Abstract
BACKGROUND
The impact of serum potassium on mortality is inadequately defined.
OBJECTIVE
To determine the association of serum potassium with mortality.
METHODS
We analyzed NHANES I Epidemiological Follow-up Study data from 1974–1992. Of 2,992 subjects with baseline serum potassium, 156 were excluded because their vital status was not known. A total of 2,836 subjects with serum potassium within 2.7–5.4 mmol/L were studied. All-cause and cardiovascular mortality were assessed controlling for sociodemographic status, smoking, medical history, and clinical characteristics.
RESULTS
At baseline, mean age was 46.6 years, and mean serum potassium was 4.07 mmol/L. Subjects were stratified into three groups by mean ±1 standard deviation of serum potassium: low, 2.7–3.7 mmol/L (N = 477); middle, 3.8–4.4 mmol/L (N = 1,982); and high, 4.5–5.4 mmol/L (N = 377). The cardiovascular mortality rate per 1,000 person-years adjusted for age, gender, and race for the high serum potassium group (8.1) was significantly higher than the middle (5.3) and low (6.5) serum potassium groups. Further analysis, controlling for age, gender, race, smoking status, cholesterol, and history of diabetes, renal disease, and cardiovascular disease, revealed that the increased cardiovascular mortality among subjects with moderately increased serum potassium was most prominent in those reporting use of diuretics (hazard ratio, 2.65; 95% confidence interval [95% CI], 1.20 to 5.85) and those with abnormal renal function (hazard ratio, 1.89; 95% CI, 1.05 to 3.41).
CONCLUSION
In this general population sample with mostly normal serum potassium, higher serum potassium was independently associated with increased cardiovascular mortality.
Keywords: serum potassium, NHANES I, epidemiological follow-up, cardiovascular disease, mortality, renal function, diuretics
Although only 2% of total body potassium is present in the vascular compartment, the accessibility of serum potassium (SK) may explain its clinical utility.1–3 Indeed, timely assessment of SK is often critical to the proper management of diabetes and heart disease.1,4 Normally, SK exists in the relatively small range of 3.5 to 5.5 mmol/L.1–3 While substantial evidence supports the hazards of very low (<2.0 mmol/L) and very high (>6.0 mmol/L) SK,1–3 there is little information about the health consequences of differences within or near the normal range. In fact, most concern has focused upon relatively low SK associated with antihypertensive therapy.5–7 However, nearly two decades ago, some observers raised concern about the possibility that elevated SK might be as great a health risk as reduced SK.8 Recently, Wannamethee et al. reported that low SK (<3.7 mmol/L) was not associated with increased mortality. Instead, they found that a moderately elevated SK (>5.2 mmol/L), as previously postulated,8 was actually associated with a significant increase in mortality, particularly among smokers.9
To explore the relationship of SK to mortality in a U.S. general population, we examined the experience of 2,836 participants in the first National Health and Nutrition Examination Survey (NHANES I), in whom SK was measured at baseline (1974–1975) and mortality ascertained through 1992. We now report the association of SK to cardiovascular and all-cause mortality.
MATERIALS AND METHODS
Study Design
The NHANES I, sponsored by the National Center for Health Statistics, was conducted from 1971 to 1975 on a nationwide probability sample of the noninstitutionalized civilian population in the United States.10–12 The NHANES I Epidemiological Follow-up Survey (NHEFS) is a longitudinal study of 14,407 participants in NHANES I who were 25 to 74 years of age at the time of survey.13 The personal interviews and physical and laboratory examinations of NHANES I provided the baseline data for the NHEFS, which comprises a series of follow-up surveys, the most recent in 1992.14 Detailed information on sampling frame is available in the plan and operation series reports of the NHANES I.10–14
Serum potassium was determined in 3,059 participants from the augmentation survey of NHANES I during 1974 to 1975.12 Of this sample, 2,992 had an available SK measure, and 156 were excluded due to missing vital status data for 1992. The remaining 2,836 subjects were the subjects of this analysis.
