Abstract
OBJECTIVE
To assess the feasibility and efficacy of two interventions for improving adherence to antiretroviral therapy regimens in HIV-infected subjects compared with a control intervention.
DESIGN
Randomized, controlled, pilot study.
SETTING
Department of Veterans Affairs HIV clinic and community-based HIV clinical trials site.
PARTICIPANTS
Fifty-five HIV-infected subjects on stable antiretroviral therapy regimens. Subjects were predominantly male (89%) and African American (69%), and had histories of heroin or cocaine use (80%).
INTERVENTIONS
Four weekly sessions of either nondirective inquiries about adherence (control group, C), cue-dose training, which consisted of the use of personalized cues for remembering particular dose times, and feedback about medication taking using Medication Event Monitoring System (MEMS) pill bottle caps, which record time of bottle opening (CD group), or cue-dose training combined with cash reinforcement for correctly timed bottle opening (CD+CR).
MEASUREMENTS
Opening of the pill bottle within 2 hours before or after a predetermined time was measured by MEMS.
RESULTS
Adherence to the medication as documented by MEMS was significantly enhanced during the 4-week training period in the CD+CR group, but not in the CD group, compared with the control group. Improvement was also seen in adherence to antiretroviral drugs that were not the object of training and reinforcement. Eight weeks after training and reinforcement were discontinued, adherence in the cash-reinforced group returned to near-baseline levels.
CONCLUSIONS
Cue-dose training with cash reinforcement led to transient improvement in adherence to antiretroviral therapy in a population including mostly African Americans and subjects with histories of drug abuse. However, we were not able to detect any sustained improvement beyond the active training period, and questions concerning the timing and duration of such an intervention require further study. Randomized, controlled clinical studies with objective measures of adherence can be conducted in HIV-infected subjects and should be employed for further evaluation of this and other adherence interventions.
Keywords: adherence, MEMS, contingency management, HIV infection, antiretroviral
Highly active antiretroviral therapy (HAART) lowers plasma levels of HIV and reduces morbidity and mortality from HIV infection.1–3 Current HAART regimens require the use of 3 or more medications, most with 2 or more daily doses. The effectiveness of antiretroviral therapy for HIV infection is believed to depend heavily on patients' ability to adhere closely to complicated multidrug regimens. Although there has been considerable effort devoted to quantifying adherence and to establishing correlations between adherence and virologic outcomes, there has been little attempt to apply rigorous clinical research methodology to the study of interventions designed to improve adherence. In a recent review of studies to improve adherence in a number of areas, only 13 randomized, controlled clinical studies were identified.4 Although lack of adherence to treatment regimens is felt to be the most serious limitation of HAART, no intervention has been shown to improve objectively verified adherence in a randomized, controlled clinical trial.
Several features of HAART pose particular problems for testing adherence interventions. First, it is difficult to define a single summary measure of adherence, given multidrug regimens and the need for adherence to dose interval as well as total daily dose. Second, although adherence interventions would optimally be targeted at nonadherent patients, there is no reliable way to identify such patients, as both provider and patient estimates of adherence overestimate true adherence.5–7 Finally, because there are no published examples of interventions to improve adherence to HAART, there is no way to predict the effect size of such interventions on adherence. Nor is there any way to predict the effect of improving adherence on downstream measures such as virologic or clinical outcomes.
Recognizing these challenges, we developed an intervention that stressed adherence to dose interval (rather than total daily dose) and piloted this intervention in a randomized, controlled study enrolling any patient prescribed any antiretroviral therapy. Review of Medication Event Monitoring System (MEMS; Aprex Corp., Menlo Park, Calif) and reinforcement were based upon a single “primary” antiretroviral drug, and patients were encouraged to cue other medications to this one. The primary outcome was adherence to dose and time interval for the primary drug. Secondary outcomes included adherence to other prescribed antiretrovirals and changes in HIV viral load. In a subset of patients who were prescribed zidovudine as part of their antiretorviral regimen, we also obtained zidovudine plasma levels to obtain confirmation that subjects were actually taking medication when they opened pill bottles.
