Abstract
BACKGROUND
Prostate-specific antigen (PSA) levels between 4.0 to 10.0 ng/ml have poor specificity in prostate cancer screening, leading to unnecessary biopsies.
OBJECTIVE
To determine whether the free-to-total PSA ratio (F/T PSA) improved the diagnostic accuracy of these nonspecific PSA levels.
MEASUREMENTS AND MAIN RESULTS
Medline was searched from 1986 to 1997. Additional studies were identified from article bibliographies and by searching urology journals. Two investigators independently identified English-language studies providing F/T PSA ratio test-operating characteristics data on ≥10 cancer patients with PSA values between 2.0 and 10.0 ng/ml. Twenty-one of 90 retrieved studies met selection criteria. Two investigators independently extracted data on methodology and diagnostic performance. Investigator-selected cut points for the optimal F/T PSA ratio had a median likelihood ratio of 1.76 (interquartile range, 1.40 to 2.11) for a positive test and 0.27 (0.20 to 0.40) for a negative test. Assuming a 25% pretest probability of cancer, the posttest probabilities were 37% following a positive test and 8% following a negative test. The summary receiver operating characteristic curve showed that maintaining test sensitivity above 90% was associated with false positive rates of 60% to 90%. Methodologic problems limited the validity and generalizability of the literature.
CONCLUSIONS
A negative test reduced the posttest probability of cancer to approximately 10%. However, patients may find that this probability is not low enough to avoid undergoing prostate biopsy. The optimal F/T PSA ratio cut point and precise estimates for test specificity still need to be determined.
Keywords: prostatic neoplasm, prostate-specific antigen, diagnostic accuracy, free PSA
The prostate-specific antigen (PSA) assay is currently considered the most useful tumor marker for detecting prostate cancer. Both the American Cancer Society and the American Urologic Association recommend annual cancer screening with both PSA and digital rectal examinations.1,2 However, not all observers find the data on PSA persuasive. The National Cancer Institute, the American College of Physicians, and the U.S. Preventive Services Task Force have all refused to recommend routine screening because there is no conclusive evidence that PSA testing reduces disease-specific morbidity or mortality.3–5 Another major concern is that PSA lacks specificity and screening leads to many unnecessary prostate biopsies, particularly for PSA values between 4.0 and 10.0 ng/ml. In this range, Catalona et al. found that the positive predictive value for PSA was only about 26%, although nearly 80% of the cancers were organ confined.6
This diagnostic “gray zone” (PSA values between 4.0 to 10.0 ng/ml) has led to different strategies to improve the specificity of PSA, including measuring PSA velocity (rate of change over time),7 PSA density (PSA per unit of prostate volume),8 and age-specific reference ranges.9 However, none of these strategies have been widely accepted or proven effective in prospective trials. Recently, investigators have begun measuring the ratio of free-to-total PSA. Serum PSA exists in a free form as well as complexed to a number of protease inhibitors.10–13 Most PSA is bound to alpha-1-antichymotripsin (ACT),11,14 and assays for total PSA measure both this bound fraction and free PSA. Empirical evidence has shown that cancer patients have a higher percentage of PSA bound to ACT than normal controls.11,15,16 While the PSA-ACT complex can be measured directly, these assays have very high intra-assay and inter-assay coefficients of variation and are considered unreliable.12,17,18 Most investigators recommend measuring free and total PSA and calculating the free-to-total ratio.13,16,18
Initially, studies using free PSA assays focused on PSA ranges between 4.0 to 10.0 ng/ml because men with levels ≥10.0 ng/ml are at high risk for cancer and men with levels below 4.0 ng/ml—the upper limit of normal—would not routinely be biopsied. Subsequently, however, a 7.9% prevalence of prostate cancer was reported in men with PSA levels between 2.9 to 4.0 ng/ml,19 and men with levels between 2.0 to 3.0 ng/ml were found to have an increased risk of developing cancer compared with men with levels less than 1.0 ng/ml.20 Consequently, some investigators now recommend measuring free PSA when total PSA levels are between 2.0 and 10.0 ng/ml.21,22
In 1998, the U.S. Food and Drug Administration approved the Hybritech Tandem free PSA assays.23 Using the free-to-total PSA ratio as a criterion for prostate biopsy could substantially change prostate cancer screening practices. We conducted a meta-analysis to evaluate the methodologic quality of the free PSA literature and to determine the diagnostic performance of the free-to-total PSA ratio for detecting prostate cancer when PSA levels are between 2.0 and 10.0 ng/ml.
METHODS
Literature Search and Data Abstraction
Medline was searched from January 1986 through July 1997, combining the MeSH headings “prostate-specific antigen” and “prostatic neoplasm” and then linking them with the MeSH heading “alpha-1-antichymotrypsin” or with the text words “free” or “gamma-seminoprotein.” Articles were also identified from bibliographies of review articles and retrieved articles, and the tables of contents from the January 1994 through December 1997 issues of the journals UrologyJournal of Urology.
Article selection criteria included English-language studies using free PSA assays and providing data on sensitivity and specificity. Studies had to evaluate at least 10 prostate cancer patients and 10 histologically confirmed noncancer controls. Studies using only gamma-seminoprotein assays or the ratio of alpha-1-antichymotrypsin to total PSA (neither of which directly correlate with the free-to-total PSA ratio) were excluded as were studies that did not provide diagnostic performance data. Two investigators reviewed all titles and abstracts, retrieving all articles that potentially met the selection criteria. Studies reported only in abstract form were retrieved but not included in the analysis; however, medline author searches were performed to see if the results were subsequently published. We retrieved one study first identified only as an abstract.24 Retrieved articles were abstracted for study design features and data on test operating characteristics for the free-to-total PSA ratio. Reviewers examined articles independently; if there were any disagreements on data abstraction, the reviewers tried to reach consensus or used a third reviewer to referee.
Quality Assessment
All studies meeting selection criteria were included in the meta-analysis. However, we also used methodologic quality criteria based on published guidelines to evaluate study validity and generalizability.25–30 Study validity was assessed by whether a study selected an appropriate reference (gold) standard, appropriately performed the diagnostic test, independently interpreted test results, and avoided work-up bias. Generalizability was assessed by the spectrum of study patients and the technical details of the test. Precision of results was based on the number of subjects with cancer. We described the number of studies meeting methodologic criteria and used these classifications for sensitivity analyses.
The most appropriate reference standard was considered to be either radical prostatectomy or multiple systematic transrectal prostate needle biopsies with long-term clinical follow-up for men with negative biopsies. Studies using either transurethral resections of the prostate or biopsies without long-term clinical follow-up have a moderate risk of bias because sampling errors can affect diagnostic test performance. Appropriately performing the free PSA assay was based on specimen handling, including storage temperature and duration, and the molar response of the immunoassay. Specimens retained beyond 24 hours should be frozen, and free PSA remains significantly more stable when frozen at −70°C than at −20°C.31,32 Equimolar antibodies—directed at two distinct epitopes that are not blocked by ACT binding—most accurately determine the free-to-total PSA ratio.13,17,33
Independent interpretation of test results (blinding) is defined by the absence of test-review or diagnostic-review bias.25 Test-review bias occurs when the diagnostic test interpretation is influenced by the results of the reference standard test. Diagnostic review bias occurs when the results of the diagnostic test affect the interpretation of the reference standard test. We looked for explicit statements that the study was blinded. Work-up bias (verification bias) was considered possible when the reference standard was not uniformly applied to all patients undergoing the diagnostic test, especially if patients with positive (or negative results) were preferentially referred for further testing.25,29,30 Work-up bias was minimized when the reference standard was uniformly applied to consecutive or randomly selected subjects.