Baseline Measurements
Blood samples were obtained from nonfasting persons, and all frozen serum was sent to the Centers for Disease Control for analysis. The baseline medical history questionnaire provided information about history of selected conditions, such as heart disease and stroke. For example, a positive history of heart disease at baseline was defined as having had a heart attack or heart failure or having used any medicine, drugs, or pills for a weak heart during the 6 months prior to the baseline interview. At the beginning of the baseline physical examination, blood pressure was measured by a physician with the subject seated. Smoking status for current smokers was available at baseline for only 50% of participants. For the remaining subjects, the smoking status at baseline was derived from a questionnaire answered during the 1982–1984 follow-up interview on lifetime smoking history. The validity of this approach has been documented.15,16 The smoking status in this analysis was therefore combined from two sources, and valid information on smoking was available for more than 95% of the subjects.
Outcome Measures
Follow-up data, based on interviews, medical records, and death certificates, were collected in 1982–1984, 1986, 1987, and 1992. In the 1982–1984 follow-up, a two-hour subject interview was conducted in person; in the 1986, 1987, and 1992 follow-ups, each interview averaged 30 minutes and was conducted by telephone. Death was ascertained by either death certificate, proxy interview, or both. In the former case, the underlying cause of death was coded according to the International Classification of Diseases, Ninth Revision. Deaths were analyzed for all causes, total cardiovascular disease (codes 390–459), ischemic heart disease (codes 410–414), stroke (code 430–438), and cancer (code 140–208). The number of years of follow-up for each individual was calculated from baseline to the date of death for decedents and to the date of follow-up for survivors. Of 2,836 subjects, there were 578 (20.4%) confirmed deaths through the 1992 follow-up.
Statistical Analysis
Mean SK level was determined by age, race, and gender. Subjects were stratified into three groups by baseline SK level with cut points at the mean ±1 standard deviation. Baseline characteristics of subjects by SK level were estimated. Furthermore, we used the mean SK ±2 standard deviations as another set of cut points (3.4 and 4.8 mmol/L).
For the longitudinal analyses, mortality rates for all-cause, total cardiovascular disease, ischemic heart disease, stroke, and cancer, expressed as per 1,000 person-years and adjusted for age, gender, and race, were calculated by the direct method of standardization with the total study sample serving as the standard population. Cox proportional hazard regression models were constructed separately to examine the relation of SK levels to each cause-specific mortality rate, adjusting for the following baseline characteristics: age, gender, race, blood pressure, cholesterol, smoking status, diuretic use, renal function, renal disease, and heart disease. In these models, SK was entered as a categorical variable. Hazard ratios and 95% confidence intervals were estimated for both low and high SK group, with the middle range group as reference. To test the J-shaped association of SK and cardiovascular mortality, the mortality risk associated with SK as a continuous variable was explored by choosing the median (SK = 4.1 mmol/L) as a cut point to stratify the subjects into two groups. A Cox regression model was constructed for each stratum.
Because some clinical characteristics, such as diuretic use, renal function, and cardiovascular risk status, are related to both cardiovascular mortality and SK, interaction terms of these variables with SK were included in the Cox regression models to test the joint effects on cardiovascular mortality. In addition, the population was stratified by diuretic use (nonuser vs user), renal function (normal vs abnormal), and cardiovascular risk factor status (low risk status vs high risk status). Diuretic use was defined by whether the participant was using diuretics during the past 30 days. Abnormal renal function was defined as blood urea nitrogen (BUN)>20 mg/dL and/or serum creatinine>1.2 mg/dL for women and>1.6 mg/dL for men. The high risk status was defined as having one or more of the following risk factors: blood pressure ≥160 over 95 mmHg or taking antihypertensive medication; cholesterol ≥245 mg/dL; and a history of at least one of the following conditions: myocardial infarction, stroke, renal disease, and diabetes. Cox regression models were used to examine the effect of SK level on total cardiovascular mortality, adjusting for age, gender, race, smoking status, cholesterol, renal disease, and heart disease.
RESULTS
Serum Potassium and Baseline Characteristics
The mean level of SK for the 2,836 subjects was 4.07 mmol/L, with a range of 2.7 to 5.4 mmol/L. Mean age was 46.6 ± 13.9 years. Men had significantly (though slightly) higher SK levels than did women (4.12 ± 0.34 vs 4.03 ± 0.35 mmol/L, P < .001). African-American (243 subjects) and white (2,593) subjects had similar SK levels (4.07 ± 0.36 vs 4.07 ± 0.35, P = .848). Mean levels of SK increased with age from 4.05 mmol/L for those under 45 years of age to 4.14 mmol/L for those 65 years and above (P < .001). For those using diuretics, SK levels were significantly, but modestly, lower than those not using diuretics (3.91 vs 4.09 mmol/L, P < .001) and did not differ according to cardiovascular risk status (low vs high: 4.08 vs 4.06, P = .217). Those with abnormal renal function had significantly higher SK, compared with those with normal renal function (4.12 vs 4.06, P = .003).