MATERIALS AND METHODS
The study was conducted at two sites: the HIV clinic of VA Connecticut Health Care System in West Haven and the University of Connecticut Infectious Disease Study Center, which is located in the City of Hartford Health Department in Hartford, Conn. The study was approved by institutional review boards at both of the participating sites.
Patients at these two sites were invited to participate if they were currently being treated with any antiretroviral medication. Antiretroviral regimens were not altered for study participation, but enrollment was deferred if the subject's provider anticipated a change in regimen during the next 12 weeks. Once enrolled in the study, however, treatment changes for clinical reasons were allowed at the discretion of the primary provider and did not require removal from the study.
Subjects who scored 23 or less on the Folstein Mini Mental Status Exam, relied on others to administer medications, or could not accommodate the use of MEMS devices in their daily routine were excluded. After giving informed consent, eligible subjects were administered a modification of a 5-item neuropsychological test battery used previously,8 the Functional Assessment of HIV Infection Quality of Life (FAHI),9 the Symptom Checklist 90 (SCL-90),10 an 11-item measure of medication side effects, the Addiction Severity Index Version 5,11 the Multidimensional Health Locus of Control Scale,12 and the Beck Depression Inventory.13 A urine toxicology screen (for opiates, cocaine, cannabis, and amphetamines) was also obtained.
Subjects were asked to generate a personalized time-of-day schedule for taking each prescribed antiretroviral medication. Study personnel reviewed the schedule to make sure that it was consistent with prescription instructions for each medication. This schedule became the basis for determination of individual adherence. If a subject taking a thrice-daily medication normally opened a bottle twice daily and removed a third dose at one of those times, the schedule reflected only the two scheduled bottle openings, thus allowing patients to continue their usual routines. Subsequently, all antiretroviral medications were dispensed in bottles with MEMS caps. Subjects took medications without any other intervention for a 1-week baseline period.
After the 1-week baseline period, MEMS cap data were downloaded. The percentage of correct doses, defined as the number of bottle openings within 2 hours before or after the agreed upon time, was calculated for each antiretroviral drug. The drug with the lowest baseline adherence was the focus of subsequent training and was designated as “primary.” Urn randomization14 was employed to ensure comparable baseline adherence to the primary medication in each study group. Subjects were randomized to 1 of 3 interventions to begin at the randomization visit (week 0) and at 4 weekly visits (weeks 1 to 4). Data were collected at visits 8 and 12 weeks after randomization (follow-up period). To minimize differential attrition among the groups, all subjects were compensated for attending each study visit (a maximum of $280 if all study visits were kept). Two research assistants without medical training and having no affiliation with the HIV clinic conducted all of the screening, baseline assessments, and training sessions.
The interventions were based upon Cramer's method of cue-dose training which involves linking medication-taking to individualized daily habits (e.g., meals, tooth brushing) which then serve as cues for taking medication.7 A trainer works with individual patients to develop a schedule for taking medication and reminders for dose times (“cues”) that were customized to the patient's lifestyle. The MEMS device records bottle-opening dates and times through a microprocessor in the medication bottle cap. This record is the basis for corrective feedback at meetings with a trainer. We employed this method with and without an additional contingency reinforcement of graduated cash payments based on consecutive correctly timed bottle openings. The specific intervention arms, which are summarized below, were described in detail in a study manual which was used by trainers to minimize variation in the interventions among subjects or between trainers.
Control training (C): Subjects were asked about medication adherence during the week preceding each visit and were encouraged in their efforts to improve adherence. No MEMS calendar was generated for feedback, but MEMS data were collected for measurement of adherence.