Generalizability of study results depends upon the clinical spectrum of study subjects.25 The important patient characteristics for prostate cancer testing include age, race, digital rectal examination findings, urinary symptoms, presence of benign prostatic hyperplasia, and cancer stage.34 Additionally, investigators should explicitly describe study eligibility criteria. Finally, the generalizability of free PSA immunoassays can be further increased by calibrating against a purified standard of PSA-ACT and free PSA, thus minimizing interassay variability.18,35,36
Diagnostic Performance
Diagnostic performance was assessed according to standard epidemiologic definitions.37 Sensitivity is the proportion of cancer cases with abnormal free-to-total PSA ratios. Specificity is the proportion of noncancer controls with normal free-to-total PSA ratios. We determined the likelihood ratio, which compares the proportion of people with and without the target disorder within a stratum of diagnostic test results. For each study where investigators selected a single best free-to-total PSA ratio cut point, we computed the likelihood ratio for positive and negative tests, the associated 95% confidence intervals, and the nonparametric trapezoidal area under the receiver operating characteristic curve.38 These diagnostic performance data were described by median values and interquartile ranges.
We used the median likelihood ratios to evaluate the relative effects of positive and negative test results on probability revision with Bayes' theorem37:

For PSA levels between 4.0 and 10.0 ng/ml, the probability of cancer is approximately 25%,6,39 which becomes the pretest probability for the above equation. Probability is converted to odds with the equation:
![]()
Using Bayes' theorem, we plotted the investigator-selected cut point for each study against the posttest probabilities for both positive and negative results. Regression lines, fitted with Statistica (Statsoft, Inc., Tulsa, OK), were not extrapolated beyond the range of empiric data.
Summary Receiver Operating Characteristic Curves
Summary receiver operating characteristic curves were obtained following the methods of Moses and Littenberg.40,41 The true positive (TPR) and false positive rates (FPR) from each study were converted to their logistic transforms using the following equations:
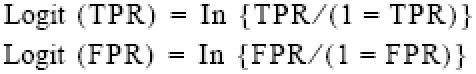
The purpose of this transformation was to linearize the data for linear regression analysis. To avoid having cells with zero, we added one-half to all counts in each cell. Two additional terms were defined:
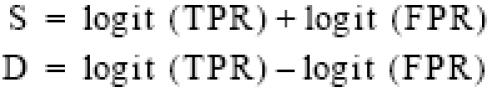
S was the sum of the two transforms and was related to the diagnostic cut point selected by the investigators. D, the logarithm of the ratio TPR/FPR, was a measure of how well the test discriminated between diseased and nondiseased subjects. The relationship between S and D was estimated with SAS42 by using a weighted least squares regression to fit the linear model: D = bS + i. After estimating the slope and intercept of the transformed line, we back-transformed the line to yield a summary curve consistent with the TPR and FPR reported for each study.
We tested for homogeneity by plotting the 95% confidence intervals for the TPR and FPR for individual studies against the summary receiver operating characteristic curve. If the confidence intervals for all studies overlapped the summary curve, then the studies were considered to be homogeneous. Sensitivity analyses were performed by classifying studies into subgroups according to methodologic criteria and comparing the D statistics. The nonparametric Mann Whitney U test was used for statistical comparisons.
RESULTS
Overall, we retrieved 90 articles from an initial 252 references identified by the literature search, but only 54 studies presented original diagnostic performance data. An additional 16 studies were excluded because we could not abstract data for PSA values between 2.0 and 10.0 ng/ml.14,16,43–56 We also excluded 5 studies with inadequate sample sizes,57–61 6 with superseded data,62–67 3 reporting only gamma-seminoprotein data,68–70 and 3 reporting only ACT ratio data.11,16,71 The remaining 21 studies reported diagnostic performance data for the free-to-total PSA ratio when total PSA levels ranged from either 4.0 to 10.0 ng/ml,35,73–88 2.5 to 10.0 ng/ml,89,90 or 2.6 to 4.0 ng/ml.91 Seventeen of the 21 studies presented data on investigator-selected cut points.73–81,83,84,86–90
Table 1 shows the number of studies meeting the methodologic criteria used to evaluate validity and generalizability. Thirteen studies used needle biopsy as the single reference standard, but none of them used long-term clinical follow-up to define true negative test results. The other studies used a combination of reference standards including 472,80,85,86 using radical prostatectomy. Two studies did not perform biopsies on all control subjects.75,86 The majority of studies used appropriate specimen handling and equimolar assays, but only 1 study calibrated the free PSA assay against a reference standard.35 Only 3 studies explicitly indicated that test interpretations were blinded.77,81,83 Nine studies evaluated fewer than 30 cancer cases. Six studies used free PSA testing in screening populations74,76,77,83,89,91; the remaining studies either tested referral populations, often with frozen stored serum samples, or did not describe indications for testing. The majority of studies failed to either describe eligibility criteria or to report on age, race, symptoms, digital rectal examination findings, and cancer stage.
Table 1.
Number of Studies Meeting Criteria for High Quality by Methodologic Category (N = 21)
| Methodologic Category | Criteria for Acceptable Quality | n (%) |
|---|---|---|
| Reference standard | Radical prostatectomy or systematic prostate biopsies with ≥1 year of clinical follow-up | 4 (19) |
| Avoidance of work-up bias | Uniform application of reference standard | 13 (62) |
| Consecutive or random sampling | 8 (38) | |
| Free PSA assay | Specimen handling: fresh specimen or long-term storage at −70°C | 14 (67) |
| Assay: equimolar | 15 (71) | |
| Calibrated against a reference standard | 1 (5) | |
| Independence of interpretations | Explicit statement of binding | 3 (14) |
| Sample size | ≥30 cancers | 12 (57) |
| Spectrum of patients | Age | 17 (81) |
| Race | 4 (19) | |
| Asymptomatic (screening) | 6 (29) | |
| Digital rectal examination results | 6 (29) | |
| Benign prostatic hyperplasia | 18 (86) | |
| Cancer stage | 16 (76) | |
| Study eligibility criteria presented | 15 (71) |
Table 2 shows the diagnostic performance data for the 17 studies presenting an investigator-selected cut point for PSA values between 2.0 to 10.0 ng/ml. Investigators generally selected these cut points to maximize sensitivity, although several studies selected cut points to maximize accuracy (overall proportion of true positive and true negative tests)75,80,90 and 1 study maximized specificity.79 In these studies, the median likelihood ratio of a positive test was 1.76 (25th percentile, 1.40; 75th percentile, 2.11) and the median likelihood ratio of a negative test was 0.27 (0.20, 0.40). The associated median area under the receiver operating characteristic curve was 0.68 (0.64, 0.71). Assuming a 25% pretest probability of cancer, Bayesian analysis with these median likelihood ratios led to a posttest cancer probability of 37% (32%, 41%) following a positive test and 8% (6.2%, 11.7%) following a negative test.
