Abstract
This study was undertaken in an effort to improve the identification scheme of catalase-negative, non-beta-hemolytic, gram-positive cocci isolated from milk samples obtained from cows. First, the sensitivity and specificity of the identification procedure currently in use in our laboratory were compared to the results obtained with API 20 STREP strips which were set as the gold standard. Second, a number of other identification tests, which could contribute to increase the sensitivity and specificity of the identification procedure of these microorganisms, were evaluated and selected. The data have shown that there is a necessity to review the identification procedure. Some modifications are suggested to laboratories doing milk sample analyses. A standardized procedure, using the CAMP test, esculin and sodium hippurate hydrolysis, the presence of the enzymes pyrolidonyl arylaminase and leucine aminopeptidase, and acid production from 1% inulin and raffinose broth, would not only improve the results of the identification process of gram-positive cocci isolated from milk samples but also ensure greater uniformity of the epidemiological data.
Several laboratory tests are suggested in the National Mastitis Council (NMC) guide for the identification of streptococci and enterococci isolated from bovine milk samples. These tests include the CAMP test, esculin hydrolysis (ESC), sodium hippurate hydrolysis (HIP), acid production from inulin (INU) and trehalose, growth in a broth containing 6.5% NaCl, and Lancefield grouping (6). For several years, at the clinical microbiology laboratory of the Faculté de Médecine Vétérinaire (FMV), Université de Montréal, identification of streptococci and other gram-positive, catalase-negative cocci isolated from milk samples has been carried out with the following tests: CAMP, ESC, acid production from raffinose (RAF), growth in a broth containing 6.5% NaCl, and Lancefield grouping. When required, additional tests were done. These tests were chosen from among those suggested by the NMC or were taken from publications on the subject (1, 6).
Recent findings have prompted microbiologists to question the routine procedures being used for the identification of non-beta-hemolytic, catalase-negative, gram-positive cocci isolated from milk samples. For example, it was found that growth in a broth containing 6.5% NaCl was not a valuable discriminatory test for enterococci and streptococci (5). The identification of Streptococcus uberis, a frequent agent of bovine mastitis, is even more problematic since this species does not react with any of the Lancefield-group antisera. Also, with the progress made in the taxonomic study of gram-positive cocci and improvement of the identification tools, scientists in several laboratories realize that many species belonging to genera such as Aerococcus, Lactococcus, Leuconostoc, and other Streptococcus that differ from Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus bovis, and S. uberis can be found in milk samples and are not part of the standard identification scheme for bacteria isolated from milk samples (1). The main objective of this study was to improve the identification scheme of non-beta-hemolytic, catalase-negative, gram-positive cocci isolated from milk samples by proposing a protocol utilizing appropriate tests.
MATERIALS AND METHODS
Origin of isolates.
The initial sampling plan was to collect approximately 100 isolates from each of the following bacterial species: S. dysgalactiae, S. uberis, S. bovis, and Enterococcus spp. Over a period of 9 months (August 1999 to April 2000), a total of 406 isolates were collected. For each of the bacterial species, only one isolate per dairy farm was kept. A portion of the isolates collected (80%) were from milk samples received at the clinical microbiology laboratory of the FMV. The remaining isolates (20%) were obtained from milk samples sent to the Saint-Hyacinthe and Sainte-Foy laboratories of the Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec. All isolates were kept frozen at −70°C until used.
The identities of the isolates received from external sources were confirmed by the biochemical tests in use at the FMV. These tests included CAMP and ESC that were done on a blood agar plate (heart infusion agar [Difco, Detroit, Mich.] supplemented with 5% citrated sheep blood) to which 1% esculin (Sigma-Aldrich Canada Ltd., Oakville, Ontario, Canada) was added; acid production from 1% RAF in a purple broth base (Difco); growth in brain heart infusion with 1% glucose (Difco), bromocresol purple as a pH indicator, and 6.5% NaCl (Difco); and Lancefield grouping with the Patho-DX kit (Inter Medico, Marklam, Ontario, Canada). With these tests, the number of isolates identified according to the initial identification scheme (Table 1) were 102 S. dysgalactiae, 101 S. uberis, 103 S. bovis, and 100 Enterococcus spp.