Stratifying the population into three groups by mean ±1 standard deviation (low, 2.7–3.7 mmol/L; middle, 3.8–4.4 mmol/L; and high, 4.5–5.4 mmol/L) revealed significant differences in baseline characteristics with relevance to cardiovascular risk (Table 1). Those with the highest level of SK (4.5–5.4 mmol/L) were older, had a higher prevalence of cardiovascular disease history (myocardial infarction, stroke, and congestive heart failure), and were more likely to be male and a smoker. They also had a higher BUN and serum creatinine than those in the low and middle SK groups. The percentage with an elevation of serum creatinine and BUN was highest in the high SK group, and this was true for both diuretic and nondiuretic users (data not shown here). By contrast, those with the lowest SK had the highest systolic blood pressure and pulse pressure. In the low SK group, 21.3% had used diuretics during the past 30 days, compared with 7.7% and 7.0% of subjects in the middle and high SK groups, respectively.
Table 1.
Baseline Characteristics of Study Subjects by Serum Potassium: Augmentation Survey, Baseline 1974–1975
| Serum Potassium (mmol/L) | ||||
|---|---|---|---|---|
| Characteristic | Low 2.7 to 3.7 (n = 477) | Medium 3.8 to 4.4 (n =1,982) | High 4.5 to 5.4 (n = 377) | P Value (Low vs High) |
| Age, y | 45.6 | 46.2 | 49.6 | <.0001 |
| Blood pressure, mmHg | ||||
| Systolic | 134 | 130 | 130 | .001 |
| Diastolic | 84.5 | 83.1 | 84.3 | .48 |
| Pulse pressure | 49.4 | 45.8 | 46.1 | <.0001 |
| Cholesterol, mg/dL | 218 | 220 | 227 | .019 |
| BUN, mg/dL | 14.1 | 14.8 | 16.0 | <.0001 |
| Serum creatinine, mg/dL | 0.97 | 1.01 | 1.04 | .014 |
| Male, % | 31.9 | 45.7 | 53.1 | <.0001 |
| African American, % | 9.2 | 8.2 | 9.5 | .40 |
| Alcohol use, % | 23.7 | 32.2 | 32.4 | .008 |
| Diuretic use in past 30 days, % | 21.3 | 7.7 | 7.0 | <.0001 |
| History of CVD*, % | 5.2 | 4.9 | 8.8 | <.0001 |
| History of renal disease, % | 10.8 | 7.3 | 8.6 | .10 |
| Abnormal renal function†, % | 13.2 | 12.8 | 18.8 | .011 |
| Smoker, % | 29.0 | 33.4 | 39.5 | .001 |
Including past history of myocardial infarction, stroke, and congestive heart failure.
Blood urea nitrogen>20 mg/dL and/or serum creatinine>1.2 mg/dL for females and>1.6 mg/dL for males.
BUN indicates blood urea nitrogen; CVD, cardiovascular disease.
Mortality
Mortality Among Overall Population
During an average of 15.9 years follow-up, there were 578 deaths (20.4% of the study group). The average follow-up was 17.4 and 10.0 years for survivors and decedents, respectively. Of total deaths, 272 (47.1%) were ascribed to cardiovascular disease (ischemic heart disease, 172; stroke, 33; other cardiovascular deaths, 67), and 150 (26%) were cancer-related. The remaining 156 deaths were associated with causes other than cardiovascular disease or cancer.
Mortality rates adjusted for age, gender, and race are shown in Table 2). A J-shaped association was observed between SK and all-cause, total cardiovascular disease, and ischemic heart disease mortality. However, only the high SK group (4.5–5.4 mmol/L) had significantly higher mortality than those in the middle SK group (3.8–4.4 mmol/L). In contrast, the low SK group (2.7–3.7 mmol/L) did not differ significantly in mortality from the middle SK group. For stroke, mortality rates were similar for low and middle SK groups, but were significantly higher for the high SK group. Of note, cancer death rates did not differ by SK. The counts in both the lowest and highest groups were reduced substantially when we used SK ±2 standard deviations as the cutoffs (52; 2,740; 44). Nevertheless, the hazard ratios for low and high SK and cardiovascular disease mortality were 1.37 (95% confidence interval [95% CI], 0.51 to 3.71) and 2.62 (95% CI, 1.34 to 5.13), respectively, with the middle range of SK as the reference.