Cue-dose training (CD): Subjects were asked to identify cues which would help them remember to take their medications correctly. Subjects were shown a MEMS-generated calendar of their taking of the primary medication during the previous week. If MEMS data revealed missed doses, the counselor would query the subject about the cue for that dose. If there was a consistent pattern of missed doses, the trainer would suggest alternative cues. Subjects were encouraged to take other medications at the cue for the primary medication if possible or to develop alternate cues.
Cue-dose training plus cash reinforcement (CD+CR): Subjects received cue-dose training as above. At weekly meetings, they were also given cash reinforcement for each dose of the primary medication taken within 2 hours of the agreed-upon time. The reinforcement began at $2 per correct dose and increased with each consecutive correct dose to a maximum of $10 per day. If a dose was not taken within 2 hours of the agreed-upon time, the reinforcement was reset to $2. Thus, a subject in this group who took 100% of doses within 2 hours of the agreed-upon time over 4 weeks would receive approximately $280 in addition to the reimbursement for study visits that all subjects received.
Viral load data were collected at baseline (or within 4 weeks prior to study entry) and at the week 12 study visit. HIV RNA values were measured using the Amplicor RNA-PCR Assay (Roche; Alameda, Calif) with lower limit of quantifiable values of 400 copies/mL. Samples that produced a signal below this were assigned an intermediate value of 200 copies (2.3 log 10), and samples that produced no detectable signal were assigned a value of 0.5 log 10 copies/mL.
Zidovudine concentrations were measured in a subset of subjects by radioimmunoassay using the ZDV-Trac kit (DiaSorin, Inc., Stillwater, Minn) as previously described in a study of methadone effects on zidovudine pharmacokinetics.15 Using the time-concentration data from the control group of that study, 98% confidence intervals were constructed for zidovudine concentrations at each time point. Concentration intervals for later time points were determined by log-linear extrapolation from the upper and lower bounds. Measured zidovudine concentrations falling within the 98% confidence limits for the time since last MEMS-recorded opening were interpreted as confirming medication ingestion at the time of the bottle opening.
Data Analysis
Adherence (percentage of prescribed doses taken on time) as measured by MEMS was calculated separately for the primary and mean of all nonprimary antiretroviral medications for each subject at weekly intervals. In order to examine the change in adherence over time for subjects in each group, we used random effects regression modeling. Random effects regression, a method similar to repeated measures analysis of variance, was used to model group and time effects because it allows use of all available data, including data from subjects who did not complete the study.16,17 Four subjects stopped using the MEMS devices during the active intervention period (weeks 0 to 4) and 5 stopped during the follow-up period (weeks 5 to 12).
After exploratory analyses, planned comparisons of each of the active interventions to the control group were included in the model. Weeks 0 to 4 were analyzed to determine acute effects of the interventions. Weeks 4 to 12 were analyzed separately to determine if there were significant treatment effects after training had ended. At each week, mean adherence for each active treatment group was compared to the control group using Wilcoxon 2-sample test for nonnormally distributed data. Error terms (±) reflect standard errors of the mean. Baseline characteristics and change in viral load were compared using χ2and analysis-of-variance tests. Log units were used for viral load analysis.
For the analysis of correlates of baseline adherence, adherence was defined as the percentage of dosages of the primary antiretroviral taken within 2 hours of the prescribed time. Subjects whose adherence was less than the median (76%) were classified as noncompliant and those above were classified as compliant. Point biserial correlation and phi coefficients were calculated between independent variables and adherence.
Sample Size
Due to the exploratory nature of this study, the sample size was sufficient to detect only large treatment effects. A sample size of 18 subjects per group would have 80% power to detect an effect size of 0.7 at a significance of 0.05 based on power calculations for two-group contrasts. Due to the small sample size, no subgroup analyses were performed. As there were no significant differences in the pattern of outcomes between the subjects enrolled in West Haven and those enrolled in Hartford, all analyses include the combined subjects from both sites.
