Abstract
Two recently produced monoclonal antibodies were used to develop an antigen capture enzyme-linked immunosorbent assay (ELISA) for rapid diagnosis of Penicillium marneffei. The method was evaluated with 53 patients with culture-confirmed penicilliosis and 240 controls. The diagnostic sensitivity, specificity, and accuracy of the ELISA were 92.45, 97.5, and 96.59%, respectively.
In recent years, Penicillium marneffei has been gaining prominence as an opportunistic pathogen of AIDS patients in Southeast Asia, particularly Thailand (3, 16, 18). The fungus is the only known thermally dimorphic Penicillium species (14) and is immunologically distinct from all other Penicillium species (2, 11). Preliminary diagnosis of P. marneffei is established on the basis of a patient's clinical symptoms in conjunction with direct microscopic identification of fission yeasts from tissue specimens (6, 20). Direct fungal culture and species identification, which are rather time consuming, are then used to confirm the diagnosis (22). Despite the relative effectiveness of this approach, a limitation in the diagnosis of invasive penicilliosis remains. Successful management of invasive penicilliosis can be hampered by nonspecific symptoms of P. marneffei infection that often mimic those of tuberculosis, pneumocystosis, histoplasmosis, and several other mycotic infections, all of which are seen in patients infected with human immunodeficiency virus (HIV) (9). In addition, our group has previously presented evidence that initial, asymptomatic forms of penicilliosis do exist in HIV-seropositive individuals in areas where the disease is endemic (4). Hence, serological tests are still needed.
A number of diagnostic methods based on antibody detection have been developed. However, they have potential limitations because the majority of immunosuppressed AIDS patients have abnormal antibody response. Other limitations include false positivity due to prior exposure and low specificity due to cross-reactivity to other fungal pathogens (9). In the case of antigen detection, Kaufman and colleagues have developed an immunodiffusion assay and a latex agglutination test which uses polyclonal antibody against yeast culture filtrate of P. marneffei. Although both tests demonstrated high specificity, the sensitivities were rather low (12).
Here, we report the development and prospective evaluation of an enzyme-linked immunosorbent assay (ELISA) which employs two monoclonal antibodies (MAbs), namely, 8B11 and 8C3, that were produced and characterized earlier (19) for the detection of P. marneffei antigens in sera of humans in areas where the organism is endemic. A standard strain of P. marneffei (ATCC 64102) and other fungi were cultured and maintained under aerobic conditions on Sabouraud dextrose agar at 25°C. P. marneffei mycelial culture was converted to monomorphic yeast phase as described previously (4). Mycelial culture filtrate antigens (MCFAg) and yeast exoantigens (YEAg) were prepared as described by Chongtrakool et al. (4) and by Kaufman and Standard (10), respectively.
To generate polyclonal antibodies, rabbits were immunized with 108 P. marneffei yeast cells mixed with 0.5 ml of 1-mg/ml YEAg and suspended in complete Freund's adjuvant both subcutaneously and through footpads. Incomplete Freund's adjuvant was used in the second immunization. The rabbits then received a monthly intramuscular injection with the same antigen mixture but suspended in phosphate-buffered saline. A total of four inoculations were completed in 3 months. Serum titers against P. marneffei YEAg and MCFAg were evaluated by using indirect ELISA (19). Rabbit serum was purified by ion-exchange chromatography (17), and the purified rabbit immunoglobulin G was subsequently biotinylated as previously described (15).
A total of 293 serum specimens were used in the analysis. Of these, 53 were from HIV-seropositive adult Thai patients with culture-confirmed P. marneffei, a majority of whom were from areas where the organism is endemic. Thirty-eight specimens were from HIV-seropositive patients with other fungal infections, i.e., candidiasis, cryptococcosis, and histoplasmosis. Negative controls consisted of sera from healthy adults from both areas where P. marneffei is endemic (59 samples) and areas where it is not endemic (143 samples). For the penicilliosis antigen test, each well of a Nunc immunoplate was coated with 50 μl of 10-μg/ml rabbit anti-mouse immunoglobulins suspended in carbonate buffer. After washing, 50 μl of a MAb solution (a mixture of the two Mabs, each at a concentration of 10 μg/ml) was added. Then, the antibody-coated well was blocked with 5% nonfat dried milk suspension for 1 h at 37°C. The well was washed and 50 μl of human serum (1:10 dilution) was added, followed by incubation at 4°C overnight. Fifty microliters of 1.5-μg/ml biotinylated anti-P. marneffei antibody was added, and 3,3′,5,5′-tetramethyl benzidine was used as a chromogen to detect streptavidin-horseradish peroxidase reaction. The enzymatic reaction was determined from the optical density (OD) value measured at 450 nm.