Table 2.
Adjusted Mortality Rates per 1,000 Person-years by Serum Potassium: NHANES I Augmentation Survey (1974–1975) to 1992 Follow-up
| Serum potassium (mmol/L) | ||||
|---|---|---|---|---|
| Cause | Low 2.7 to 3.7 (N = 477) | Medium 3.8 to 4.4 (N = 1,982) | High 4.5 to 5.4 (N = 377) | P Value* |
| All-cause | 12.0 (86) | 12.0 (377) | 17.0 (115) | .0002 |
| CVD | 6.5 (46) | 5.3 (170) | 8.1 (56) | .0001 |
| IHD | 4.2 (30) | 3.3 (107) | 5.1 (35) | .005 |
| Stroke | 0.5 (5) | 0.6 (18) | 1.5 (10) | .019 |
| Cancer | 2.4 (17) | 3.4 (108) | 3.9 (25) | NS |
CVD, total cardiovascular disease; IHD, ischemic heart disease. Numbers in parentheses are deaths.
P value for high vs medium. P value for low vs medium not significant.
The results of the Cox regression revealed a higher SK to be associated with increased cardiovascular mortality, with a hazard ratio of 1.54 (95% CI, 1.03 to 2.31). However, low SK was not associated with the risk (Table 3). The increased risk of death for high SK was also observed for all-cause and stroke mortality, with hazard ratios of 1.51 (95% CI, 1.15 to 1.97) and 2.94 (95% CI, 1.10 to 7.80), respectively.
Table 3.
Hazard Ratios and 95% Confidence Intervals for Cardiovascular Mortality Associated with Serum Potassium and Other Risk Factors, NHANES I (1974–1992)
| Characteristic | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| Age (≥55 y = 1) | 7.4 | 5.1 to 11 |
| Gender (male =1) | 1.8 | 1.4 to 2.4 |
| Race (African American = 1) | 0.75 | 0.46 to 1.2 |
| Systolic BP (20 mmHg) | 2.7 | 2.2 to 3.4 |
| Cholesterol (45 mg/dL) | 1.7 | 1.2 to 2.2 |
| Diuretic use (yes = 1) | 1.6 | 1.1 to 2.4 |
| Renal function (abnormal = 1) | 1.2 | 0.86 to 1.7 |
| History of myocardial infarction/stroke (yes = 1) | 2.5 | 1.4 to 4.7 |
| History of kidney disease (yes = 1) | 1.5 | 0.99 to 2.2 |
| Smoke (yes = 1) | 2.8 | 2.1 to 3.7 |
| Serum potassium (low vs middle) | 0.96 | 0.61 to 1.5 |
| Serum potassium (high vs middle) | 1.5 | 1.0 to 2.3 |
For subjects with SK below the median, SK was negatively (not significantly) associated with cardiovascular mortality, with a hazard ratio of 0.77 (95% CI, 0.30 to 1.98). However, for subjects with SK at or above median, SK was positively associated with cardiovascular mortality, with a hazard ratio of 2.71 (95% CI, 1.41 to 5.23).
Mortality According to Clinical Characteristics
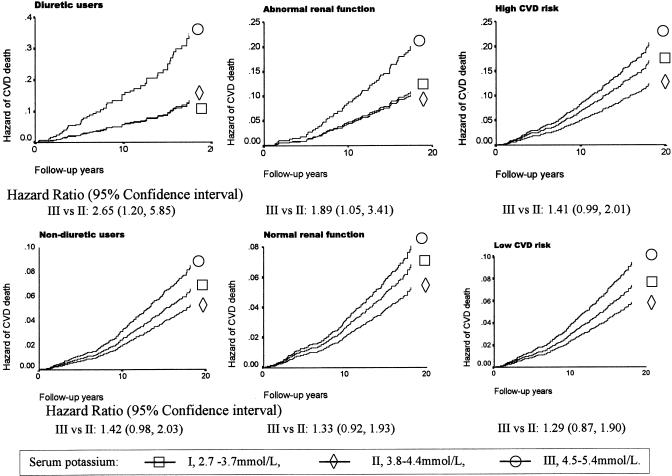
After introduction of interaction terms into the models, we observed that the interaction between SK and diuretic use was significantly associated with cardiovascular mortality (Table 4). With the middle range of SK as reference, the hazard curves of cardiovascular mortality for each level of SK are presented in Figure 1. Among subjects not using diuretics, with normal renal function, and with either low or high cardiovascular risk status, neither high SK nor low SK was associated with cardiovascular mortality. However, among those using diuretics and with abnormal renal function, a high SK was associated with sharply increased cardiovascular mortality compared with the middle SK group, with hazard ratios of 2.65 (95% CI, 1.20 to 5.85) and 1.89 (95% CI, 1.05 to 3.41) for diuretic users and those with abnormal renal function, respectively.