RESULTS
Fifty-five subjects were enrolled and randomized. Characteristics of the subjects in each of the three groups are detailed in Table 1. There were no significant differences among the three groups in any of the baseline measures.
Table 1.
Baseline Characteristics of the Study Population*
| Characteristic | C (N = 18) | CD (N = 22) | CD+CR (N = 15) | Total (N = 55) |
|---|---|---|---|---|
| Age (mean ± SEM), y | 47.2 ± 1.9 | 44.6 ± 1.6 | 43.9 ± 2.5 | 44.2 ± 1.1 |
| Race, n (%) | ||||
| White, not Hispanic | 3 (17) | 4 (18) | 4 (27) | 11 (20) |
| African American | 13 (72) | 16 (73) | 9 (60) | 38 (69) |
| Other | 2 (11) | 2 (9) | 2 (13) | 6 (11) |
| Gender, n (%) | ||||
| Male | 18 (100) | 19 (86) | 12 (80) | 49 (89) |
| Female | 0 | 3 (14) | 3 (20) | 6 (11) |
| History of heroin or cocaine use, n (%)† | 14 (78) | 18 (82) | 12 (80) | 44 (80) |
| Antiretroviral drugs | ||||
| Mean n ± SE | 2.67 ± 0.9 | 2.68 ± 0.6 | 2.47 ± 0.7 | 2.60 ± 0.10 |
| No. (%) with 3 or more | 10 (56) | 15 (68) | 7 (47) | 32 (58) |
| Baseline viral load | ||||
| Mean log 10 value ± SE | 2.70 ± 0.4 | 3.16 ± 0.4 | 2.86 ± 0.5 | 2.93 ± 0.2 |
| n (%) < 400 copies | 8 (44) | 6 (22) | 5 (33) | 19 (36) |
| Baseline adherence to primary medication ± SE | 68% ± 7 | 68% ± 6 | 70% ± 5 | 69% ± 3 |
P >.05 for all comparisons. C indicates control; CD, cue-dose training; CD+CR, cue-dose training plus cash reinforcement.
Lifetime history of at least thrice-weekly use for 1 month.
Thirty-two subjects (58%) were on regimens including at least three antiretroviral drugs. Nineteen (35%) were on two-drug regimens, and 4 (7%) were on single-drug therapy. Twenty-nine (53%) were on regimens that contained at least one drug that was dosed 3 times a day.
Baseline Adherence
Mean adherence during the baseline period (1 week before randomization) for the primary medication was 69% (±27%), including 11 subjects with 100% adherence and 7 subjects with 90% to 99% adherence. All but one of the subjects with high baseline adherence maintained over 90% adherence during almost the entire study.
Several of the baseline measurements collected were significantly correlated with baseline adherence. Baseline adherence lower than the group median was associated with external health locus of control-doctor (P = .013), higher ASI composite scores for drug abuse (P = .048) or alcohol abuse (P = .007), higher positive symptom distress index on the SCL-90 (P = .039), and positive urine toxicology screen for any substance other than amphetamines (P = .015). We were not able to detect any significant associations between baseline adherence and site of enrollment, race, gender, number of medications prescribed, or number of doses per day for the primary medication, although the small sample size limited our ability to detect any but the strongest associations. In stepwise regression, only external health locus of control-doctor and the composite alcohol score (from the ASI) were independently correlated with baseline adherence (adjusted R2for regression model = 0.203)
Changes in Adherence
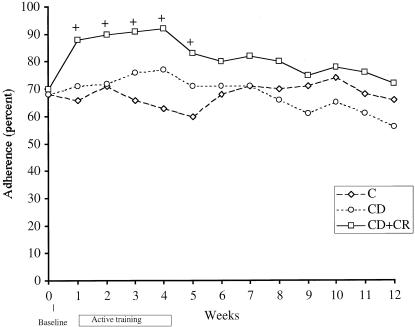
During each week of the active training, mean adherence in the CD+CR group was significantly higher than the control group, but there were no significant differences in mean adherence between the CD group and control group (Fig. 1). Adherence during the follow-up period (weeks 5 to 12) remained numerically higher in the CD+CR group, although comparisons with the control group did not attain statistical significance except for week 5. Prior to training, adherence to nonprimary medications was highly correlated with adherence to primary medications (Pearson correlation 0.95, P < .001). In response to training, adherence to nonprimary antiretrovirals followed the same pattern as the primary medication (Fig. 2).
