Abstract
Human immunodeficiency virus type 1 (HIV-1) RNA was detected by means of ultrasensitive reverse transcription-PCR assay of 19 cervicovaginal lavage, 21 cervical mucus, 18 vaginal wall, and 17 vaginal fornix paired samples from 25 asymptomatic HIV-1-infected women (76, 84, 72, and 68%, respectively; P > 0.5). Levels of HIV-1 RNA in cervicovaginal wash samples were highly correlated with those in paired endocervical mucus samples (r = 0.71; P = 0.0006), indicating that cervicovaginal washing and endocervical swabbing are equivalent collection procedures.
The viral infectivity of genital secretions of human immunodeficiency virus type 1 (HIV-1)-infected women is usually assessed by measuring the size of the inoculum. Determination of HIV-1 RNA genital load is needed for studying the pathophysiology of female-to-male sexual transmission (9, 13, 14, 15) and mother-to-child perinatal transmission (5, 7) to assess the possible effect of antiretroviral drugs on HIV genital shedding (17) and to evaluate the influence of various cofactors of transmission on genital HIV replication (6, 10, 11, 16). Evaluation of female genital infectivity by HIV-1 RNA detection or quantitative reverse transcription-PCR assays remains to be standardized (1). An accurate collection procedure for genital fluids and well-standardized and reproducible qualitative and quantitative assays are prerequisites for the evaluation of HIV genital shedding (8). In the present study, two sample collection procedures for female genital secretions were compared to estimate the genital shedding of HIV-1 RNA in asymptomatic, untreated HIV-1-infected women.
Patients were selected from a cohort of 213 HIV-1-infected women (Bangui, Central African Republic), provided they were nonpregnant and free of cervicitis, sexually transmitted diseases, genital bleeding, and seminal pollution in the genital tract. The main demographic and biological characteristics of the study population have been previously reported (10).
Sterile dry swabs (Eurotubo, Rubi, Spain) were first used to collect genital secretions on the endocervix, the vaginal wall, and the vaginal fornix. The swabs were gently applied to the mucosal walls, and a slight pressure was applied by partly rotating the swabs without any mucosal traumatism. All the swab samples were rapidly stored dry at −30°C until use. Following genital swab sampling, whole vaginal secretions were collected by a standardized 60-s vaginal washing with 3 ml of phosphate saline buffer (pH 7.2), as previously described (2). After the cervicovaginal lavage sample was centrifuged at 1,000 × g for 10 min, the cell-free supernatant and the cellular pellet were collected and stored separately at −30°C. The presence of contaminating semen in vaginal secretions was determined by a Y-chromosome PCR assay of DNA extracted from the cellular pellet produced from the cervicovaginal secretion as described previously (4). Traces of hemoglobin in vaginal secretions were detected by spectrophotometry of the acellular fraction of genital fluids as described previously (17).
To quantify HIV-1 RNA levels from the swab samples, the tips of the swabs were first treated for 20 min in 200 μl of lysis buffer obtained from the Qiagen (Courtaboeuf, France) viral RNA minikit. Then, the following steps of RNA extraction were carried out according to the manufacturer's instructions. The ultrasensitive assay (HIV-1 Monitor Test 1.5; Roche Diagnostic Systems, Branchburg, N.J.) was used to quantify HIV-1 RNA. Briefly, 2 μg of total RNA extracted from each swab sample was diluted in a total volume of 50 μl of specimen diluent (provided by the manufacturer) containing a known number of copies of the RNA internal standard of the kit and amplified as recommended by the manufacturer's instructions (HIV-1 Monitor Test 1.5). We estimated that this assay has an analytical sensitivity of 25 input HIV RNA copies per amplification reaction tube containing 2 μg of total RNA extracted from female genital secretions, which corresponds to a threshold of 12.5 copies of HIV RNA per μg of total RNA. Results were expressed as log copies per microgram of total extracted RNA, allowing comparison of results obtained by the different swab collection procedures. To quantify HIV-1 RNA levels in the cervicovaginal wash samples, 500 μl of the acellular part of the vaginal secretions was initially precipitated by centrifugation at 23,600 × g for 60 min at 4°C, and the total RNA was extracted from the resulting pellet with the Qiagen viral RNA minikit (18). The HIV-1 Monitor Test 1.5 assay was again used for HIV-1 RNA quantitation. The threshold of the HIV RNA detection assay was estimated to be 50 copies per ml of cervicovaginal wash (18).