Table 2.
Performance Characteristics of Investigator-Selected Optimal Free-To-Total Ratio Cut Points*
| Study | Subjects | Cancers (%) | PSA Range(ng/ml) | F/T PSA Ratiocut point, % | LR Positive(95% CI) | LR Negative(95% CI) | AUROC(95% CI) |
|---|---|---|---|---|---|---|---|
| Alivizatos et al.73 | 102 | 22 (22) | 4.0 to 10.0 | 20 | 2.08 (1.41 to 3.06) | 0.42 (0.22 to 0.82) | 0.69 (0.57 to 0.81) |
| Bangma et al.74 | 427 | 99 (23) | 4.0 to 10.0 | 20 | 1.67 (1.44 to 1.93) | 0.37 (0.24 to 0.56) | 0.66 (0.60 to 0.72) |
| Bjork et al.75 | 31 | 12 (39) | 4.0 to 10.0 | 17 | 2.11 (1.01 to 4.40) | 0.49 (0.22 to 1.08) | 0.68 (0.48 to 0.88) |
| Catalona et al. 76 | 113 | 50 (44) | 4.0 to 10.0 | 20.3 | 1.49 (1.21 to 1.83) | 0.21 (0.08 to 0.53) | 0.65 (0.55 to 0.75) |
| Catalona et al. 77 | 773 | 379 (49) | 4.0 to 10.0 | 25 | 1.18 (1.12 to 1.25) | 0.27 (0.17 to 0.43) | 0.57 (0.53 to 0.61) |
| Egawa et al.78 | 78 | 28 (36) | 4.0 to 10.0 | 17 | 2.88 (1.75 to 4.77) | 0.34 (0.18 to 0.64) | 0.75 (0.63 to 0.85) |
| Filella et al.79 | 59 | 11 (19) | 4.0 to 10.0 | 8 | 10.9 (2.82 to 42.1) | 0.57 (0.34 to 0.95) | 0.71 (0.51 to 0.91) |
| Jung et al.80 | 43 | 26 (60) | 4.0 to 10.0 | 16 | 3.95 (1.73 to 9.07) | 0.20 (0.10 to 0.43) | 0.87 (0.75 to 0.99) |
| Luderer et al.81 | 57 | 25 (44) | 4.0 to 10.0 | 20 | 1.76 (1.22 to 2.54) | 0.24 (0.09 to 0.67) | 0.69 (0.55 to 0.83) |
| Partin et al.83 | 217 | 139 (64) | 4.0 to 10.0 | 20 | 1.35 (1.16 to 1.56) | 0.17 (0.08 to 0.37) | 0.62 (0.54 to 0.70) |
| Prestigiacomo et al.72 | 46 | 18 (39) | 4.0 to 10.0 | 15 | 2.05 (1.35 to 3.11) | 0.10 (0.02 to 0.47) | 0.74 (0.60 to 0.88) |
| Prestigiacomo et al.84 | 98 | 44 (45) | 4.0 to 10.0 | 20 | 1.63 (1.27 to 2.11) | 0.20 (0.08 to 0.52) | 0.68 (0.58 to 0.78) |
| Van Cangh et al.86 | 185 | 61 (33) | 4.0 to 10.0 | 25 | 1.38 (1.19 to 1.61) | 0.28 (0.13 to 0.60) | 0.62 (0.54 to 0.70) |
| Vashi et al.87 | 248 | 117 (47) | 4.0 to 10.0 | 24 | 1.09 (1.01 to 1.18) | 0.40 (0.17 to 0.94) | 0.54 (0.46 to 0.62) |
| Wang et al.88 | 62 | 23 (37) | 4.0 to 10.0 | 15 | 1.77 (1.31 to 2.39) | 0.09 (0.02 to 0.46) | 0.71 (0.59 to 0.83) |
| Reissigl et al.89 | 106 | 37 (35) | 2.5 to 10.0 | 22 | 1.40 (1.19 to 1.65) | 0.09 (0.02 to 0.44) | 0.64 (0.54 to 0.74) |
| Toubert et al.90 | 161 | 62 (39) | 2.5 to 10.0 | 15 | 5.81 (3.27 to 10.3) | 0.40 (0.28 to 0.56) | 0.77 (0.69 to 0.85) |
| Catalona et al.91 | 317 | 72 (23) | 2.6 to 4.0 | 27 | 1.10 (1.00 to 1.21) | 0.54 (0.26 to 1.12) | 0.54 (0.46 to 0.62) |
Figure 1 shows the investigator-selected cut points plotted against posttest probabilities, again assuming a pretest probability of 25%. For negative tests (the lower line), the relationship was linear with a slope of approximately zero (−0.002, SE = 0.002), indicating that the posttest probability did not depend on the cut point. Following a negative test, the probability of cancer was reduced by over 50%. We found a logarithmic relationship between the cut point and posttest probability for positive tests. The probability of cancer was greater than 70% for cut points less than 10%, but less than 40% for cut points above 20%.
FIGURE 1.
Investigator-selected cut points for the free-to-total PSA ratio are plotted against the posttest probabilities for positive and negative tests. Curves are based on a pretest probability of 25%.
Figure 2 shows the estimated summary receiver operating characteristic curve based on the 17 studies with investigator-selected cut points. The summary curve shows that setting the free-to-total PSA ratio cut point to achieve a true positive rate above 90% led to false positive rates ranging from 60% to 90%. Conversely, setting the cut point to achieve a false positive rate less than 10% led to true positive rates ranging from 30% to 50%.
FIGURE 2.
The estimated summary receiver operating characteristic curve based on the 17 studies presenting data on investigator-selected cut points for the free-to-total PSA ratio.
Graphical tests showed no significant heterogeneity among studies, implying that between-study differences in true positive and false positive rates arose from the different cut points selected by the investigators. As shown in Table 3, we also performed sensitivity analyses based on avoidance of work-up bias, specimen handling, type of free PSA assay, blinding, purpose of testing, cohort assembly, avoidance of spectrum bias, and sample size. Although discriminating power as represented by the intercept (D) of the (S, D) space regression line was consistently lower in studies with greater methodologic rigor (except for specimen handling), the differences did not achieve statistical significance.
Table 3.
