Abstract
Strains of Cryptococcus neoformans expressing heteroresistance to fluconazole have been described previously. The present study was conducted to investigate the prevalence of heteroresistance among clinical isolates of C. neoformans and to characterize the heteroresistant phenotypes. A total of 107 clinical isolates of C. neoformans for which the MICs of fluconazole ranged from 0.25 to 32 μg/ml were selected. The isolates were chosen to represent a broad geographic distribution. Of the 107 C. neoformans isolates tested, 4 grew on medium containing fluconazole at concentrations that were four to eight times higher than the MICs for each strain. A fifth isolate, for which the fluconazole MIC was 32 μg/ml, grew on agar with 64 μg of fluconazole per ml. These five isolates (4.7% of the total number) were confirmed to exhibit heteroresistant compositions by population analysis. The degree and frequency of resistance varied among the isolates. Stepwise selection by exposure to fluconazole resulted in subclones of all five strains for which the fluconazole MIC was >64 μg/ml. Subclones of three strains demonstrated a homogenous population of resistant cells on medium containing 64 μg of fluconazole/ml. The resistance was sensitive to incubation temperature, that is, heteroresistance was demonstrable only at 30°C by agar-based tests, and was reversible through serial transfers on fluconazole-free medium over a period of 8 days. These results suggest that the fluconazole-heteroresistant phenotype of C. neoformans exists in a significant proportion of clinical isolates and that fluconazole resistance can be developed by selection from heteroresistant clones and induction by exposure to fluconazole.
Cryptococcus neoformans has a worldwide distribution and is one of the most important agents of life-threatening infection among the community-acquired opportunistic fungal pathogens (8). Since the 1980s, the incidence of Cryptococcus infections in some countries has increased dramatically as a result of AIDS (8, 12). In the United States, the majority of studies report a prevalence of C. neoformans infection in the range of 5 to 10% among patients infected with human immunodeficiency virus (8, 17). In AIDS patients, cryptococcosis is considered incurable and requires lifelong antifungal therapy (17). Current regimens for treatment of the disease remain focused on amphotericin B, with or without flucytosine, for induction treatment, while fluconazole remains the agent of choice for long-term maintenance treatment (6, 17, 23). Previous studies have demonstrated that most recurrences of cryptococcal meningitis during maintenance therapy were due to the persistence of the original infecting strains rather than reinfection with a new cryptococcal strain (5, 8, 21). The MICs for serial isolates from patients with persistent cryptococcosis generally did not change significantly, and strains resistant to fluconazole were infrequently isolated from these patients during episodes of recurrent cryptococcal meningitis (5, 6, 8). However, concerns regarding fluconazole-resistant strains of C. neoformans have been expressed by several investigators (1, 4, 5, 6, 24; J. P. Viard, C. Hennequin, N. Fortineau, N. Pertuiset, C. Rothschild, and H. Zylberberg, Letter, Lancet 346:118, 1995).
Recently, strains of C. neoformans expressing heteroresistance to fluconazole have been described (18). Mondon et al. (18) investigated serial isolates from two infected patients and demonstrated that each isolate produced cultures with heterogeneous compositions of fluconazole susceptibility and that the proportion of subpopulations resistant to fluconazole (64 μg/ml) increased steadily over time. This selection process was reproduced in vitro.
The present study was performed to investigate the prevalence of heteroresistant strains of C. neoformans among clinical isolates and to characterize the heteroresistant phenotypes.
MATERIALS AND METHODS
Microorganisms.
A total of 107 clinical isolates of C. neoformans for which the MICs of fluconazole ranged from 0.25 to 32 μg/ml were selected. Since our purpose was to investigate the prevalence of heteroresistant strains of C. neoformans among clinical isolates, the isolates in this study were selected from diverse geographic areas to include fluconazole-susceptible (MICs, <8 μg/ml) and fluconazole-susceptible dose-dependent (MICs, 16 to 32 μg/ml) isolates as determined by reference broth microdilution testing as recommended by the NCCLS. The isolates used in this study included strains for which fluconazole MICs were 0.25 μg/ml (1 isolate), 0.5 μg/ml (2 isolates), 1 μg/ml (12 isolates), 2 μg/ml (45 isolates), 4 μg/ml (18 isolates), 8 μg/ml (19 isolates), 16 μg/ml (7 isolates), and 32 μg/ml (3 isolates). These isolates were from the United States (84 isolates) and Africa (23 isolates). The isolates from the United States were obtained from AIDS patients located in California, Iowa, Texas, and Georgia as part of a population-based survey of cryptococcal disease conducted by the Centers for Disease Control and Prevention (5, 6). The African isolates were obtained from AIDS patients with cryptococcal meningitis in Kampala, Uganda (21). All isolates were stored as suspensions in sterile distilled water at room temperature until the study was performed. Prior to testing, each isolate was subcultured at least twice on potato dextrose agar (PDA) plates (Remel, Lenexa, Kans.) to ensure purity and viability.