TABLE 1.
Initial identification schemea used for identification of non-beta-hemolytic, catalase-negative, gram-positive cocci recovered from milk specimens
| Strain identification | Result from test:
|
||||
|---|---|---|---|---|---|
| CAMP | ESC | NaCl | RAF | Lancefield group | |
| Enterococcus spp. | NAb | + | + | − | NA |
| S. uberis | −Vc | + | − | − | NA |
| S. bovis | − | + | − | + | NA |
| S. dysgalactiae | − | −V | − | − | C |
Adapted from reference 6 with permission of the publisher.
NA, not applicable.
−V, 80 to 90% were negative.
Tests used in this study.
In addition to the five tests mentioned above that were used at the FMV as standard tests for the identification of streptococci isolated from milk samples, five other tests were evaluated. These tests are the hydrolysis of 1% sodium hippurate (Sigma), the presence of the enzymes pyrolidonyl arylaminase (PYR) (R7112 disk; PML Microbiologicals, Saint-Laurent, Quebec, Canada) and leucine aminopeptidase (LAP) (DLDL 1050 discs; Oxoid, Nepean, Ontario, Canada), acid production from 1% INU in a purple broth base (Difco), and growth at 45°C in brain heart infusion (Difco) in a water bath. Finally, API strips (20 STREP and 32 STREP; BioMérieux Canada, Inc., Saint-Laurent, Quebec, Canada) were chosen as the gold standard for identification.
Statistical analyses.
Sensitivity and specificity of the initial identification results relative to the identification by using the API 20 STREP strip were estimated. A discriminatory function was estimated in order to decide which tests would allow better discrimination between the catalase-negative, gram-positive cocci relative to the results obtained with the API 20 STREP strip (version 7.0; BMDP Statistical Software, Inc., Los Angeles, Calif.). Sensitivity and specificity of the initial identifications relative to the identifications obtained by using the API 20 STREP strip were calculated and compared to the relative sensitivity and specificity determined by using the discriminatory function. The McNemar chi-square test was used to compare the proportions.
RESULTS
The identification results of the 406 isolates with the five tests used currently at the FMV were compared only to those on the API 20 STREP strip. The color changes in the API 32 STREP strip were more difficult to interpret than those in the API 20 STREP strip. For this reason, it was decided that the results of the conventional tests would be compared only to the results of the API 20 STREP strip test.
Only 23% of Enterococcus spp. were correctly identified by the conventional procedure (Table 2). Other isolates identified by the conventional procedure as Enterococcus spp. were identified by API 20 STREP as Aerococcus spp., S. uberis, or Lactococcus spp. There was agreement between the two identification procedures for 70% of the S. bovis isolates and 69% of the S. uberis isolates. For S. dysgalactiae, no major discrepancies were noted, as the agreement between the two methods was 91%.
TABLE 2.