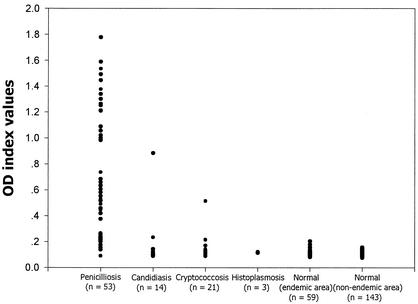
The ELISA cutoff value was chosen as the mean OD plus 3 standard deviations (SD). The MAb-based antigen capture ELISA was able to detect levels of P. marneffei MCFAg as low as 10 pg/ml and YEAg as low as approximately 200 pg/ml. The mean OD ± SD of the background in the test was 0.11 ± 0.02. No cross-reactivity was demonstrated when the ELISA was employed to detect various concentrations of other fungal antigens (Table 1). Subsequently, the diagnostic value of the ELISA was evaluated by using clinical specimens from patients whose cultures were positive for P. marneffei, Cryptococcus neoformans, Candida albicans, and Histoplasma capsulatum. The results are shown in Fig. 1. Sera of 202 healthy adults from both areas where P. marneffei is endemic and areas where it is not endemic as well as sera of patients with histoplasmosis were negative by the antigen capture ELISA. There were false-positive sera from six individuals who lived in areas of P. marneffei endemicity. Two were from patients diagnosed with cryptococcosis, while two others were from individuals with recurrent oral candidiasis. Since the two MAbs have been shown previously (19) to have no cross-reactivity to C. neoformans and C. albicans antigens, it was likely that subclinical or mixed fungal infections involving P. marneffei were present. The data support our earlier finding that P. marneffei antibody is present in asymptomatic, HIV-seropositive individuals from areas where P. marneffei is endemic (4). Additionally, sera from two healthy individuals who lived in areas of P. marneffei endemicity also demonstrated false positivity. It would therefore be interesting to observe whether these two patients will subsequently develop clinical penicilliosis.
TABLE 1.
Immunoreactivity of an antigen capture ELISA to crude preparations of fungal antigens
| Antigena | OD at 450 nmb |
|---|---|
| P. marneffei MCFAg | 1.540 |
| P. marneffei YEAg | 1.417 |
| C. albicans | 0.125 |
| C. neoformans | 0.151 |
| H. capsulatum | 0.154 |
| A. fumigatus | 0.163 |
| A. flavus | 0.194 |
| Culture medium (SDA) | 0.110 |
Fungal strains were obtained from the American Type Culture Collection, Rockville, Md.; the Microbiology Reference Center, Thailand Institute of Science and Technological Research, Bangkok; and Department of Clinical Pathology, Ramathibodi Hospital Faculty of Medicine, Mahidol University. The protein content of each fungal antigen preparation was adjusted to 1 μg/ml. A 50-μl volume was used for analysis. SDA, Sabourand dextrose agar.
Mean OD determined by duplicate assays performed on three separate occasions.
FIG. 1.
Scattergram showing the P. marneffei antigen OD index values of serum samples obtained from patients with P. marneffei, C. neoformans, and C. albicans infections as measured by the MAb-based antigen capture ELISA. Sera from healthy adults from both areas where P. marneffei is endemic and areas where it is not endemic were included as test controls.
