorylated proteins but also regulate SHP-2 phosphatase activity (Huyer and Alexander, 1999). SHP-2 interacts with the phosphorylated tyrosine residues in exons 13 and 14 of PECAM-1, which form an ITIM, resulting in SHP-2 activation (Huyer and Alexander, 1999; Newman, 1999). The cytoplasmic domains of PECAM-1 isoforms that lack exon 14 lack the ITIM, and these fail to associate with SHP-2 even though other tyrosines are phosphorylated (our unpublished results). SHP-2 is a major regulator of cell motility, and its localization to focal adhesions allows fine tuning of integrin-mediated cell adhesion signals to stimulate or inhibit cell migration by modulating phosphorylation of focal adhesion kinase (Huyer and Alexander, 1999). The ability of cells to migrate is linked to the MAPK/ERKs pathway (Klemke et al., 1997). Focal adhesion kinase can activate MAPK/ERKs through its interaction with Shc/Grb2/SOS or p130cas/crk (Guan, 1997). In addition, SHP-2 also can interact directly with Grb2/SOS and activate MAPK/ERKs (Huyer and Alexander, 1999). The ability of the Δ15 PECAM-1 isoform to bind SHP-2 and its proximity to focal adhesions may enhance focal adhesion turnover and stimulate cell migration (Manes et al., 1999). The cytoplasmic domain of PECAM-1 isoforms that contain exon 14 can also interact directly with Shc/Grb2 upon tyrosine phosphorylation and thus activate MAPK/ERKs (our unpublished results). This is consistent with the inability of the mutant Δ15 PECAM-1 (Y→F Δ15) to activate MAPK/ERKs in MDCK cells. Therefore, PECAM-1 isoforms containing exon 14 can, either directly or indirectly, activate the MAPK/ERKs pathway.
Activation and inhibition of MAPK/ERKs play a central role in the control of angiogenesis, a cell migration–dependent process (D'Angelo et al., 1995; Eliceiri et al., 1998). The down-regulation of cadherins in epithelial and endothelial cell tumors is consistent with the invasive and migratory phenotype of these cells (Dejana et al., 1995). The ability of PECAM-1 isoforms to differentially modulate cadherin-mediated cell adhesion may play an important role during angiogenesis. Isoforms that contain exon 14 may function in early stages of angiogenesis when cell motility is necessary and strong cell–cell interactions are undesirable, whereas later in development of the vasculature these PECAM-1 isoforms would be replaced with those that lack exon 14 to promote and perhaps stabilize cell–cell junctions. Indeed, this pattern of PECAM-1 isoform switching is observed during development of the kidney vasculature (Sheibani et al., 1999). We have recently shown that in the developing kidney, PECAM-1 isoform(s) that contain exon 14 are expressed early in vascular development, when there is a high degree of cell migration and low levels of stable cell–cell adhesion. These isoforms are later replaced by PECAM-1 isoform(s) that lack exon 14, thus favoring formation of strong cell–cell interactions in the maturing blood vessels (Sheibani et al., 1999). Therefore, PECAM-1 emerges not as a mechanical component of the adhesion mechanism but as a signaling component that can regulate an important adhesive and junctional apparatus. This raises the interesting possibility that PECAM-1 isoform switching may play an important role during developmental and reparative angiogenesis in a number of situations.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants CA 65872 (to W.A.F.) and AR 45599 (to N.S.). C.M.S. is supported by a grant from the American Heart Association
Abbreviations used:
- ERK
extracellular regulated kinase
- HGF/SF
hepatocyte growth factor/scatter factor
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- MDCK
Madin-Darby canine kidney
- PECAM-1
platelet endothelial cell adhesion molecule-1
- TBS
Tris-buffered saline
REFERENCES
- Albelda SM, Oliver PD, Romer LH, Buck CA. EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990;110:1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi D, Cuenda A, Cohen P, Dudley D, Saltiel A. PD98059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Struman I, Martial J, Weiner RI. Activation of mitogen-activated protein kinases by vascular endothelial growth factor and basic fibroblast growth factor in capillary endothelial cells is inhibited by the antiangiogenic factor 16-kDa N-terminal fragment of prolactin. Proc Natl Acad Sci USA. 1995;92:6374–6378. doi: 10.1073/pnas.92.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998;160:395–402. [PubMed] [Google Scholar]
- DeLisser HM, Yan HC, Newman PJ, Muller WA, Buck CA, Albelda SM. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J Biol Chem. 1993;268:16037–16046. [PubMed] [Google Scholar]
- DeLisser HM, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J. 1995;9:910–918. [PubMed] [Google Scholar]
- Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin αvβ3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti J, Sun J, DeLisser HM, Albelda SM. Tyrosine residue in exon 14 of the cytoplasmic domain of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) regulates ligand binding specificity. J Cell Biol. 1997;138:1425–1435. doi: 10.1083/jcb.138.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J-L. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- Huyer G, Alexander DR. Immune signaling: SHP-2 docks at multiple ports. Curr Biol. 1999;9:R129–R132. doi: 10.1016/s0960-9822(99)80080-3. [DOI] [PubMed] [Google Scholar]
- Jackson DE, Kupcho KR, Newman PJ. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J Biol Chem. 1997;272:24868–24875. doi: 10.1074/jbc.272.40.24868. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem. 1998;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Lehmann K, Marte BM, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junction: differential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TL, Yan L, Madri J. Integrin engagement mediates tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1. Proc Natl Acad Sci USA. 1996;93:11808–11813. doi: 10.1073/pnas.93.21.11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S, Mira E, Gomez-Mouton C, Zhao ZJ, LaCalle RA, Martinez AC. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol Cell Biol. 1999;19:3125–3135. doi: 10.1128/mcb.19.4.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 1997;408:331–336. doi: 10.1016/s0014-5793(97)00457-2. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-α-catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ. Switched at birth: a new family for PECAM-1. J Clin Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedboeuf B, Gamache M, Frenette J, Horowitz S, Baldwin HS, Petrov P. Increased endothelial cell expression of platelet-endothelial cell adhesion molecule-1 during hyperoxic lung injury. Am J Respir Cell Mol Biol. 1998;19:543–553. doi: 10.1165/ajrcmb.19.4.2349. [DOI] [PubMed] [Google Scholar]
- Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal I, Park M. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- Schramek H, Feifel E, Healy E, Pollack V. Constitutively active mutant of the mitogen-activated protein kinase kinase MEK1 induces epithelial dedifferentiation and growth inhibition in Madin-Darby canine kidney–C7 cells. J Biol Chem. 1997a;272:11426–11433. doi: 10.1074/jbc.272.17.11426. [DOI] [PubMed] [Google Scholar]
- Schramek H, Wilflingseder D, Pollack V, Freudinger R, Mildenberger S, Gekle M. Ochratoxin A-induced stimulation of extracellular signal-regulated kinases 1/2 is associated with Madin-Darby canine kidney-C7 cell dedifferentiation. J Pharmacol Exp Ther. 1997b;283:1460–1468. [PubMed] [Google Scholar]
- Sheibani N, Frazier WA. Down-regulation of platelet endothelial cell adhesion molecule-1 results in thrombospondin-1 expression and concerted regulation of endothelial cell phenotype. Mol Biol Cell. 1998;9:701–713. doi: 10.1091/mbc.9.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N, Frazier WA. Thrombospondins, PECAM-1, and regulation of angiogenesis. Histol Histopathol. 1999;14:285–294. doi: 10.14670/HH-14.285. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Newman PJ, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol Biol Cell. 1997;8:1329–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N, Sorenson CM, Frazier WA. Tissue specific expression of alternatively spliced murine PECAM-1 isoforms. Dev Dyn. 1999;214:44–54. doi: 10.1002/(SICI)1097-0177(199901)214:1<44::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Staddon JM, Rubin LL. Cell adhesion, cell junctions and the blood-brain barrier. Curr Opin Neurobiol. 1996;6:622–627. doi: 10.1016/s0959-4388(96)80094-8. [DOI] [PubMed] [Google Scholar]
- Sun J, Paddock C, Shubert J, Zhang HB, Amin K, Newman PJ, Albelda SM. Contributions of the extracellular and cytoplasmic domains of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell-cell localization. J Cell Sci. 2000;113:1459–1469. doi: 10.1242/jcs.113.8.1459. [DOI] [PubMed] [Google Scholar]
- Sun J, Williams J, Yan HC, Amin KM, Albelda SM, DeLisser HM. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- Tanimura S, Chatani Y, Hoshino R, Sato M, Watanabe S-I, Kataoka T, Nakamura T, Kohno M. Activation of the 41/43 kDa mitogen-activated protein kinase signaling pathway is required for hepatocyte growth factor-induced cell scattering. Oncogene. 1998;17:57–65. doi: 10.1038/sj.onc.1201905. [DOI] [PubMed] [Google Scholar]
- Virtanen I, Lehto VP, Lehtonen E, Vartio T, Stenman S, Kurki P, Wager O, Small JV, Dahl D, Badley RA. Expression of intermediate filaments in cultured cells. J Cell Sci. 1981;50:45–63. doi: 10.1242/jcs.50.1.45. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rose B. An inhibition of gap-junctional communication by cadherins. J Cell Sci. 1997;110:301–309. doi: 10.1242/jcs.110.3.301. [DOI] [PubMed] [Google Scholar]
- Yan HC, Baldwin HS, Sun J, Buck CA, Albelda SA, DeLisser HM. Alternative splicing of a specific cytoplasmic exon alters the binding characteristics of murine platelet/endothelial cell adhesion molecule-1 (PECAM-1) J Biol Chem. 1995;270:23672–23680. doi: 10.1074/jbc.270.40.23672. [DOI] [PubMed] [Google Scholar]