Table 4.
Hazard Ratios and 95% Confidence Intervals for Cardiovascular Mortality Associated with Serum Potassium from Cox Regression Models*
| Characteristic | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| SK × diuretic use | ||
| Interaction term | 2.9 | 1.0 to 8.0 |
| SK (low vs middle) | 1.2 | 0.73 to 1.9 |
| SK (high vs middle) | 1.5 | 0.99 to 2.2 |
| SK × renal function | ||
| Interaction term | 1.8 | 0.82 to 4.1 |
| SK (low vs middle) | 1.0 | 0.64 to 1.6 |
| SK (high vs middle) | 1.4 | 0.91 to 2.2 |
| SK × CVD risk status | ||
| Interaction term | 0.99 | 0.90 to 1.1 |
| SK (low vs middle) | 0.95 | 0.61 to 1.5 |
| SK (high vs middle) | 1.4 | 0.93 to 2.2 |
| SK × history of renal disease | ||
| Interaction term | 0.78 | 0.29 to 2.1 |
| SK (low vs middle) | 0.93 | 0.59 to1.5 |
| SK (high vs middle) | 1.6 | 1.04 to 2.4 |
Controlling for age, gender, race, blood pressure, cholesterol, diuretic use, renal function, cardiovascular (CVD) risk status and history of diabetes, and history of renal disease. SK indicates serum potassium.
FIGURE 1.
Hazard ratio of total cardiovascular mortality associated with serum potassium level, adjusting for age, gender, race, smoking status, diuretic use, renal function, cholesterol, and history of diabetes, renal disease, and cardiovascular disease, from Cox regression analysis among subjects stratified by diuretic use, renal function, and cardiovascular risk status.
DISCUSSION
These data do not support concerns that a moderately low SK (2.7–3.7 mmol/L) might increase all-cause or cardiovascular mortality. In fact, both all-cause and cardiovascular mortality were greatest in those with a high normal SK (4.5–5.4 mmol/L). No association of increased cardiovascular mortality and moderately low SK was observed here. Further analysis revealed that the increased cardiovascular mortality associated with high SK was driven by those using diuretics or who had abnormal renal function, after accounting for other known risk factors.
Our findings in a general population are consistent with two Scottish reports regarding SK in treated hypertensive patients. One longitudinal study at the Glasgow Blood Pressure Clinic found no relationship between low potassium concentrations (<3.7 mmol/L) and ischemic heart disease mortality.17 A second report from the Scotland Clinic Study of 7,636 middle-aged men treated for hypertension followed for a mean of 11.5 years revealed that an SK level <3.7 mmol/L was not associated with increased mortality.9
Serum potassium levels below 3.5 and above 5.5 mmol/L have traditionally been labeled as hypokalemia and hyperkalemia, respectively.1–3 It is generally recognized that more severe hypokalemia or hyperkalemia is life-threatening.1–3 Little attention has been focused on mild elevation in SK, in contrast to mild hypokalemia, which often occurs in hypertensive patients and is a common clinical consequence of diuretic treatment.7 Since diuretic-related hypokalemia has been linked to cardiac arrhythmia, it has often been viewed as a hazardous and potentially lethal consequence of such therapy. Interest has been centered on the increased incidence of ventricular ectopic beats in hypokalemic patients taking diuretics and on the possibility that this might predispose the patient to sudden death.5–7 Indeed, observational studies have reported an association of increasing diuretic dosage with sudden death.5–7 However, in none of these studies have SK levels been reported. There are, of course, other possible explanations for increasing cardiovascular mortality among diuretic users, including confounding by selection bias, as well as perturbation of magnesium, uric acid, blood glucose, and lipids.18–22
The robust association of high SK and increased cardiovascular mortality among those using diuretics cannot be explained by the available data. Perhaps an elevated SK in such patients marks subtle renal disease, and it is the renal disease that is responsible both for the higher SK and the increased cardiovascular mortality. In any event, the data here reveal a link between moderate increased SK with either diuretic use or abnormal renal function. In either case, an elevated SK raises the risk for cardiovascular mortality. Somewhat surprisingly, however, an elevated SK in subjects with high cardiovascular risk was not related to increased cardiovascular mortality. Also, increased SK was not a risk marker in nondiuretic users or those with normal renal function, after accounting for other known risk factors. The limited study population precluded further stratification of these groups.