Sensitivity Analysis for Summary Reciever Operating Characteristic Curve: Comparison of Median D Values* for Studies Stratified by Presence and Absence of Methodologic Features. (N = 17)
| Feature Present | |||||
|---|---|---|---|---|---|
| Yes, n | No, n | ||||
| Methodologic Feature | Studies (subjects) | Median D Value | Studies (subjects) | Median D Value | P Value† |
| Avoidance of work-up bias‡ | 11 (2,262) | 1.86 | 6 (540) | 2.35 | .31 |
| Appropriate specimen handling § | 10 (2,048) | 1.99 | 7 (754) | 1.86 | .84 |
| Equimolar assay | 11 (2,047) | 1.86 | 6 (755) | 2.59 | .19 |
| Explicit blinding | 3 (1,047) | 1.86 | 14 (1,755) | 2.03 | .45 |
| Screening cohort | 5 (1,636) | 1.86 | 12 (1,166) | 2.03 | .34 |
| Consecutive or random selection | 6 (1,005) | 1.76 | 11 (1,797) | 2.01 | .48 |
| Avoidance of spectrum bias‖ | 6 (1,658) | 1.97 | 11 (1,144) | 1.98 | .76 |
| Sample size ≥30 | 9 (2,325) | 1.86 | 8 (477) | 2.20 | .15 |
The D value is the logarithm of the true positive rate/false positive rate and is a measure of the test's discriminating power.
Mann-Whitney U test comparing median D values.
All subjects within a study underwent the same reference test evaluation.
Assay performed on specimens that were fresh or stored at −70°C.
Clinical description included age, digital rectal examination finding, and cancer stage.
Several studies provided data for PSA levels below 4.0 ng/ml.78,86,87,91 Median likelihood ratios were 1.64 (25th percentile, 1.28; 75th percentile, 2.56) for positive tests, 0.27 (0.16, 0.45) for negative tests, and 0.67 (0.59, 0.76) for the area under the receiver operating characteristic curve. The literature suggested a 10% pretest probability of prostate cancer for PSA values less than 4.0 ng/ml.92 Therefore, the posttest cancer probability was 15.4% (12.5%, 22.1%) following a positive test and 2.9% (1.7%, 4.8%) following a negative test. However, the only screening study, which evaluated 317 men with PSA values between 2.6 to 4.0 ng/ml, had a likelihood ratio of only 1.10 (95% confidence interval, 1.00 to 1.21) for a positive test and a likelihood ratio of 0.54 (0.26, 1.12) for a negative test. In this screening population, the posttest cancer probability was 10.9% (10.0%, 11.9%) following a positive test and 5.7% (2.8%, 11.1%) following a negative test. The area under the receiver operating characteristic curve was 0.54 (0.46, 0.62).
DISCUSSION
The free-to-total PSA ratio has been recommended as an effective strategy to improve the specificity of total PSA for “gray zone” values between 2.0 and 10.0 ng/ml. Our meta-analysis showed that using the investigator-selected free-to-total PSA cut point yielded modest revisions of probability estimates for cancer. The median likelihood ratio for a positive test was 1.76 (interquartile range, 1.40 to 2.11), a value which generates minimal changes in posttest probabilities.27 The median likelihood ratio for a negative test was 0.27 (0.20, 0.40). Although this likelihood ratio is considered to generate only small probability changes,27 a negative test substantially reduced the probability of prostate cancer from 25% to 8%.
When we plotted the investigator-selected cut points against posttest probabilities, we found that the probability revision following a negative test was independent of the study cut point within the range of cut points that were considered. In contrast, the posttest probability following a positive test depended upon the cut point. The lower the cut point, the more likely that a patient had prostate cancer, with the probability nearly doubling as the cut point dropped from 20% to 10%. However, we cannot endorse using a lower cut point because few studies selected cut points less than 15% and more cancers will be missed at lower cut points. Nonetheless, these results suggested that using multiple cut points, especially for evaluating positive tests, may provide more precise information about the posttest probability for cancer.
Our results indicated that the free-to-total PSA ratio did not have a high discriminating power. This finding was supported by the relatively low median area under the receiver operating characteristic curve of 0.68. Most of the investigators chose an optimal free-to-total PSA cut point that set the sensitivity around 95% to minimize the chance of missing a cancer. The summary receiver operating characteristic curve showed that sensitivities above 90% were associated with very high false positive rates.
Investigators were willing to accept poor specificity for the free-to-total PSA ratio because measuring free PSA could reduce the number of unnecessary biopsies. However, potential spectrum bias and imperfect reference standards made the estimates of specificity unreliable for a screening population. Spectrum bias25,30,93 was possible because the majority of studies evaluated subjects referred to urologists with prostate abnormalities. The magnitude and direction of this bias was difficult to assess because the indications for enrolling patients and performing biopsies usually were not provided, and few studies presented complete demographic and clinical descriptions. Another source of bias came from relying on the relatively insensitive prostate needle biopsy for a reference standard. The transrectal prostate needle biopsy has false negative rates of at least 20%.94,95 Diagnostic test properties can change with disease prevalence when the reference test negative group contains many diseased subjects.96,97 Because the median cancer prevalence in these studies of men with PSA values between 4.0 and 10.0 ng/ml was 39%, specificity might be expected to differ in a screening population in which disease prevalence is much lower.97
The literature also provided no consensus on the optimal free-to-total PSA cut point because assays and specimen handling were not comparable across studies. Only 4 studies clearly performed assays on fresh specimens; the remaining studies either did not describe specimen handling or else used specimens frozen for unreported lengths of time. However, free PSA and PSA have been shown to undergo significant degradation during frozen storage. This implies that the free-to-total PSA ratios reported for samples with long-term or uncertain storage may be unreliable.31,32,76 Inter-assay differences in immunoresponsiveness between the skewed and equimolar response assays can also affect the estimated ratios.13,17,33,98 Stamey has reported overcoming this problem by calibrating against a PSA-ACT and free PSA standard.36 However, only 1 group of investigators35 calibrated their assay against such a standard.
Test-retest variability is an important problem with free PSA assays. While the studies in our meta-analysis generally reported a coefficient of variation for percent free PSA less than 8% for control specimens, other investigators have shown higher coefficients of variation, ranging from 10% to 16%, with serial blood sampling.99,100 Without further data, investigators cannot yet establish an optimal cut point for using the free-to-total PSA ratio in prostate cancer screening.
We found additional methodologic flaws that threatened the validity and generalizability of study results. Work-up bias potentially occurred in the studies failing to test all subjects with the same reference standard and when subjects were not consecutively or randomly selected. Many studies were retrospective and patient selection was based on having both a biopsy and enough stored serum to run assays. Additionally, the selection of reference standards was flawed. The definitive reference standard, radical prostatectomy, was used in only a few studies and was not applied to all subjects. Transrectal prostate needle biopsy, the most frequently used reference standard, has a high false negative rate.94,95 However, no study used long-term clinical follow-up to determine the validity of the false negative biopsy.
Generalizing study results was difficult because few studies provided explicit eligibility criteria or described the subjects' ages, clinical symptoms, digital rectal examination findings, and cancer stages. Although investigators reported on various different free PSA assays, only 1 study calibrated their results against a reference standard. Finally, few studies had large enough sample sizes to ensure adequate precision for estimating diagnostic accuracy.
Although sensitivity analyses did not show statistically significant differences between subgroups defined by quality criteria, our power to detect such an effect was low with only 17 eligible studies. Nonetheless, the discriminating power was consistently lower in studies with greater methodologic rigor.