FIGURE 1.
Mean adherence to primary medication for treatment groups and controls. (+) indicates significant (P < .05) differences from control group value at indicated week; C, control, CD; cue-dose training; CD+CR, cue-dose training plus cash reinforcement.
FIGURE 2.
Mean adherence to nonprimary medications for treatment groups and controls. Adherence to other antiretroviral medications increased in CD+CR group and remained numerically higher throughout the study period compared with controls. C indicates control, CD; cue-dose training; CD+CR, cue-dose training plus cash reinforcement.
The effect of the two interventions over time was assessed using a random effects regression model which included baseline adherence as a covariate. During the active training period (weeks 0 to 4), there was a significant increase in adherence over time for the CD+CR group (z = 3.5, P = .0005) but not the CD group (z = 0.27, P = .79), compared with the control group. The durability of change was assessed with a separate regression model for weeks 5 to 12 (follow-up period) with week 4 adherence as a covariate. During weeks 5 to 12, there was a significant decrease in adherence over time in the CD+CR group (z = −2.2, P = .026), compared with the control group, but no significant change in the CD group compared with controls. Thus, in the cash-reinforced group, there was both a significant improvement in adherence during the active training period and a significant loss of these gains in the same group during the follow-up period, compared with the control group.
The random effects regression procedure makes no assumptions about the value of missing data in subjects who did not complete the study. In order to evaluate the possibility that these missing data may have affected the conclusions of the study, we conducted a sensitivity analysis substituting missing data points with the highest and then the lowest of the measured weekly adherence values for the relevant subject. With either of these extreme assumptions, the significant improvement in the CD+CR group during weeks 1 to 4 was maintained. Therefore, the significant effects of the CR+CR intervention were unlikely to have been the result of differential retention.
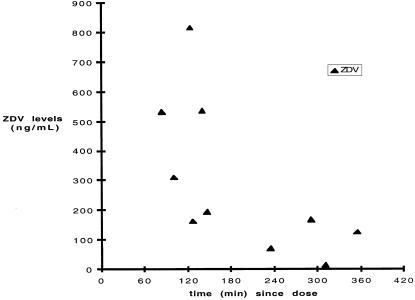
In order to determine whether opening the pill bottle reflected taking the medication, 10 subjects who were prescribed zidovudine had serum samples collected at week 4 for determination of zidovudine drug levels. In all cases, the levels, when compared to time between phlebotomy and previous MEMS-documented bottle opening, were consistent with known pharmacokinetics of zidovudine (Fig. 3). Thus, there was evidence that subjects were actually taking medication when they opened pill bottles.
FIGURE 3.
Zidovudine (ZDV) levels compared to time interval between phlebotomy and last MEMS-documented bottle opening. Zidovudine levels for all time points were consistent with known pharmacokinetics of zidovudine.
Virologic Effects
At baseline, 19 subjects (36%) had viral loads less than 400 copies/mL (the lower limit of quantitation in the assay used), including 15 who had undetectable viremia. The mean adherence to primary medications during the baseline observation period in subjects with less than 400 copies/mL was 80% (±46%, range 30% to 100%), which was significantly higher than baseline adherence in subjects with higher viral loads (mean 64% ± 28%, P = .023). In 43 subjects who had serum samples collected at baseline and at week 12, mean change in viral load was 0.64 log increase in the CD+CR group, 0.29 log decrease in the CD group, and 0.34 log increase in the control group. The differences in viral load change between each of the training groups and the control group were not statistically significant. Two subjects in the CD+CR group had more than a 1 log increase in viral load. One subject's viral load increased from undetectable to 24,672 copies, despite maintaining high baseline adherence (92%) during the study. Six subjects had more than a 0.5 log decrease in viral load (1 in control group, 4 in CD, and 1 in CD+CR) and 8 had more than a 0.5 log increase (2 in control group, 3 in CD, and 3 in CD+CR). Thus, high levels of baseline adherence were associated with low viral loads, but there was no consistent effect of improved adherence on viral replication.