Chi-square, Fischer's exact, and Spearman's rank order correlation tests were used for statistical analyses. Linear regression analysis on log-transformed values was performed with the software Stat View SE.1 (Abacus Concepts, Inc., Berkeley, Calif.).
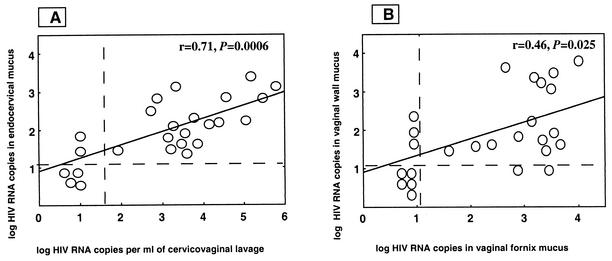
HIV-1 RNA was evidenced with similar rates of detection in 21 (84%) of endocervical swab samples, in 18 (72%) of vaginal wall samples, and in 17 (68%) of vaginal fornix paired samples (84% vs. 72% vs. 68%; P = 0.4). All patients whose endocervical secretion samples were without detectable HIV-1 RNA also produced negative vaginal wall and fornix swab samples. When cervicovaginal secretions were collected from the genital tract by a washing procedure, HIV-1 RNA was detected in 19 of 25 (76%) samples (Table 1), a rate similar to that obtained by swab collection (P = 0.47). Figure 1 shows the distributions of the HIV-1 RNA levels according to the sampling procedures. Levels of HIV-1 RNA in cervicovaginal lavage samples were highly correlated with HIV-1 RNA levels in paired endocervical mucus samples (r = 0.71; P = 0.0006) (Fig. 1A). In contrast, the levels of HIV-1 RNA in cervicovaginal samples did not appear correlated with HIV-1 RNA loads in paired vaginal wall secretion samples (r = 0.40; P = 0.055) (not shown) and with those measured in paired vaginal fornix secretion samples (r = 0.30; P = 0.14) (not shown). HIV-1 RNA levels in vaginal wall swab samples were correlated with those found in fornix swab samples (r = 0.46; P = 0.025) (Fig. 1B). In contrast, amounts of HIV-1 RNA in endocervical mucus samples were not correlated with HIV-1 RNA levels in paired vaginal wall swab samples (r = 0.39; P = 0.06) (not shown) and with those in paired vaginal fornix secretion samples (r = 0.37; P = 0.08) (not shown).
TABLE 1.