Four studies provided data on using the free-to-total PSA ratio when the total PSA was less than 4.0 ng/ml. The only screening study, which excluded men with abnormal digital rectal examinations, reported likelihood ratios that generated extremely small probability revisions. Additionally, the area under the receiver operating characteristic curve was 0.54, indicating poor discriminating power. None of the other studies stratified data by digital rectal examination findings, leaving them susceptible to patient selection bias because men undergoing biopsies with a normal PSA level are more likely to have abnormal digital rectal examinations. Therefore, using free-to-total PSA ratios when total PSA is less than 4.0 ng/ml is not supported by the available literature.
Our study results potentially could be limited by missing relevant studies. However, we conducted an exhaustive literature search, including a hand search of leading urology journals. We did not include foreign language studies, although we reviewed the English-language abstracts. The homogeneity of study results seen in the summary receiver operating characteristic curve and the lack of significant differences in the sensitivity analyses suggest that we have appropriately summarized the available literature.
Based on our meta-analysis of the free-to-total PSA ratio, we concluded that the test did not have good discriminating power and that likelihood ratios for positive tests had minimal effect on probability revision. A negative test result in a screening population could reduce the posttest probability for cancer to approximately 10%. This information may be helpful in clinical decision making and could reduce the number of unnecessary biopsies. However, patients may find that this probability is not low enough to avoid undergoing a prostate biopsy.
Methodologic flaws in reference standards and the potential for work-up and spectrum biases limited the validity and generalizability of the free PSA literature. No optimal cut point could be determined from the meta-analysis and estimates for test specificity—the potential reduction in unnecessary biopsies—were imprecise.
Further research is needed to accurately assess the diagnostic performance and utility of the free-to-total PSA ratio. The test should be evaluated in prospective studies consecutively enrolling subjects from screening populations. Data should be reported on age, digital rectal examination findings, symptoms, and ethnicity; the most important population to study is men with indeterminate PSA values and normal digital rectal examinations. Using free PSA assays calibrated against a purified reference standard would increase the generalizability of recommended cut points. Investigators should also consider reporting diagnostic performance data for multiple cut points. Similar design criteria should be applied for evaluating other recently proposed strategies for improving the specificity of PSA, including prostate-specific membrane antigen, human kallikrein 2, and newer assays of complexed PSA.101
Acknowledgments
This work was supported by the VA Medical Center, Albuquerque, NM.
The authors thank Daniel Kent, MD, for his insightful comments on an earlier draft of this paper.
REFERENCES
- 1.Mettlin C, Jones G, Averette H, Gusberg SB, Murphy GP, et al. Defining and updating the American Cancer Society guidelines for the cancer related check-up: prostate and endometrial cancers. CA Cancer J Clin. 1993;43:42–6. doi: 10.3322/canjclin.43.1.42. [DOI] [PubMed] [Google Scholar]
- 2.American Urological Association. Early detection of prostate cancer and use of transrectal ultrasound. American Urological Association 1992 Policy Statement Book. Baltimore, Md: Williams & Wilkins; 1992. [Google Scholar]
- 3. PDQ. (Physician Data Query) [database online]. Bethesda, Md: National Cancer Institute: 1984 – [updated 9/99]. Screening for prostate cancer. Available from: National Cancer Institute; National Library of Medicine, Bethesda, Md: CDP Technologies, Inc., New York, NY; Lexis-Nexis, Miamisburg, Ohio.
- 4.American College of Physicians. Screening for prostate cancer. Ann Intern Med. 1997;126:480–4. [PubMed] [Google Scholar]
- 5.U.S. Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- 6.Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–90. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 7.Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267:2215–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Benson MC, Whang IS, Olsson CA, McMahon DJ, Cooner WH, et al. The use of prostate specific antigen density to enhance the predictive value of intermediate levels of serum prostate specific antigen. J Urol. 1992;147:817–21. doi: 10.1016/s0022-5347(17)37394-9. [DOI] [PubMed] [Google Scholar]
- 9.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860–4. [PubMed] [Google Scholar]
- 10.Lilja H, Christensson A, Dahlén U, et al. Prostate-specific antigen in serum occurs predominantly in complex with α1-antichymotrypsin. Clin Chem. 1991;37:1618–25. [PubMed] [Google Scholar]
- 11.Stenman UH, Leinonen J, Alfthan H, Rannikko S, Tuhkanen K, Alfthan O, et al. A complex between prostate-specific antigen and α1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–6. [PubMed] [Google Scholar]
- 12.Christensson A, Laurell CB, Lilja H, et al. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem. 1990;194:755–63. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- 13.McCormack RT, Rittenhouse HG, Finlay JA, et al. Molecular forms of prostate-specific antigen and the human kallikrein gene family: a new era. Urology. 1995;45:729–44. doi: 10.1016/s0090-4295(99)80076-4. [DOI] [PubMed] [Google Scholar]
- 14.Lilja H, et al. Regulation of the enzymatic activity of prostate-specific antigen and its reactions with extracellular protease inhibitors in prostate cancer. Scand J Clin Lab Invest Suppl. 1995;220:47–56. [PubMed] [Google Scholar]
- 15.Leinonen J, Lövgren T, Vornanen T, Stenman UH, et al. Double-label time-resolved immunofluorometric assay of prostate-specific antigen and of its complex with α1-antichymotrypsin. Clin Chem. 1993;39:2098–103. [PubMed] [Google Scholar]
- 16.Christensson A, Björk T, Nilsson O, et al. Serum prostate specific antigen complexed to α1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150:100–5. doi: 10.1016/s0022-5347(17)35408-3. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamsson PA, Lilja H, Oesterling JE, et al. Molecular forms of serum prostate-specific antigen. The clinical value of percent free prostate-specific antigen. Urol Clin North Am. 1997;24:353–65. doi: 10.1016/s0094-0143(05)70382-7. [DOI] [PubMed] [Google Scholar]
- 18.Vessella RL, Lange PH, et al. Issues in the assessment of prostate-specific antigen immunoassays. An update. Urol Clin North Am. 1997;24:261–8. doi: 10.1016/s0094-0143(05)70371-2. [DOI] [PubMed] [Google Scholar]
- 19.Colberg JW, Smith DS, Catalona WJ, et al. Prevalence and pathological extent of prostate cancer in men with prostate specific antigen levels of 2.9 to 4.0 ng/ml. J Urol. 1993;149:507–9. doi: 10.1016/s0022-5347(17)36130-x. [DOI] [PubMed] [Google Scholar]
- 20.Gann PH, Hennekens CH, Stampfer MJ, et al. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273:289–94. [PubMed] [Google Scholar]
- 21.Vashi AR, Oesterling JE, et al. Percent free prostate-specific antigen: entering a new era in the detection of prostate cancer. Mayo Clin Proc. 1997;72:337–44. doi: 10.4065/72.4.337. [DOI] [PubMed] [Google Scholar]
- 22.Catalona WJ, et al. Clinical utility of measurements of free and total prostate-specific antigen (PSA): a review. Prostate Suppl. 1996;7:64–9. doi: 10.1002/(sici)1097-0045(1996)7+<64::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Food and Drug Administration. Center for Devices and Radiological Health. Premarket Approval Decisions for March 1998. Available at: http://www.fda.gov:80/cdrh/pmamar98.html.