DISCUSSION
In this preliminary study which included two interventions of graded intensity, cue-dose training coupled with monetary reinforcement led to significant increases in adherence to antiretroviral therapy during the intervention period that were not maintained during postintervention follow-up. The effect was apparent by week 1, after the promise of payment, but no actual payment. In fact, mean adherence in the reinforced group increased from 70% at baseline to 88% at week 1. This rapid improvement suggests that it was the motivating effect of the reinforcement rather than new skill acquisition during MEMS feedback that was effective. We were not able to detect any improvement from cue-dose training without reinforcement compared with the control group, although numerical values were higher at most time points.
This study demonstrated the feasibility of comparing standardized interventions to a control condition in a randomized study design as well as the feasibility and utility of using the MEMS system in HIV adherence studies. While we cannot exclude the possibility that some subjects may have opened bottles (in order to obtain cash) without taking medications, the validity of MEMS-documented pill bottle openings as an outcome measure is supported by its concordance with expected zidovudine levels. Thus, this study provides a model for conducting rigorous clinical trials of interventions to improve adherence to antiretroviral drugs, using objective measures of adherence as the outcome of interest.
We provided training and reinforcement around a single, primary drug in a multidrug regimen and selected the primary drug based on baseline observation (the drug with the poorest adherence record). The potential validity of this approach was supported by the high degree of concordance in baseline adherence to all drugs in multidrug regimens and similar improvements in adherence to other drugs when adherence to the primary drug improved. However, factors such as the frequency of dosing and the side-effect profile of individual drugs may create significant differences in adherence. A potential limitation of selecting the drug with poorest baseline adherence as the focus of intervention is the possibility of a regression to the mean effect leading to improvement over time unrelated to the intervention. We did not observe this phenomenon. In fact, adherence in the control group tended to decrease over the course of the study. Further validation of this approach is necessary.
The sample size of this pilot study limited the power to detect small-to-moderate treatment effects. While the subjects enrolled in this study were highly representative of the HIV populations served by the participating institutions, the study group included relatively few women or men who have sex with men. Although others have reported that mode of HIV acquisition is not an important predictor of adherence, it is possible that interventions aimed at improving adherence will need to consider the cultural and demographic makeup of the targeted patient groups.
Implications for Future Studies
Several implications for future study design, interventions, and outcome measures are suggested by the results of this preliminary study. We did not exclude subjects who had high baseline adherence. Because a large percentage of subjects had excellent adherence during the baseline period (18 subjects with over 90% adherence) and maintained those high levels throughout the study (regardless of group assignment), there was a considerable ceiling effect which minimized the chances of detecting any positive effect of the study intervention. Future studies should concentrate on enrolling subjects with poor adherence during preintervention observation or who have characteristics correlated with poor adherence. Our investigation of correlates of baseline adherence produced results that were consistent with some prior studies. The association between a history of intravenous drug use and nonadherence has been widely reported.18,24 However, the negative correlation we found between adherence and a positive toxicology screen collected within the week suggests that in some patients, recent drug use is the mediating factor by which past drug use accounts for nonadherence. The use of this simple screening for recent drug use may be an effective tool for identifying patients who are unlikely to be adherent to therapy.