Comparison between results of the conventional identification procedure and the API 20 STREP system for the identification of 406 isolates of catalase-negative, non-beta-hemolytic, gram-positive cocci recovered from milk specimens
| API 20 STREP identification result | No. of results obtained by conventional identification procedures
|
|||
|---|---|---|---|---|
| Enterococcus spp. | S. bovis | S. dysgalactiae | S. uberis | |
| Aerococcus spp. | 48 | 5 | 0 | 2 |
| Enterococcus spp. | 23 | 14 | 0 | 5 |
| Lactococcus spp. | 11 | 3 | 1 | 24 |
| S. uberis | 13 | 1 | 1 | 69 |
| S. bovis | 0 | 73 | 0 | 0 |
| S. dysgalactiae | 0 | 0 | 93 | 0 |
| Others | 5 | 7 | 7 | 0 |
| Total | 100 | 103 | 102 | 101 |
The CAMP, ESC, NaCl, RAF, INU, growth at 45°C, PYR, LAP, HIP, and Lancefield grouping tests were chosen as variables for discriminatory analysis. The isolates identified as Aerococcus spp., Enterococcus spp., Lactococcus spp., and S. uberis by API 20 STREP (n = 220) were the only ones included in the discriminatory analysis. It was noted that the initial identification procedure could be misleading in regard to the identification of S. uberis. With this latter species, of the isolates initially identified as S. uberis, 24% were found to be Lactococcus spp., 5% were found to be Enterococcus spp., and 2% were found to be Aerococcus spp. (Table 2). Isolates belonging to the genera Aerococcus and Lactococcus made up an important proportion (23% of 406 isolates) of the gram-positive cocci isolated from milk samples that were initially identified as Enterococcus spp., S. uberis, or S. bovis.
Following the discriminatory analysis, only the LAP, HIP, INU, RAF, and NaCl variables were chosen to make up the classification function (Table 3). The level of exact classification was 85%. The LAP test was found to be the most discriminatory for the identification of isolates of the Aerococcus genus, which indicated that it should be the first test done. The HIP and INU tests were found to be the most discriminatory after the LAP test for the identification of S. uberis. All isolates of S. uberis gave a positive reaction with the HIP test, and most of the isolates (90%) were positive with the INU test.
TABLE 3.
Classification functionsa for different tests to sort into the groups Aerococcus spp., Enterococcus spp., Lactococcus spp., and S. uberis
| Variable | Coefficient for isolate
|
|||
|---|---|---|---|---|
| Aerococcus spp. | Enterococcus spp. | Lactococcus spp. | S. uberis | |
| NaCl | 5.68 | 5.64 | 2.18 | 0.88 |
| RAF | 1.26 | 4.83 | 1.75 | −1.62 |
| INU | 1.44 | 1.80 | 1.51 | 9.91 |
| LAP | 2.42 | 112.47 | 111.94 | 111.03 |
| HIP | 6.56 | −0.85 | 1.22 | 7.76 |
| Constant | −6.75 | −61.13 | −58.17 | −65.21 |
A classification function was computed for each group of bacteria by adding coefficients for each positive test. The bacterial group with the highest classification function was the most likely classification of the bacteria examined.
A retrospective redistribution of the Enterococcus and S. uberis isolates was done according to the results obtained with the original identification scheme and completed with the results of the additional tests done with the discriminatory function. The specificity for the identification of S. uberis increased from 90.4 to 99.4% (P < 0.0001), while the sensitivity did not vary significantly (82.1 to 88.1%, P = 0.36). For Enterococcus spp., the sensitivity increased from 54.8 to 83.3% (P = 0.006) and the specificity increased from 79.5 to 97.9% (P < 0.0001). Members of the genus Aerococcus that were initially identified as Enterococcus spp. could be distinguished from actual Enterococcus spp. on the basis of a negative LAP test. Based on the discriminatory function, 90% of the Aerococcus isolates were correctly identified.
The new tests used did not bring about any change in the identification of S. bovis. However, upon examination of our records, we realized that some isolates had been classified on the basis of three tests (CAMP, ESC, and RAF), the result of the NaCl test not having been considered. Since the reaction was always negative with S. bovis, the NaCl test must be part of the identification scheme for this bacterial species. A new classification taking the result of this test into consideration increased the specificity to 96.5% up from 91.3% (P < 0.0001) for S. bovis.
It should be noted that in several identification tables, including those found in the NMC guide, results of the NaCl test for S. uberis and S. dysgalactiae were negative when they should have been indicated as variable. In this study, almost 32% of the S. uberis isolates and 40% of the S. dysgalactiae isolates were NaCl positive.