Antifungal agents.
Reagent grade fluconazole powder was obtained from Pfizer. Stock solutions were prepared in water. The fluconazole stock solution was diluted with RPMI 1640 medium (Sigma, St. Louis, Mo.) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma) and was dispensed into 96-well microdilution trays. Trays containing an aliquot of 0.1 ml in each well were sealed and frozen at −70°C until needed.
E test strips containing fluconazole (0.016 to 256 μg/ml) were obtained from AB BIODISK (Solna, Sweden).
Susceptibility testing.
Broth microdilution MICs were determined according to the method of the NCCLS (20, 25). The yeast inoculum was adjusted spectrophotometrically to match the turbidity of a 0.5 McFarland standard and diluted to a concentration of 1 × 103 to 5 × 103 cells/ml in RPMI 1640 medium. Each well of the microdilution tray containing 0.1 ml of RPMI 1640 medium with antifungal agents was inoculated with a 0.1-ml aliquot of the yeast preparation. The final concentrations of fluconazole ranged from 0.125 to 128 μg/ml. In each case, the inoculum size was verified by colony counting. The microdilution trays were incubated at 35°C. The MIC endpoints were read visually following 72 h of incubation. The MIC of fluconazole was defined as the lowest concentration that produced a 50% reduction in growth (prominent decrease in turbidity) compared with that of the drug-free growth control (5, 20, 22, 25).
E tests (11) and agar dilution screening (13) were also performed to determine susceptibility to fluconazole. For the E test, 90-mm-diameter plates containing agar at a depth of 4.0 mm were used. The agar formulations used for the E test consisted of RPMI 1640 medium supplemented with 1.5% agar and 2% glucose and buffered with MOPS. The agar surface was inoculated by using a nontoxic swab dipped in a cell suspension adjusted spectrophotometrically to the turbidity of a 1.0 McFarland standard. After excess moisture was absorbed into the agar and the surface was completely dry, an E test strip was applied to each plate. The plates were incubated at 35°C and read after 48 and 72 h. The MIC was read at the lowest concentration at which the border of the elliptical inhibition zone intercepted the scale on the strip. Any growth, such as microcolonies, throughout a discernible inhibition ellipse was ignored (11).
The agar dilution method was performed as previously described by Kirkpatrick et al. (13).
Screening of heteroresistant isolates.
Cell suspensions (1 × 103 to 5 × 103 CFU/ml) in sterile saline were plated on PDA plates containing fluconazole at concentrations four to eight times higher than the MICs for the respective isolates. The isolates for which fluconazole MICs were 16 to 32 μg/ml were plated on plates containing 64 μg of fluconazole per ml. The growth pattern was read after 72 h of incubation at 30°C, and the isolates were regarded as possibly heteroresistant when colonies grew on a plate containing fluconazole.
Resistance of clonal populations.
Analysis of fluconazole heteroresistance was performed by the method of Mondon et al. (18). A single colony from the growth of each isolate was suspended in sterile saline. Cells were counted with a hemocytometer and diluted to approximately 3 × 103 CFU per ml. An aliquot of 100 μl of the suspension was plated on PDA without fluconazole or with 8, 16, 32, 64, or 128 μg of fluconazole/ml. For each concentration, three plates were inoculated. The number of colonies that grew on plates containing fluconazole at 30°C for 72 h was compared with the number that grew on plates without fluconazole. When heteroresistant clones were selected, the clones were also passaged on PDA with stepwise increasing concentrations of fluconazole (up to 64 μg/ml). At each passage, the plates were incubated at 30°C for 72 h.