Medication adherence is a complex behavior that involves motivation, education, skill, and reinforcement. In a major review of interventions to improve adherence, Roter et al. found that interventions that address more than one dimension (affective, cognitive, and behavioral) are more effective than those that target a single aspect.23 In our study, the monetary reinforcer probably served as an important motivational factor. While cue-dose training has been used effectively in other settings, the important role of monetary reinforcement in our study speaks to the importance of addressing motivation and reinforcement as well as skill building in adherence interventions.
The issue of using a monetary reinforcement has not been widely discussed, and there may be objections to consideration of widespread use of such an intervention. However, various types of incentives have been used in a number of other health care settings, including use of cash or vouchers to improve adherence to naltrexone maintenance, tuberculosis treatment, or breast cancer screening.19–22 Given the apparently strong effect of monetary reinforcement, it may be possible to retain its benefits with more socially sanctioned reinforcers such as vouchers, fee reductions, nonmonetary reinforcers, or donated prizes.
Ultimately, the goal of intervening to improve adherence is to improve medical outcomes of treatment. Our study was not ideally designed to test this possibility because treatment regimens were not optimized as part of the study, and in many cases, maximum antiviral effect may have already been achieved from the regimens prescribed. However, the ready availability of viral load markers allowed us to make some preliminary observations. First, as others have observed, high baseline adherence was associated with lower viral loads. However, the observed increase in viral load in the CD+CR group during the course of the study suggests that the relationship of adherence to favorable health outcomes is not a simple one. In this study, the ability to detect a decreased viral load associated with improved adherence was additionally constrained by the short follow-up, the relatively low “baseline” viral loads (floor effect), and the diminution in adherence improvement during weeks 5 through 12 of the study. Future studies of adherence interventions, which should aim to assess their effect on both adherence and virologic outcomes, will need to address the issues of the efficacy of regimens employed and the timing of the intervention as it relates to initiation of therapy.
Conclusions
The importance of medication adherence behavior to medical treatment outcomes is widely recognized, and there is understandable interest in interventions to improve adherence, particularly with highly active antiretroviral therapy. However, because of the complexity of this relationship and the relative lack of previous experience with studying adherence interventions, it is necessary to address methodologic issues in study design and execution as well as to develop and test promising interventions. We report the results of a pilot study that addressed some of these methodologic questions and examined the effects of two interventions of graded intensity in a randomized, controlled study design.
The findings of this study are preliminary, and additional, larger studies will be needed to determine how these findings generalize to other populations such as patients with lower baseline adherence, men who have sex with men, and women, who made up a small percentage of our subjects. Important questions also remain about whether training involving monetary incentives would be cost-effective or might have unexpected and undesirable consequences. We suggest, however, that because the benefits of improved adherence may be considerable, additional studies should be undertaken to study strategies such as cue-dose training with cash reinforcement for improving adherence to HIV therapy.
Acknowledgments
This study was supported by grants K20-DA000191-05 (MIR) and VA Merit Review 1241 (MIR), P50-DA09241 (BJR) and a General Clinical Research Center grant (M01RR06192) to the University of Connecticut Health Center, Farmington, Conn.
We gratefully acknowledge the contributions of Rhonda L. Pruzinsky, Jennifer Howden, and Charla Nich.
REFERENCES
- 1.Ioannidis JPA, Sacks HS, Cappellen JC, et al. Presented at the Fourth Conference on Retroviruses and Opportunistic Infections. Washington, DC: 1997. Clinical efficacy of antiretroviral changes in treatment-experienced HIV-infected patients: a meta-analysis. [Google Scholar]
- 2.Hammer SM, Squires KE, Hughes D, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:734–9. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 3.Cameron DW, Heath-Chiozzi M, Danner S, et al. Randomized placebo-controlled trial of ritonavir in advanced HIV-1 disease. Lancet. 1998;351:543–9. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- 4.Haynes RB, McKibbon KA, Kanani R. Systematic review of randomized trials of interventions to assist patients to follow prescriptions for medications. Lancet. 1996;348:383–6. doi: 10.1016/s0140-6736(96)01073-2. [DOI] [PubMed] [Google Scholar]
- 5.Patterson DL, Swindels S, Mohm JA, et al. Adherence with protease inhibitor therapy for human immunodeficiency virus infections. Presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Diego. 1998. Calif.