DISCUSSION
This study has shown that the procedure commonly used to identify non-beta-hemolytic, catalase-negative, gram-positive cocci isolated from milk samples and based on the CAMP, ESC, NaCl, RAF, and Lancefield grouping tests can result in a relatively high number of misidentifications. Sensitivity and specificity were at their lowest value in the identification of Enterococcus spp. The following test result profile is inadequate for the identification of Enterococcus spp.: CAMP, negative; ESC, positive; NaCl, positive; and RAF, negative. The use of the NaCl test in the Enterococcus identification scheme has to be questioned. In a previous study, it was mentioned that identification of Enterococcus spp. is difficult and that species identification is considered a prerequisite to genus identification (2).
It was found that several isolates identified as S. uberis were in fact Lactococcus spp. On the other hand, isolates identified as Enterococcus spp., based on an NaCl positive result, were in fact S. uberis. In several diagnostic laboratories, it was often concluded that gram-positive cocci isolated from cows with clinical or subclinical mastitis that are negative with the catalase and CAMP tests and positive with the ESC test are S. uberis (1). This bacterial species is known to be, along with S. agalactiae and S. dysgalactiae, an important cause of bovine mastitis (6), and it is in the best interest of diagnostic laboratories to increase the sensitivity and specificity of their identification procedure by adding the HIP and INU tests for the identification of S. uberis. In the case where the INU test result is negative (10%), the mucoid appearance of the colonies of some isolates of S. uberis as well as the presence of long strings of gram-positive cocci can be indicative of the species identity. Use of the API 20 STREP test may be useful to confirm the identity of an isolate obtained from a cow with mastitis when routine tests give a doubtful identification.
The most common mistake made in identifying S. bovis was to identify it as Enterococcus spp. In reviewing older cases, it has been noted that isolates positive in the NaCl and RAF tests were identified as S. bovis based on the fact that a great number of streptococcal species are known to grow in a broth containing 6.5% NaCl. However, it is currently accepted that S. bovis always gives a negative result by the NaCl test and that this test is crucial for differentiation from Enterococcus spp. (4). As for the RAF test, all S. bovis isolates are positive and it is a good test to include in the identification scheme (S. Larivière, personal communication). The test results for S. bovis were as follows: CAMP, positive; ESC, positive; RAF, positive; and NaCl, negative.
For S. dysgalactiae, the tests used in the initial identification procedure had good specificity and sensitivity. In a retrospective study, it was found that nine isolates were misidentified as S. dysgalactiae and in all cases, the agglutination test to confirm that the isolate belonged to Lancefield group C was not done. It was found that none of the nine isolates belonged to group C and should have been identified as a species different from S. dysgalactiae, indicating the importance of serogrouping to confirm the identity of this species.
Identification of Aerococcus spp. is rarely done in a veterinary diagnostic laboratory. Their distinctive microscopic morphology (gram-positive cocci in tetrads) and a negative LAP test result allow for adequate identification.
In recent years, taxonomic studies of bacteria have allowed for the characterization of an even greater number of potential pathogenic agents (1, 3). This has prompted microbiologists to propose that a greater number of tests be used to better identify catalase-negative, gram-positive cocci isolated from milk samples (1, 3). The API 20 STREP test has proven to be a suitable alternative to fulfill this need (1, 3). However, its cost may be too high for most laboratories that wish to offer analyses at an affordable cost, especially in herd-monitoring programs where regular sampling of all cows is done at the start of the milk production period.