Detection of HIV-1 RNA in cervicovaginal secretions from 25 asymptomatic, untreated HIV-1-infected African women collected by vaginal washing or by endocervical, vaginal wall, and vaginal fornix swabbing
| Patient no. | Age (yr) | HIV-1 RNA quantitative detectionc by:
|
|||
|---|---|---|---|---|---|
| Cervicovaginal washing procedure (log HIV RNA copies/ml)a | Swabbing procedure (log HIV RNA copies/μg of total RNA)b
|
||||
| Endocervix | Vaginal wall | Vaginal fornix | |||
| 1 | 18 | <1.69 | 1.491 | <1.1 | <1.1 |
| 2 | 24 | <1.69 | <1.1 | <1.1 | <1.1 |
| 3 | 25 | 1.924 | 1.447 | 1.255 | 3.554 |
| 4 | 20 | 3.468 | 1.613 | 3.973 | 4.014 |
| 5 | 31 | 3.279 | 2.1 | 3.358 | 3.175 |
| 6 | 28 | 3.326 | 3.122 | 3.492 | 3.556 |
| 7 | 25 | 4.348 | 2.255 | 1.929 | 3.561 |
| 8 | 28 | 3.785 | 2.32 | 1.875 | 2.928 |
| 9 | 23 | 2.881 | 2.818 | 1.322 | 1.462 |
| 10 | 32 | 5.792 | 3.127 | 3.908 | 2.663 |
| 11 | 20 | <1.69 | <1.1 | <1.1 | <1.1 |
| 12 | 30 | 5.045 | 2.238 | 1.633 | 3.568 |
| 13 | 25 | 2.716 | 2.481 | 2.176 | 3.122 |
| 14 | 24 | 5.456 | 2.838 | 1.301 | 2.176 |
| 15 | 24 | 5.173 | 3.389 | 1.756 | 3.538 |
| 16 | 30 | <1.69 | 1.973 | <1.1 | 3.447 |
| 17 | 27 | 3.21 | 1.477 | 1.826 | 2.897 |
| 18 | 26 | 3.459 | 1.732 | 1.924 | <1.1 |
| 19 | 20 | 3.609 | 1.491 | 3.289 | 3.312 |
| 20 | 21 | <1.69 | <1.1 | <1.1 | <1.08 |
| 21 | 30 | 3.875 | 1.613 | 1.633 | 2.382 |
| 22 | 22 | 4.565 | 2.843 | 2.364 | <1.1 |
| 23 | 25 | <1.69 | <1.1 | <1.1 | <1.1 |
| 24 | 29 | 4.388 | 2.188 | <1.1 | <1.1 |
| 25 | 23 | 3.56 | 1.892 | 3.066 | 3.498 |
| Mean ± SD | 3.191 ± 1.53 | 2.018 ± 0.72 | 1.963 ± 0.99 | 2.434 ± 1.13 | |
Threshold of detection, 50 copies of viral RNA (1.69 log HIV RNA copies) per ml. The data are given per milliliter of vaginal lavage without taking into account the dilution factor introduced by the vaginal washing procedure (1:10).
A threshold of 12.5 copies of HIV RNA per μg of total RNA (1.1 log HIV RNA copies/μg of total RNA) was estimated in swab samples.
An arbitrary value of 10 HIV-1 RNA copies was used when HIV RNA was undetectable in the vaginal lavage or in genital swab samples.
FIG. 1.
Distribution of HIV-1 RNA levels in genital secretions from 25 HIV-1-infected African women. (A) Correlation between levels of HIV-1 RNA in cervicovaginal secretion lavage samples (acellular fraction) and in the endocervical secretions collected by swabbing. (B) Correlation between levels of HIV-1 RNA in vaginal fornix secretions and in vaginal wall secretions collected by swabbing. Vertical and horizontal dashed lines, threshold of positive detection. The threshold of HIV-1 RNA detection is 1.69 log copies/ml for wash samples and 1.1 log copies/μg of total RNA for swab samples. An arbitrary value of 10 HIV-1 RNA copies was used when HIV-RNA was undetectable in the vaginal washing or in genital swab samples.
In the present study, our results indicated that the endocervical swabbing procedure was equivalent to the vaginal washing procedure for detecting HIV-1 RNA and for quantifying the HIV-1 RNA levels in the genital tracts of 25 clinically asymptomatic, untreated HIV-1-infected African women. To reduce the possible methodological bias in data interpretation, criteria for the selection of the study patients included the lack of genital infection or inflammation and the lack of recent sexual intercourse as assessed by the negativity of a Y-chromosome PCR assay for cervicovaginal secretions. The endocervical swabbing procedure did not appear to be significantly more sensitive than the vaginal washing procedure for qualitatively detecting HIV-1 RNA in genital fluids. Our results did not confirm previous studies showing higher rates of HIV-1 RNA detection in cervical swab samples than in paired vaginal wash samples (1, 8). In these two previous studies, the authors did not use an ultrasensitive assay for HIV-1 RNA detection; this is the likely explanation for the lack of sensitivity to low levels of HIV-1 RNA in vaginal wash samples (1, 8).