Quality control.
Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as quality control organisms and were included each time a set of isolates was tested (20, 22).
Strain typing.
Electrophoretic karyotyping by using a contour-clamped homogeneous electrophoretic field system (CHEF-DRII; Bio-Rad, Richmond, Calif.) was performed to confirm molecular relatedness among the various heteroresistant clones. DNA samples were prepared as described previously (2, 21). Electrophoresis was performed in 1.0% agarose (Seakem GTG agarose; FMC Bioproducts) and 0.5× TBE (0.5 M Tris, 0.5 M sodium borate, 0.005 M Na2EDTA) at 14°C and 4.5 V/cm. Each gel was run for 48 h with the switch time ramping from 120 to 280 s.
Effect of temperature on expression of resistance.
The influence of temperature on the expression of heteroresistance was studied at temperatures of 30 and 35°C.
Stability of fluconazole resistance in vitro.
Resistant subclones were transferred daily (50 μl) into 5 ml of fresh fluconazole-free Sabouraud modified antibiotic medium 3 (BBL, Cockeysville, Md.) at 30 and 35°C. At each passage, the proportion of subpopulations resistant to fluconazole (64 μg/ml) was determined by subculturing onto fluconazole-containing agar plates. Likewise, the fluconazole MIC for each subclone was determined after each passage by using both broth microdilution and E test methods.
RESULTS
Prevalence and phenotype of heteroresistance among the clinical isolates of C. neoformans.
Of the 107 C. neoformans isolates tested, 4 grew on PDA containing fluconazole at concentrations four to eight times higher than the MICs for each strain. In addition, strain 20.074.038 was included for further evaluation since it was the only strain among the nine isolates for which the fluconazole MICs were 16 to 32 μg/ml that was able to grow on PDA with 64 μg of fluconazole per ml. Therefore, these five isolates (4.7% of the total number) represented possible fluconazole-heteroresistant strains and were used for further characterization of the heteroresistant phenotype.
A single clone of each isolate, selected at random from the colonies grown on PDA, showed a heterogeneous composition in which most of the cells were susceptible but in which some cells demonstrated the ability to grow at fluconazole concentrations that were at least fourfold higher than the original MIC (Fig. 1). For comparison, five additional isolates which failed to grow on screening plates were investigated for the existence of heteroresistant subpopulations and showed clear cutoff points, failing to grow on plates containing fluconazole at concentrations greater than two times the respective MICs (data not shown).
FIG. 1.
Population analysis of the five isolates which were screened on fluconazole (FLCZ)-containing medium. Values on the y axis indicate the percentage of clonal populations which grew on the plate containing different concentrations of fluconazole (x axis).
The highest concentration at which resistant clonal populations were able to grow differed among the five isolates. Strains 20.067.061 (fluconazole MIC, 8 μg/ml) and 20.074.039 (fluconazole MIC, 32 μg/ml) were found to have highly resistant subpopulations (selected on plates with 64 μg of fluconazole/ml), originally at frequencies of 0.3 and 1.2%, respectively. For both strain 20.021.051 and strain 20.074.013, the fluconazole MIC was 2 μg/ml, and both had minor (0.3 to 0.7%) subpopulations that grew on agar plates with 8 μg of fluconazole/ml. Strain 20.067.025 (fluconazole MIC, 4 μg/ml) demonstrated a subpopulation that grew on plates with 32 μg of fluconazole/ml. The three strains with highly resistant (selected on plates with 32 to 64 μg of fluconazole/ml) subpopulations (20.067.061, 20.74.039, and 20.067.025) were exposed to stepwise increasing concentrations of fluconazole by subculture on fluconazole containing PDA, and all isolates were subcultured successfully at fluconazole concentrations up to 64 μg/ml (Table 1).
TABLE 1.