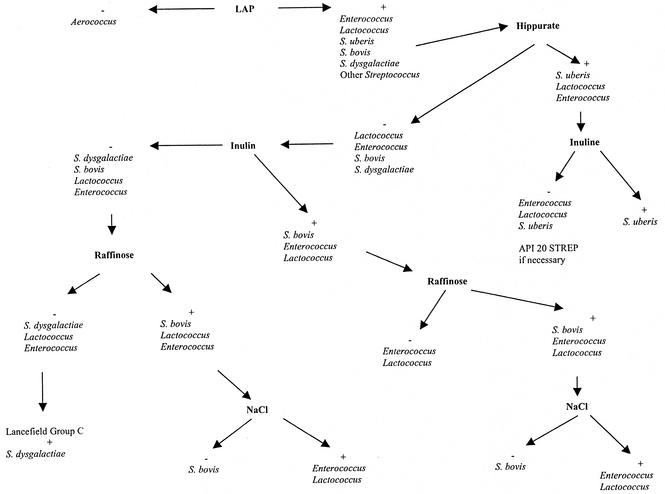
We propose an identification scheme based on standard tests that is affordable and will improve the identification of S. uberis, S. dysgalactiae, S. bovis, and Aerococcus spp. (Fig. 1). However, difficulties remain in the distinction between Lactococcus spp. and Enterococcus spp. since their test results are quite similar (5, 7).
FIG. 1.
Proposed flow chart for the identification of catalase-negative, non-beta-hemolytic, gram-positive cocci isolated from a milk sample.
The CAMP, ESC, LAP, INU, RAF, and HIP tests should be routinely done, and the NaCl test should be used occasionally only as an additional test to confirm the identification of S. bovis. Lancefield serogrouping should always be used to confirm the identification of S. dysgalactiae. As for the API 20 STREP test, it should be used to identify isolates obtained from a mastitis case and when the results of conventional tests are inconclusive.
We were able to measure the performance of our initial identification scheme and to propose an improved identification scheme that will give us a better appreciation of the prevalence of the different catalase-negative, gram-positive cocci isolated from mastitis cases. This identification scheme could also allow better monitoring of the treatment procedures applied in herds with mastitis problems.
Based on the findings of the present study, a review of the identification scheme and of the test results used for the identification of some of the bacteria associated with bovine mastitis should be done to increase the sensitivity and specificity of the identification process. This review may also result in better standardization among diagnostic laboratories.
REFERENCES
- 1.Devriese, L. A., J. Hommez, H. Leevers, B. Pot, P. Vandamme, and F. Haesebrouck. 1999. Identification of aesculin-hydrolysing streptococci, lactococci, aerococci and enterococci from subclinical intramammary infection in dairy cows. Vet. Microbiol. 70:87-94. [DOI] [PubMed] [Google Scholar]
- 2.Devriese, L. A., B. Pot, and M. D. Collins. 1993. Phenotypic identification of the genus Enterococcus and differentiation of phylogenetically distinct enterococcal species and species groups. J. Appl. Bacteriol. 75:399-408. [DOI] [PubMed] [Google Scholar]
- 3.Devriese, L. A., P. Vandamme, M. D. Collins, N. Alvarez, B. Pot, J. Hommez, P. Butaye, and F. Haesebrouck. 1999. Streptococcus pluranimalium sp. nov. from cattle and other animals. Int. J. Syst. Bacteriol. 49:1221-1226. [DOI] [PubMed] [Google Scholar]
- 4.Euzéby, J. P. 7June1998, posting date. Dictionnaire de bactériologie vétérinaire. Entries for Streptococcus bovis, Streptococcus equinus, and Streptococcus gallyticus. [Online.] Société de Bactériologie Systématique et Vétérinaire, Toulouse, France. http://www.bacterio.cict.fr/bacdico/ss/html
- 5.Facklam, R. R., D. F. Sahm, and L. M. Teixeira. 1999. Enterococcus, p. 297-305. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.) Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 6.National Mastitis Council. 13November2002, revision date. Laboratory handbook on bovine mastitis, rev. ed. [Online.] National Mastitis Council, Madison, Wis. http://www.nmconline.org.
- 7.Teixeira, L. M., M. G. Carhudo, V. L. Merquior, A. G. Steigewalt, M. G. Teixeira, D. J. Brenner, and R. R. Facklam. 1997. Recent approaches on the taxonomy of the enterococci and some related microorganisms. Adv. Exp. Med. Biol. 418:397-400. [DOI] [PubMed] [Google Scholar]