The endocervical swabbing procedure allowed an evaluation of the production of HIV at the level of the main reservoir of the virus in the female genital tract, which is mainly located within the submucosa of the cervix (12). In contrast, virus obtained from vaginal wash samples may be considered a mixture of cervix-originating HIV and HIV particles pasted on the mucous underlining the vaginal walls. The absence of correlation between HIV-1 RNA levels in secretions collected by vaginal wash and those in both vaginal wall and fornix swabs, in contrast to the high correlation between HIV-1 RNA levels in vaginal wash secretions and those in cervical swab samples, suggested that cervical HIV may represent the major origin of the virus recovered by the cervicovaginal washing procedure (Fig. 1). However, the presence of contaminated HIV-1-infected cells in the swab samples could partly explain the lack of significant correlation between HIV-1 RNA levels in vaginal wall or fornix swab samples and those in acellular vaginal wash samples.
In conclusion, cervicovaginal washing and endocervical swabbing appeared to be equivalent procedures for collecting genital fluids. Nevertheless, collection of cervicovaginal secretions by means of the vaginal washing technique may be an interesting method for performing immunological investigations of the acellular part of the genital sample (3). The endocervical swabbing procedure may be more representative of the virus directly produced by the main reservoir of HIV in the female genital tract and therefore may be particularly interesting when studying factors governing the genital infectivity of HIV-1-infected women.
Acknowledgments
We thank Fredric Eberlé from Roche Diagnostic France for providing kits for the study and all the attendees of the Centre National de Référence des Maladies Sexuellement Transmissibles et du SIDA of Bangui.
This work was supported by the Agence Nationale de Recherches sur le SIDA, Paris, France (grant FF-111-F).
REFERENCES
- 1.Baron, P., J. Bremer, S. S. Wasserman, N. Mareck, B. Driscoll, B. Polsky, A. Kovacs, and P. S. Reichelfelder. 2000. Detection and quantification of human immunodeficiency virus type I in the female genital tract. J. Clin. Microbiol. 38:3822-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bélec, L., D. Meillet, M. Levy, A. Georges, C. Tevi-Benissan, and J. Pillot. 1995. Dilution assessment of cervicovaginal secretions obtained by vaginal washing for immunological assays. Clin. Diagn. Lab. Immunol. 2:57-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bélec, L., P. D. Ghys, H. Hocini, J. N. Nkengasong, J. Tranchot-Diallo, M. O. Diallo, V. Ettiegne-Traore, C. Maurice, P. Becquart, M. Matta, A. Si-Mohamed, N. Chomont, I. M. Coulibaly, S. Z. Wiktor, and M. D. Kazatchkine. 2001. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J. Infect. Dis. 184:1412-1422. [DOI] [PubMed] [Google Scholar]
- 4.Chomont, N., G. Gresenguet, M. Levy, H. Hocini, P. Becquart, M. Matta, J. Tranchot-Diallo, L. Andreoletti, M. P. Carreno, M. D. Kazatchkine, and L. Belec. 2001. Polymerase chain reaction for Y chromosome to detect semen in cervicovaginal fluids: a prerequisite to assess HIV-specific vaginal immunity and HIV genital shedding. AIDS 15:801-802. [DOI] [PubMed] [Google Scholar]
- 5.Chuachoowong, R., N. Shaffer, W. Siriwasin, P. Chaisilwattana, N. L. Young, P. A. Mock, S. Chearskul, N. Waranawat, T. Chaowanachan, J. Karon, R. J. Simonds, T. D. Mastro, et al. 2000. Short-course antenatal zidovudine reduces both cervicovaginal human immunodeficiency virus type 1 RNA levels and risk of perinatal transmission. J. Infect. Dis. 181:99-106. [DOI] [PubMed] [Google Scholar]