- 24.Catalona WJ, Partin AW, Slawin KM, et al. A multicenter clinical trial evaluation of free PSA in the differentiation of prostate cancer from benign disease. J Urol. 1997;157(suppl):111. [Google Scholar]
- 25.Ransohoff DF, Feinstein AR, et al. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. New Engl J Med. 1978;299:926–30. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
- 26.Jaeschke R, Guyatt G, Sackett DL, et al. Users' guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? JAMA. 1994;271:389–91. doi: 10.1001/jama.271.5.389. [DOI] [PubMed] [Google Scholar]
- 27.Jaeschke R, Guyatt GH, Sackett DL, et al. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? JAMA. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 28.Mulrow CD, Linn WD, Gaul MK, Pugh JA, et al. Assessing quality of a diagnostic test evaluation. J Gen Intern Med. 1989;4:288–95. doi: 10.1007/BF02597398. [DOI] [PubMed] [Google Scholar]
- 29.Irwig L, Tosteson ANA, Gatsonis C, et al. Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med. 1994;120:667–76. doi: 10.7326/0003-4819-120-8-199404150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Reid MC, Lachs MS, Feinstein AR, et al. Use of methodological standards in diagnostic test research. Getting better but still not good. JAMA. 1995;274:645–51. [PubMed] [Google Scholar]
- 31.Arcangeli CG, Smith DS, Ratliff TL, Catalona WJ, et al. Stability of serum total and free prostate specific antigen under varying storage intervals and temperatures. J Urol. 1997;158:2182–7. doi: 10.1016/s0022-5347(01)68191-6. [DOI] [PubMed] [Google Scholar]
- 32.Woodrum D, French C, Shamel LB, et al. Stability of free prostate-specific antigen in serum samples under a variety of sample collection and sample storage conditions. Urology. 1996;48:33–9. doi: 10.1016/s0090-4295(96)00607-3. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen SJ, Lilja H, Klee GG, Wright Gl, Jr., Pettersson K, Oesterling JE, et al. Comparability of the Tandem-R and IMx assays for the measurement of serum prostate-specific antigen. Urology. 1994;44:512–8. doi: 10.1016/s0090-4295(94)80049-9. [DOI] [PubMed] [Google Scholar]
- 34.Pienta KJ, Esper PS, et al. Risk factors for prostate cancer. Ann Intern Med. 1993;118:793–803. doi: 10.7326/0003-4819-118-10-199305150-00007. [DOI] [PubMed] [Google Scholar]
- 35.Marley GM, Miller MC, Kattan MW, et al. Free and complexed prostate-specific antigen serum ratios to predict probability of primary prostate cancer and benign prostatic hyperplasia. Urology. 1996;48:16–22. doi: 10.1016/s0090-4295(96)00605-x. [DOI] [PubMed] [Google Scholar]
- 36.Stamey TA, et al. Progress in standardization of immunoassays for prostate-specific antigen. Urol Clin North Am. 1997;24:269–73. doi: 10.1016/s0094-0143(05)70372-4. [DOI] [PubMed] [Google Scholar]
- 37.Sackett DL, Haynes RB, Guyatt GH, Tugwell P, et al. Clinical Epidemiology. A basic science for clinical medicine. 2nd ed. Boston: Little, Brown; 1991. [Google Scholar]
- 38.Peirce JC, Cornell RG, et al. Integrating stratum-specific likelihood ratios with the analysis of ROC curves. Med Decis Making. 1993;13:141–51. doi: 10.1177/0272989X9301300208. [DOI] [PubMed] [Google Scholar]
- 39.Brawer MK, Chetner MP, Beatie J, Buchner DM, Vessella RL, Lange PH, et al. Screening for prostatic carcinoma with prostate specific antigen. J Urol. 1992;147:841–5. doi: 10.1016/s0022-5347(17)37401-3. [DOI] [PubMed] [Google Scholar]
- 40.Moses LE, Shapiro D, Littenberg B, et al. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12:1293–316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 41.Littenberg B, Moses LE, et al. Estimating diagnostic accuracy from multiple conflicting reports: a new meta-analytic method. Med Decis Making. 1993;13:313–21. doi: 10.1177/0272989X9301300408. [DOI] [PubMed] [Google Scholar]
- 42.SAS® Language: Reference. Version 6. Cary, NC: SAS Institute, Inc; 1990. [Google Scholar]
- 43.Elgamal AA, Cornillie FJ, Van Poppel HP, Van de Voorde WM, McCabe R, Baert LV, et al. Free-to-total prostate specific antigen ratio as a single test for detection of significant stage T1c prostate cancer. J Urol. 1996;156:1042–9. [PubMed] [Google Scholar]
- 44.Froschermaier SE, Pilarsky CP, Wirth MP, et al. Clinical significance of the determination of noncomplexed prostate-specific antigen as a marker for prostate carcinoma. Urology. 1996;47:525–8. doi: 10.1016/S0090-4295(99)80488-9. [DOI] [PubMed] [Google Scholar]
- 45.Junker R, Brandt B, Zechel C, Assmann G, et al. Comparison of prostate-specific antigen (PSA) measured by four combinations of free PSA and total PSA assays. Clin Chem. 1997;43:1588–94. [PubMed] [Google Scholar]
- 46.Mitrunen K, Pettersson K, Piironen T, Björk T, Lilja H, Lövgren T, et al. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–20. [PubMed] [Google Scholar]
- 47.Reissigl A, Klocker H, Pointner J, Ennemoser O, Falk M, Bartsch G, et al. Improvement of prostate cancer screening by determination of the ratio free/total PSA in addition to PSA levels. Prostate. 1997;30:243–7. doi: 10.1002/(sici)1097-0045(19970301)30:4<243::aid-pros3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 48.Riccardo B, Alberino D, Fabrizio T, et al. Free to total prostatic specific antigen ratio as a new diagnostic tool in prostatic carcinoma. Anticancer Res. 1997;17:1297–302. [PubMed] [Google Scholar]
- 49.Stephan C, Lein M, Jung K, Schnorr D, Loening SA, et al. The influence of prostate volume on the ratio of free to total prostate specific antigen in serum of patients with prostate cancer and benign prostate hyperplasia. Cancer. 1997;79:104–9. [PubMed] [Google Scholar]
- 50.Tarle M, Kraljic I, et al. Free and total serum PSA values in patients with prostatic intraepithelial neoplasia (PIN), prostate cancer and BPH. Is F/T PSA a potential probe for dormant and manifest cancer? Anticancer Res. 1997;17:1531–4. [PubMed] [Google Scholar]
- 51.Thiel RP, Oesterling JE, Wojno KJ, et al. Multicenter comparison of the diagnostic performance of free prostate-specific antigen. Urology. 1996;48:45–50. doi: 10.1016/s0090-4295(96)00609-7. [DOI] [PubMed] [Google Scholar]
- 52.Wolff JM, Borchers H, Effert PJ, Habib FK, Jakse G, et al. Free-to-total prostate-specific antigen serum concentrations in patients with prostate cancer and benign prostatic hyperplasia. Br J Urol. 1996;78:409–13. doi: 10.1046/j.1464-410x.1996.00095.x. [DOI] [PubMed] [Google Scholar]