As expected, those using diuretics were more likely to have a low SK level. The striking increase in cardiovascular and total mortality of those taking diuretics who fell into the high normal range of SK was unexpected. Possible explanations for this finding include failure to adhere to the diuretic regimen, or use of potassium-sparing diuretics or potassium supplements. However, there was no information regarding the class of diuretics used in NHANES data. Conversely, it is more likely that the increased mortality associated with a higher SK reflects impaired renal function. Those taking diuretics who failed to lower their SK had increased serum creatinine and BUN compared with those with low SK levels. This may have blunted the expected kaliuretic response to diuretics. Perhaps, renal dysfunction is the common factor associated with increased cardiovascular mortality.
The present study is limited by the availability of only a single baseline measure of SK. It is likely that intraindividual daily variation produced random misclassification. There may also be some random bias due to the influences of different blood drawing techniques. Clearly, the validity of an association between a single serum determination and subsequent mortality after a long delay, even when significant, specific, and independent of other known cardiovascular risk factors, must be viewed with caution. Much could have changed in the interval between baseline measure and mortality. It should also be noted that low SK might increase risk. However, over the long term, these subjects were likely to get therapy that corrected this abnormality. In addition, while a low SK (2.7 to 3.7 mmol/L) was not related to increased cardiovascular mortality, it might be if there were sufficient numbers to explore a group with a lower level, as we observed with mean SK ± 2 standard deviations, with a cut point for the low group of 3.4 mmol/L (hazard ratio, 1.37; 95% CI, 0.51 to 1.71). Another limitation is that data for history of diabetes was missing for these subjects, and we were unable to control for this important factor.
These data call attention to the possible adverse association of frequently ignored mildly elevated SK and mortality, particularly in diuretic-treated patients and those with even minimally compromised renal function.
Acknowledgments
The NHANES I Epidemiologic Follow-up Study has been developed and funded by these agencies: National Center for Health Statistics; National Institute on Aging; National Cancer Institute; National Center for Chronic Disease Prevention and Health Promotion; National Institute of Child Health and Human Development; National Heart, Lung, and Blood Institute; National Institute on Alcohol Abuse and Alcoholism; National Institute of Mental Health; National Institute of Diabetes and Digestive and Kidney Disease; National Institute of Arthritis and Musculoskeletal and Skin Disease; National Institute of Allergy and Infectious Disease; National Institute of Neurological and Communicative Disorders and Stroke; and U.S. Department of Agriculture. The field work was conducted by Westat Inc., Gaithersburg, Md.