- 6.Melbourne K, Geletko S, Brown S, Willey C, Chase S, Fisher A. Electronic adherence assessment versus self-report in HIV-infected individuals. Presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Diego. 1998. Calif.
- 7.Cramer JA. Enhancing patient adherence in the elderly. Role of packaging aids and monitoring. Drugs Aging. 1998;12:7–15. doi: 10.2165/00002512-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kosten TR, Rosen MI, McMahon TJ, et al. Treatment of early AIDS dementia in intravenous drug users: high versus low dose peptide T. Am J Drug Alcohol Abuse. 1997;23:543–53. doi: 10.3109/00952999709016894. [DOI] [PubMed] [Google Scholar]
- 9.Cella DF, Bonomi AE. The Functional Assessment of Cancer Therapy (FACT) and Functional Assessment of HIV Infection (FAHI) quality of life measurement system. In: Spiker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. 2nd ed. New York: Raven Publishers; 1995. pp. 203–14. In: [Google Scholar]
- 10.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull. 1973;4:13–28. [PubMed] [Google Scholar]
- 11.McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 12.Wallston KA, Stein MJ, Smith CA. Form C of the MHLC scales: condition-specific measure of locus of control. J Pers Assess. 1994;63:534–53. doi: 10.1207/s15327752jpa6303_10. [DOI] [PubMed] [Google Scholar]
- 13.Beck A, Ward CH, Mendelson M. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:461–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 14.Stout RL, Wirtz PW, Carbonari JP, et al. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–5. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 15.McCance-Katz EF, Rainey PM, Jatlow P, Friedland G. Methadone effects on zidovudine disposition (ACTG 262) J Acquir Immune Defic Syndr. 1998;18:435–43. doi: 10.1097/00042560-199808150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Newbury Park, Calif: Sage Publications, Inc; 1992. [Google Scholar]
- 17.Hedeker D, Gibbons RD. MIXREG: a computer program for mixed-effects regression analysis with autocorrelated errors. Comput Methods Programs Biomed. 1996;49:229–52. doi: 10.1016/0169-2607(96)01723-3. [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Squier C, Sivek C, Wagener M, Nguyen MH, Yu VL. Determinants of adherence with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing adherence. AIDS Care. 1996;8:261–9. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- 19.Preston KL, Silverman K, Umbrict A, et al. Improvement in naltrexone treatment adherence with contingency management. Drug Alcohol Depend. 1999;54:127–35. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- 20.Volmink J, Garner P. Systematic review of randomized controlled trials of strategies to promote adherence to tuberculosis treatment. BMJ. 1997;315:1403–6. doi: 10.1136/bmj.315.7120.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoner TJ, Down B, Carr WP, Maldonado G, Church TR, Mandel J. Do vouchers improve breast cancer screening rates? Results from a randomized trial. Health Serv Res. 1998;33:111–28. [PMC free article] [PubMed] [Google Scholar]
- 22.Giuffrida A, Torgerson DJ. Should we pay the patient? Review of financial incentives to enhance patient adherence. BMJ. 1997;315:703–7. doi: 10.1136/bmj.315.7110.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roter DL, Hall JA, Merisca R, Ruehle B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient adherence: a meta-analysis. Med Care. 1998;36:1138–61. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Samet JH, Libman H, Steger KA, et al. Adherence with zidovudine therapy in patients infected with human immunodeficiency virus, type 1: a cross-sectional study in a municipal hospital clinic. Am J Med. 1992;92:495–502. doi: 10.1016/0002-9343(92)90746-x. [DOI] [PubMed] [Google Scholar]