- 53.Morote J, Raventós CX, Lorente JA, et al. Measurement of free PSA in the diagnosis and staging of prostate cancer. Int J Cancer. 1997;71:756–9. doi: 10.1002/(sici)1097-0215(19970529)71:5<756::aid-ijc11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Murphy GP, Barren RJ, Erickson SJ, et al. Evaluation and comparison of two new prostate carcinoma markers. Free-prostate specific antigen and prostate specific membrane antigen. Cancer. 1996;78:809–18. doi: 10.1002/(SICI)1097-0142(19960815)78:4<809::AID-CNCR18>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 55.Prestigiacomo AF, Stamey TA, et al. Clinical usefulness of free and complexed PSA. Scand J Clin Lab Invest Suppl. 1995;221:32–4. doi: 10.3109/00365519509090561. [DOI] [PubMed] [Google Scholar]
- 56.Chen YT, Luderer AA, Thiel RP, Carlson G, Cuny CL, Soriano TF, et al. Using proportions of free to total prostate-specific antigen, age, and total prostate-specific antigen to predict the probability of prostate cancer. Urology. 1996;47:518–24. doi: 10.1016/s0090-4295(99)80487-7. [DOI] [PubMed] [Google Scholar]
- 57.Higashihara E, Nutahara K, Kojima M, et al. Significance of serum free prostate specific antigen in the screening of prostate cancer. J Urol. 1996;156:1964–8. [PubMed] [Google Scholar]
- 58.Morgan TO, McLeod DG, Leifer ES, Moul JW, Murphy GP, et al. Prospective use of free PSA to avoid repeat prostate biopsies in men with elevated total PSA. Prostate Suppl. 1996;7:58–63. [PubMed] [Google Scholar]
- 59.Akdas A, Cevik I, Tarcan T, Turkeri L, Dalaman G, Emerk K, et al. The role of free prostate-specific antigen in the diagnosis of prostate cancer. Brit J Urol. 1997;79:920–3. doi: 10.1046/j.1464-410x.1997.00183.x. [DOI] [PubMed] [Google Scholar]
- 60.Auvinen A, Tammela T, Stenman UH, et al. Screening for prostate cancer using serum prostate-specific antigen: a randomised, population-based pilot study in Finland. Br J Cancer. 1996;74:568–72. doi: 10.1038/bjc.1996.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Correale M, Pagliarulo A, Donatuti G, et al. Preliminary clinical evaluation of free/total PSA ratio by the IMMULITE system. Int J Biol Markers. 1996;11:24–8. doi: 10.1177/172460089601100105. [DOI] [PubMed] [Google Scholar]
- 62.Reissigl A, Pointner J, Horninger W, et al. Comparison of different prostate-specific antigen cutpoints for early detection of prostate cancer: results of a large screening study. Urology. 1995;46:662–5. doi: 10.1016/S0090-4295(99)80297-0. [DOI] [PubMed] [Google Scholar]
- 63.Van Cangh PJ, De Nayer P, Sauvage P, et al. Free to total prostate-specific antigen (PSA) ratio is superior to total-PSA in differentiating benign prostate hypertrophy from prostate cancer. Prostate Suppl. 1996;7:30–4. [PubMed] [Google Scholar]
- 64.Bangma CH, Kranse R, Blijenberg BG, Schröder FH, et al. The value of screening tests in the detection of prostate cancer. Part II: Retrospective analysis of free/total prostate-specific analysis ratio, age-specific reference ranges, and PSA density. Urology. 1995;46:779–84. doi: 10.1016/S0090-4295(99)80343-4. [DOI] [PubMed] [Google Scholar]
- 65.Bangma CH, Kranse R, Blijenberg BG, Schröder FH, et al. The value of screening tests in the detection of prostate cancer. Urology. 1995;46:773–8. doi: 10.1016/S0090-4295(99)80342-2. Part I: Results of a retrospective evaluation of 1726 men. [DOI] [PubMed] [Google Scholar]
- 66.Bangma CH, Kranse R, Blijenberg BG, Schröder FH, et al. Free and total prostate-specific antigen in a screened population. Br J Urol. 1997;79:756–62. doi: 10.1046/j.1464-410x.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 67.Morgan TO, McLeod DG, Leifer ES, Murphy GP, Moul JW, et al. Prospective use of free prostate-specific antigen to avoid repeat prostate biopsies in men with elevated total prostate-specific antigen. Urology. 1996;48:76–80. doi: 10.1016/s0090-4295(96)00615-2. [DOI] [PubMed] [Google Scholar]
- 68.Demura T, Shinohara N, Tanaka M, et al. The proportion of free to total prostate specific antigen: a method of detecting prostate carcinoma. Cancer. 1996;77:1137–43. doi: 10.1002/(sici)1097-0142(19960315)77:6<1137::aid-cncr20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 69.Demura T, Watarai T, Togashi M, Hirano T, Ohashi N, Koyanagi T, et al. Measurement of prostate specific antigen and γ-seminoprotein ratio: a new means of distinguishing benign prostatic hyperplasia and prostate cancer. J Urol. 1993;150:1740–5. doi: 10.1016/s0022-5347(17)35883-4. [DOI] [PubMed] [Google Scholar]
- 70.Kuriyama M, Takeuchi T, Shinoda I, Okana M, Nishiura T, et al. Clinical evaluation of γ-seminoprotein in prostate cancer. Prostate. 1986;8:301–11. doi: 10.1002/pros.2990080310. [DOI] [PubMed] [Google Scholar]
- 71.Stenman UH, Hakama M, Knekt P, Aromaa A, Teppo L, Leinonen J, et al. Serum concentrations of prostate specific antigen and its complex with α1-antichymotrypsin before diagnosis of prostate cancer. Lancet. 1994;344:1594–8. doi: 10.1016/s0140-6736(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 72.Prestigiacomo AF, Stamey TA, et al. Can free and total prostate specific antigen and prostatic volume distinguish between men with negative and positive systematic ultrasound guided prostate biopsies? J Urol. 1997;157:189–94. [PubMed] [Google Scholar]
- 73.Alivizatos G, Deliveliotis C, Mitropoulos D, et al. Does free to total ratio of prostate-specific antigen alter decision-making on prostatic biopsy? Urology. 1996;48:71–5. doi: 10.1016/s0090-4295(96)00614-0. [DOI] [PubMed] [Google Scholar]
- 74.Bangma CH, Rietbergen JBW, Kranse R, Blijenberg BG, Petterson K, Schröder FH, et al. The free-to-total prostate specific antigen ratio improves the specificity of prostate specific antigen in screening for prostate cancer in the general population. J Urol. 1997;157:2191–6. [PubMed] [Google Scholar]
- 75.Björk T, Piironen T, Pettersson K, et al. Comparison of analysis of the different prostate-specific antigen forms in serum for detection of clinically localized prostate cancer. Urology. 1996;48:882–8. doi: 10.1016/s0090-4295(96)00486-4. [DOI] [PubMed] [Google Scholar]