REFERENCES
- 1.Gabow PA, Peterson LN. Disorders of potassium metabolism. In: Schrier RW, editor. Renal and Electrolyte Disorders. Boston: Little, Brown & Company; 1986. pp. 231–85. [Google Scholar]
- 2.Young DB. Analysis of long term potassium regulation. Endocr Rev. 1985;6:24–44. doi: 10.1210/edrv-6-1-24. [DOI] [PubMed] [Google Scholar]
- 3.Hyman D, Kaplan NM. The difference between serum and plasma potassium. New Engl J Med. 1985;313:642. doi: 10.1056/nejm198509053131019. [DOI] [PubMed] [Google Scholar]
- 4.Pannall P, Rossi A. Potassium levels in serum and plasma. Clin Chim Acta. 1970;30:218–20. doi: 10.1016/0009-8981(70)90211-1. [DOI] [PubMed] [Google Scholar]
- 5.Hollifield JW, Slaton PE. Thiazide diuretics, hypokalemia and cardiac arrhythmias. Acta Med Scand. 1981;641(suppl):67–73. doi: 10.1111/j.0954-6820.1981.tb02640.x. [DOI] [PubMed] [Google Scholar]
- 6.Siscovick DS, Raghunathan TE, Psaty BM, et al. Diuretic therapy for hypertension and risk of primary cardiac death. New Engl J Med. 1994;330:1852–7. doi: 10.1056/NEJM199406303302603. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JD, Neaton JD, Prineas RJ, Daniels KA. Diuretics, serum potassium and ventricular arrhythmias in the multiple risk factor intervention trial. Am J Cardiol. 1987;60:548–54. doi: 10.1016/0002-9149(87)90303-1. [DOI] [PubMed] [Google Scholar]
- 8.Kassirer JP, Harrington JT. Fending off the potassium pushers. New Engl J Med. 1985;312:785–7. doi: 10.1056/NEJM198503213121210. [DOI] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Lever AF, Shaper AG, Whincup PH. Serum potassium, cigarette smoking, and mortality in middle-aged men. Am J Epidemiol. 1997;145:598–606. doi: 10.1093/oxfordjournals.aje.a009156. [DOI] [PubMed] [Google Scholar]
- 10.Miller HW. Plan and operation of the health and nutrition examination survey, United States, 1971-1973. National Center for Health Statistics. Vital Health Stat 1. 1978;1:1–46. [PubMed] [Google Scholar]
- 11.Miller HW. Plan and operation of the health and nutrition examination survey: United States, 1971-1973. Vital Health Stat 1. 1973;1:1–77. [PubMed] [Google Scholar]
- 12.Engel A, Murphy RS, Maurer K, Collins E. Plan and operation of the NHANES I Augmentation Survey of Adults 25-74 years, United States, 1974-1975. Vital Health Stat 1. 1978;14:1–110. [PubMed] [Google Scholar]
- 13.Cohen BB, Barbano HE, Cox CS, et al. Plan and operation of the NHANES I Epidemiologic Followup Study: 1982-84. Vital Health Stat 1. 1987;22:1–142. [PubMed] [Google Scholar]
- 14.Cox CS, Mussolino ME, Rothwell ST, et al. Plan and operation of the NHANES I Epidemiologic Followup Study: 1992. Vital Health Stat 1. 1997;35:1–231. [PubMed] [Google Scholar]
- 15.McLaughlin JK, Dietz MS, Mehl ES, et al. Reliability of surrogate information on cigarette smoking by type informant. Am J Epidemiol. 1987;126:144–6. doi: 10.1093/oxfordjournals.aje.a114647. [DOI] [PubMed] [Google Scholar]
- 16.Machlin SR, Kleinman JC, Madans JH. Validity of mortality analysis based on retrospective smoking information. Stat Med. 1989;8:997–1009. doi: 10.1002/sim.4780080810. [DOI] [PubMed] [Google Scholar]
- 17.Robertson JWK, Isles CG, Brown I, et al. Mild hypokalemia is not a risk factor in treated hypertensives. J Hypertens. 1986;4:603–8. doi: 10.1097/00004872-198610000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Siegel D, Hulley SB, Black DM, et al. Diuretics, serum and intracellular electrolyte levels, and ventricular arrhythmias in hypertensive men. JAMA. 1992;267:1083–9. [PubMed] [Google Scholar]
- 19.Hollifield JW. Potassium and magnesium abnormalities: diuretics and arrhythmias in hypertension. Am J Med. 1984;77:28–32. doi: 10.1016/s0002-9343(84)80005-4. [DOI] [PubMed] [Google Scholar]
- 20.Ames RP, Hill P. Elevation of serum lipid levels during treatment with antihypertensive drugs. Am J Med. 1976;61:748–57. doi: 10.1016/0002-9343(76)90156-x. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson C. Elevated serum uric acid levels during treatment with antihypertensive drugs. Acta Med Scand Suppl. 1979;628:69–71. doi: 10.1111/j.0954-6820.1979.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 22.Murphy MB, Lewis PJ, Kohner E, Schumer B, Dollery CT. Glucose intolerance in hypertensive patients treated with diuretics: a 14-year follow-up. Lancet. 1982;2:1293–5. doi: 10.1016/s0140-6736(82)91506-9. [DOI] [PubMed] [Google Scholar]