- 6.Clemetson, D. B. A., G. B. Moss, D. M. Willerford, M. Hensel, W. Emonyi, K. K. Holmes, F. Plummer, J. Ndinyia-Achola, P. L. Roberts, S. Hiller, and J. K. Kreiss. 1993. Detection of HIV DNA in cervical and vaginal secretions. JAMA 269:2860-2864. [PubMed] [Google Scholar]
- 7.Garcia, P. M., L. A. Kalish, J. Pitt, H. Minkoff, T. C. Quinn, S. K. Burchett, J. Kornegay, B. Jackson, J. Moye, C. Hanson, C. Zorrilla, J. F. Lew, et al. 1999. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N. Engl. J. Med. 341:394-402. [DOI] [PubMed] [Google Scholar]
- 8.John, G. C., H. Sheppard, D. Mbori-Ngacha, R. Nduati, D. Maron, M. Reiner, and J. Kreiss. 2001. Comparison of techniques for HIV-1 detection and quantitation in cervicovaginal secretions. J. Acquir. Immune Defic. Syndr. 26:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovacs, A., S. S. Wasserman, D. Burns, D. J. Wright, J. Cohn, A. Landay, K. Weber, M. Cohen, A. Levine, H. Minkoff, P. Miotti, J. Palefsky, M. Young, and P. Reichelderfer. 2001. Determinants of HIV-1 shedding in the genital tract of women. Lancet 358:1593-1601. [DOI] [PubMed] [Google Scholar]
- 10.Mbopi-Keou, F. X., G. Gresenguet, P. Mayaud, H. A. Weiss, R. Gopal, M. Matta, J. L. Paul, D. W. Brown, R. J. Hayes, D. C. Mabey, and L. Belec. 2000. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J. Infect. Dis. 182:1090-1096. [DOI] [PubMed] [Google Scholar]
- 11.Mostad, S. B. 1998. Prevalence and correlates of HIV type 1 shedding in the female genital tract. AIDS Res. Hum. Retroviruses 14:S4-S15. [PubMed] [Google Scholar]
- 12.Nuovo, G. J., A. Forde, P. MacConnell, and R. Fahrenwald. 1993. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am. J. Pathol. 143:40-48. [PMC free article] [PubMed] [Google Scholar]
- 13.Pedraza, M. A., J. del Romero, F. Roldan, S. Garcia, M. C. Ayerbe, A. R. Noriega, and J. Alcami. 1999. Heterosexual transmission of HIV-1 is associated with high plasma viral load levels and a positive viral isolation in the infected partner. J. Acquir. Immune Defic. Syndr. 21:120-125. [PubMed] [Google Scholar]
- 14.Quinn, T., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, R. H. Gray, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 15.Royce, R. A., A. Sena, W. J. Cates, and M. S. Cohen. 1997. Sexual transmission of HIV. N. Engl. J. Med. 336:1072-1078. [DOI] [PubMed] [Google Scholar]
- 16.Schacker, T., A. J. Ryncarz, J. Goddard, K. Diem, M. Shaughnessy, and L. Corey. 1998. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA 280:61-66. [DOI] [PubMed] [Google Scholar]
- 17.Si-Mohamed, A., M. D. Kazatchkine, I. Heard, C. Goujon, T. Prazuck, G. Aymard, G. Cessot, Y. H. Kuo, M. C. Bernard, B. Diquet, J. E. Malkin, L. Gutmann, and L. Bélec. 2000. Selection of drug-resistant variants in the female genital tract of human immunodeficiency virus type 1-infected women receiving antiretroviral therapy. J. Infect. Dis. 182:112-122. [DOI] [PubMed] [Google Scholar]
- 18.Si-Mohamed, A., L. Andréoletti, I. Colombet, M.-P. Carreno, G. Lopez, G. Chatelier, M. D. Kazatchkine, and L. Bélec. 2001. Quantitation of human immunodeficiency virus type 1 (HIV-1) RNA in cell-free cervicovaginal secretions: comparison of reverse transcription-PCR amplification (AMPLICOR HIV-1 MONITOR 1.5) with enhanced-sensitivity branched-DNA assay (Quantiplex 3.0). J. Clin. Microbiol. 39:2055-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]