The sensitivity, specificity, and accuracy of the ELISA when compared with the specimen culture method were 92.45, 97.5, and 96.59%, respectively (8). The sensitivity of the ELISA in this study was higher than those reported by Kaufman et al. (11, 12). The positive predictive value of the test was 89.09%, while the negative predictive value was 98.32% (Table 2). The kappa coefficient (k) and kappa probability value (Z) were calculated by the methods of Cohen (5) and Fleiss (7). k was 0.89, which indicates a degree of agreement between the ELISA and the culture that is beyond chance (13). Z was 15.18, which indicates that the ELISA results were reliable (P < 0.001) (5, 7). The reproducibility, which was calculated from OD readings of a reference sample and determined by duplicate assays performed on 20 separate occasions, was 92.47% (7).
TABLE 2.
Results of P. marneffei antigen capture ELISA compared with disease status as confirmed by specimen culturesa
| ELISA result | Disease status
|
Total | |
|---|---|---|---|
| Present | Absent | ||
| Positive | 49 | 6 | 55b |
| Negative | 4 | 234 | 238c |
| Total | 53 | 240 | 293 |
k = 0.89 and Z = 15.18.
Positive predictive value = 89.09%.
Negative predictive value = 98.32%.
The effectiveness of the ELISA implies the presence of P. marneffei carbohydrate antigens, mycelial glycoproteins, and two YEAg in the patients' sera, as has been reported previously (19). In immunosuppressed patients, the antigenemia test would be more useful when there was an increase in fungal antigen load. The only distinct problem of fungal antigen detection was the presence of cross-reactivity to antigens of closely related fungi. The previously developed latex agglutination test (21) and immunohistochemical staining (1) displayed some cross-reactivity between the common cell wall antigens of Aspergillus fumigatus and P. marneffei. In our case, no cross-reaction to A. fumigatus was detected. Although the two Mabs reacted weakly with Penicillium pinophilum antigens (19), there has been, fortunately, no reported case of P. pinophilum to date. Seronegative results obtained from healthy individuals who lived in areas where P. marneffei is prevalent would therefore be likely to represent true negative results.
In summary, the antigen capture ELISA described here should prove useful for the rapid serodiagnosis of P. marneffei and may be modifiable into a more convenient diagnostic kit. The assay method could be readily adapted for use in peripheral laboratories lacking well-trained microscopists. More importantly, the test should be applicable for the identification of individuals with nonspecific symptoms of P. marneffei infection as well as those who have subclinical forms of the disease.
Acknowledgments
This work was supported by the National Science and Technology Development Agency (Thailand) and the Chulabhorn Research Institute, Bangkok, Thailand.
We thank V. Vithayasai (Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand), R. Teanpaisan (Prince of Songkla University, Songkla, Thailand), and P. Chongtrakool (Department of Clinical Pathology, Ramathibodi Hospital, Mahidol University) for supplying serum. We appreciate the valuable suggestions on statistical analysis made by Montip Tiensuwan (Department of Mathematics, Faculty of Science, Mahidol University).
REFERENCES
- 1.Arrese Estrada, J., D. Stynen, J. Van Cutsem, C. Pierard-Franchimont, and G. E. Pierard. 1992. Immunohistochemical identification of Penicillium marneffei by monoclonal antibody. Int. J. Dermatol. 31:410-412. [DOI] [PubMed] [Google Scholar]
- 2.Brouwer, J. 1996. Cross reactivity between Aspergillus fumigatus and Penicillium. Int. Arch. Allergy Immunol. 110:166-173. [DOI] [PubMed] [Google Scholar]
- 3.Chiewchanvit, S., P. Mahanupab, P. Hirunsri, and N. Vanittanakorn. 1991. Cutaneous manifestations of disseminated Penicillium marneffei mycosis in five HIV-infected patients. Mycoses 34:245-249. [DOI] [PubMed] [Google Scholar]
- 4.Chongtrakool, P., S. C. Chaiyaroj, V. Vithayasai, S. Trewatcharegon, R. Teanpaisan, S. Kalnawakul, and S. Sirisinha. 1997. Immunoreactivity of a 38-kilodalton Penicillium marneffei antigen with human immunodeficiency virus-positive sera. J. Clin. Microbiol. 35:2220-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, J. A. 1960. A coefficient of agreement for nominal scale. Educ. Psychol. Meas. 20:37-46. [Google Scholar]
- 6.Drouhet, E. 1993. Penicilliosis due to Penicillium marneffei: a new emerging systemic mycosis in AIDS patients travelling or living in southeast Asia. Review of 44 cases reported in HIV infected patients during the last 5 years compared to 44 cases of non-AIDS patients reported over 20 years. J. Med. Mycol. 4:195-224. [Google Scholar]
- 7.Fleiss, J. L. 1981. The measurement of interrater agreement, p. 212-235. In Statistical methods for rates and proportions, 2nd ed. John Wiley & Sons, New York, N.Y.