- 76.Catalona WJ, Smith DS, Wolfert RL, et al. Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA. 1995;274:1214–20. [PubMed] [Google Scholar]
- 77.Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease. A prospective multicenter clinical trial. JAMA. 1998;279:1542–7. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 78.Egawa S, Soh S, Ohori M, et al. The ratio of free to total serum prostate specific antigen and its use in differential diagnosis of prostate carcinoma in Japan. Cancer. 1997;79:90–8. [PubMed] [Google Scholar]
- 79.Filella X, Alcover J, Molina R, et al. Clinical usefulness of free PSA fraction as an indicator of prostate cancer. Int J Cancer. 1995;63:780–4. doi: 10.1002/ijc.2910630605. [DOI] [PubMed] [Google Scholar]
- 80.Jung K, Stephan C, Lein M, et al. Analytical performance and clinical validity of two free prostate-specific antigen assays compared. Clin Chem. 1996;42:1026–33. [PubMed] [Google Scholar]
- 81.Luderer AA, Chen YT, Soriano TF, et al. Measurement of the proportion of free to total prostate-specific antigen improves diagnostic performance of prostate-specific antigen in the diagnostic gray zone of total prostate-specific antigen. Urology. 1995;46:187–94. doi: 10.1016/s0090-4295(99)80192-7. [DOI] [PubMed] [Google Scholar]
- 82.Mione R, Aimo G, Bombardieri E, et al. Preliminary results of clinical evaluation of the free/total prostate-specific antigen ratio in a multicentric study. Tumori. 1996;82:543–9. doi: 10.1177/030089169608200606. [DOI] [PubMed] [Google Scholar]
- 83.Partin AW, Catalona WJ, Southwick PC, Subong ENP, Gasior GH, Chan DW, et al. Analysis of percent free prostate-specific antigen (PSA) for prostate cancer detection: influence of total PSA, prostate volume, and age. Urology. 1996;48:55–61. doi: 10.1016/s0090-4295(96)00611-5. [DOI] [PubMed] [Google Scholar]
- 84.Prestigiacomo AF, Lilja HJ, Pettersson K, Wolfert RL, Stamey TA, et al. A comparison of the free fraction of serum prostate specific antigen in men with benign and cancerous prostates: the best case scenario. J Urol. 1996;156:350–4. doi: 10.1097/00005392-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Roehrborn CG, Gregory A, McConnell JD, Sagalowsky AI, Wians Fh, Jr, et al. Comparison of three assays for total serum prostate-specific antigen and percentage of free prostate-specific antigen in predicting prostate histology. Urology. 1996;48:23–32. doi: 10.1016/s0090-4295(96)00606-1. [DOI] [PubMed] [Google Scholar]
- 86.Van Cangh PJ, De Nayer P, De Vischer L, et al. Free to total prostate-specific antigen (PSA) ratio improves the discrimination between prostate cancer and benign prostatic hyperplasia (BPH) in the diagnostic gray zone of 1.8 to 10 ng/mL total PSA. Urology. 1996;48:67–70. doi: 10.1016/s0090-4295(96)00613-9. [DOI] [PubMed] [Google Scholar]
- 87.Vashi AR, Wojno KJ, Henricks W, et al. Determination of the “reflex range” and appropriate cutpoints for percent free prostate-specific antigen in 413 men referred for prostatic evaluation using the AxSYM system. Urology. 1997;49:19–27. doi: 10.1016/S0090-4295(96)00511-0. [DOI] [PubMed] [Google Scholar]
- 88.Wang TJ, Hill TM, Sokoloff RL, Frankenne F, Rittenhouse HG, Wolfert RL, et al. Dual monoclonal antibody immunoassay for free prostate-specific antigen. Prostate. 1996;28:10–6. doi: 10.1002/(SICI)1097-0045(199601)28:1<10::AID-PROS2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 89.Reissigl A, Klocker H, Pointner J, et al. Usefulness of the ratio free/total prostate-specific antigen in addition to total PSA levels in prostate cancer screening. Urology. 1996;48:62–6. doi: 10.1016/s0090-4295(96)00612-7. [DOI] [PubMed] [Google Scholar]
- 90.Toubert ME, Guillet J, Chiron M, et al. Percentage of free serum prostate-specific antigen: a new tool in the early diagnosis of prostatic cancer. Eur J Cancer. 1996;32A:2088–93. doi: 10.1016/s0959-8049(96)00245-6. [DOI] [PubMed] [Google Scholar]
- 91.Catalona WJ, Smith DS, Ornstein DK, et al. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/ml and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997;277:1452–5. [PubMed] [Google Scholar]
- 92.Schröder FH, van der Cruijsen-Koeter I, de Koning HJ, Vis AN, Hoedemaker RF, Kranse R, et al. Prostate cancer detection at low prostate specific antigen. J Urol. 2000;163:806–12. [PubMed] [Google Scholar]
- 93.Lachs MS, Nachamkin I, Edelstein PH, Goldman J, Feinstein AR, Schwartz JS, et al. Spectrum bias in the evaluation of diagnostic tests: lessons from the rapid dipstick test for urinary tract infection. Ann Intern Med. 1992;117:135–40. doi: 10.7326/0003-4819-117-2-135. [DOI] [PubMed] [Google Scholar]
- 94.Stroumbakis N, Cookson MS, Reuter VE, Fair WR, et al. Clinical significance of repeat sextant biopsies in prostate cancer patients. Urology. 1997;49(suppl):113–8. doi: 10.1016/s0090-4295(97)00178-7. [DOI] [PubMed] [Google Scholar]
- 95.Ellis WJ, Brawer MK, et al. Repeat prostate needle biopsy: who needs it? J Urol. 1995;153:1496–8. [PubMed] [Google Scholar]
- 96.Buck AA, Gart JJ, et al. Comparison of a screening test and a reference test in epidemiologic studies. Indices of agreement and their relation to prevalence. Am J Epidemiol. 1966;83:586–92. doi: 10.1093/oxfordjournals.aje.a120609. [DOI] [PubMed] [Google Scholar]
- 97.Boyko EJ, Alderman BW, Baron AE, et al. Reference test errors bias the evaluation of diagnostic tests for ischemic heart disease. J Gen Intern Med. 1988;3:476–81. doi: 10.1007/BF02595925. [DOI] [PubMed] [Google Scholar]
- 98.Nixon RG, Petteway JC, Meyer GE, Brawer MK, et al. Comparison of three investigative assays for the free form of prostate-specific antigen. J Urol. 1997;157(suppl):255. [Google Scholar]
- 99.Ornstein DK, Smith DS, Rao GS, Basler JW, Ratliff TL, Catalona WJ, et al. Biological variation of total, free and percent free serum prostate specific antigen levels in screening volunteers. J Urol. 1997;157:2179–82. [PubMed] [Google Scholar]
- 100.Nixon RG, Lilly JD, Liedtke R J, Batjer JD, et al. Variation of free and total prostate-specific antigen levels: The effect on percent free/total prostate-specific antigen. Arch Pathol Lab Med. 1997;121:385–91. [PubMed] [Google Scholar]
- 101.Brawer MK, et al. Prostate-specific antigen: current status. CA Cancer J Clin. 1999;49:264–81. doi: 10.3322/canjclin.49.5.264. [DOI] [PubMed] [Google Scholar]