- 8.Galen, R. S. 1980. The predictive values and efficiency of laboratory testing. Clin. North Am. 2:861-869. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton, A. J. 1998. Serodiagnosis of histoplasmosis, paracoccidiomycosis and penicilliosis marneffei: current status and future trends. Med. Mycol. 36:351-364. [PubMed] [Google Scholar]
- 10.Kaufman, L., and P. G. Standard. 1987. Specific and rapid identification of medically important fungi by exoantigen detection. Annu. Rev. Microbiol. 41:209-225. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman, L., P. G. Standard, A. Anderson, M. Jalbert, and B. L. Swisher. 1995. Development of specific fluorescent-antibody test for tissue form of Penicillium marneffei. J. Clin. Microbiol. 33:2136-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman, L., P. G. Standard, M. Jalbert, P. Kantipong, K. Limpakarnjanarat, and T. D. Mastro. 1996. Diagnostic antigenemia tests for penicilliosis marneffei. J. Clin. Microbiol. 34:2503-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landis, A. R., and G. G. Koch. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159-174. [PubMed] [Google Scholar]
- 14.LoBuglio, K. F., and J. W. Taylor. 1995. Phylogeny and PCR identification of human pathogenic fungus Penicillium marneffei. J. Clin. Microbiol. 33:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nerurkar, L. S., M. Namba, G. Brashears, A. J. Jacob, Y. J. Lee, and J. L. Sever. 1984. Rapid detection of herpes simplex virus in clinical specimens by use of a capture biotin-streptavidin enzyme-linked immunosorbent assay. J. Clin. Microbiol. 20:109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirisanthana, T., K. Suppataratpinyo, J. Perriens, and K. E. Nelson. 1998. Amphotericin B and itraconazole for treatment of disseminated Penicillium marneffei infection in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 26:1107-1110. [DOI] [PubMed] [Google Scholar]
- 17.Sirisinha, S., R. Chawengkirttikul, R. Sermswan, S. Amornpant, S. Mongkolsuk, and S. Panyim. 1991. Detection of Opisthorchis viverrini by monoclonal antibody-based ELISA and DNA hybridization. Am. J. Trop. Med. Hyg. 44:140-145. [DOI] [PubMed] [Google Scholar]
- 18.Suppataratpinyo, K., C. Kwamwan, V. Baosoung, K. E. Nelson, and T. Sirisanthana. 1994. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet 344:1739-1743. [DOI] [PubMed] [Google Scholar]
- 19.Trewatcharegon, S., S. C. Chaiyaroj, P. Chongtrakool, and S. Sirisinha. 2000. Production and characterization of monoclonal antibodies reactive with the mycelial and yeast phases of Penicillium marneffei. Med. Mycol. 38:91-96. [DOI] [PubMed] [Google Scholar]
- 20.Tsui, W. M. S., K. F. Ma, and D. N. Tsang. 1992. Disseminated Penicillium marneffei infection in HIV-infected subject. Histopathology 20:287-293. [DOI] [PubMed] [Google Scholar]
- 21.Van Cutsem, J., L. Meulemans, F. Van Gervan, and D. Stynen. 1990. Detection of circulating galactomannan by Pastorex Aspergillus in experimental invasive aspergillosis. Mycoses 33:61-69. [DOI] [PubMed] [Google Scholar]
- 22.Yuen, K. Y., S. S. Wong, D. N. Tsang, and P. Chau. 1994. Serodiagnosis of Penicillium marneffei infection. Lancet 344:444-445. [DOI] [PubMed] [Google Scholar]