Comparison of antifungal susceptibilities of parent isolates and their heteroresistant clones
| Strain | Isolate or clonea | Fluconazole MIC (μg/ml) according to:
|
||
|---|---|---|---|---|
| Broth microdilution | E test | Agar dilution | ||
| 20.021.051 | Parent | 2 | 16 | 32 |
| 8 | 4 | 64 | 32 | |
| 16 | 16 | >256 | 64 | |
| 32 | 16 | >256 | 128 | |
| 64 | 16 | >256 | 128 | |
| 20.067.025 | Parent | 4 | 24 | 64 |
| 32 | 4 | 32 | 128 | |
| 64 | 16 | >256 | 128 | |
| 20.067.061 | Parent | 8 | >256 | 128 |
| 64 | 32 | >256 | 128 | |
| 20.074.013 | Parent | 2 | 1 | 16 |
| 8 | 4 | 6 | 32 | |
| 16 | 4 | 12 | 32 | |
| 32 | 4 | 16 | 32 | |
| 64 | 16 | NAb | 64 | |
| 20.074.039 | Parent | 32 | >256 | 128 |
| 64 | 64 | >256 | 128 | |
Clones were selected on PDA supplemented with different concentrations of fluconazole. Numbers indicate the fluconazole concentration (micrograms per milliliter) at which each subclone was obtained.
NA, not available.
In vitro susceptibility results of the parent isolates and their resistant clones.
Fluconazole susceptibility was assayed by the broth microdilution method, the E test, and the agar dilution method. Although the three methods found different MICs, a trend characterized by stepwise reduction of the fluconazole susceptibility of the serially selected subclones was apparent (Table 1). Most of the resistant clones of each isolate selected on plates with 64 μg of fluconazole/ml had MICs of ≥128 μg/ml by both the E test and the agar dilution method. Broth microdilution MICs for resistant clones were two- to eightfold higher than those for parent isolates, again suggesting heteroresistance.
The wide variance between MICs obtained by the broth microdilution and agar-based methods may be attributed to the enhanced growth produced by the increased amount of glucose (2% supplementation) contained in the agar medium. Similar medium-specific differences have been observed previously (11, 13, 18, 25).
Resistant clones.
With three strains, clones selected on PDA with 64 μg of fluconazole per ml demonstrated a homogenous population of highly resistant cells (Table 2). One hundred percent of the inoculated cells were able to grow on agar with 64 μg of fluconazole per ml at 30°C. In contrast, the resistant clone of strain 20.074.013 failed to grow on agar with 64 μg of fluconazole per ml. Although the resistant clone of strain 20.074.039 grew on agar with 64 μg of fluconazole per ml, growth represented only 3.4% of the starting inoculum, similar to that of the parent strain (2.1%).
TABLE 2.
Percentage of subpopulation of resistant clones which grew on agar plates supplemented with 64 μg of fluconazole per ml
| Strain | Isolate or clonea | % Growth |
|---|---|---|
| 20.021.051 | Parent | 0 |
| 64 | 100 | |
| 20.067.025 | Parent | 0 |
| 64 | 117 | |
| 20.067.061 | Parent | 0.3 |
| 64 | 105 | |
| 20.074.013 | Parent | 0 |
| 64 | 0 | |
| 20.074.039 | Parent | 2.1 |
| 64 | 3.4 |
Subclones were selected on plates with 64 μg of fluconazole per ml.
Stability of resistance in vitro.
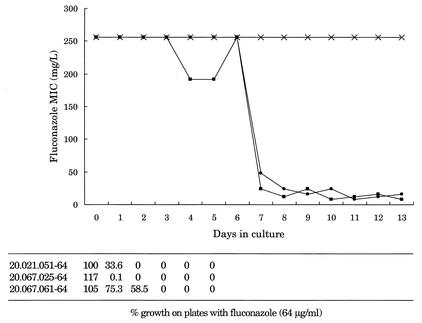
The highly resistant subclones of strains 20.021.051, 20.067.025, and 20.067.061 were passed in drug-free medium to assess the stability of resistance. Strains 20.074.013 and 20.074.039 were not included since a homogeneously resistant population could not be obtained from these isolates. With all three strains, the resistance as determined by growth on fluconazole-containing agar was reversible through serial transfers in fluconazole-free medium at 30°C (Fig. 2). The fluconazole MICs (determined by E test) for strains 20.021.051 and 20.067.025 reverted to the baseline over 8 passages, whereas strain 20.067.061 remained highly resistant though 13 passages.
FIG. 2.
Stability of fluconazole resistance after daily transfers in drug-free medium at 30°C. Shown are the MICs determined from E tests and the percentage of positive growth on medium containing 64 μg of fluconazole per ml for homogeneously resistant clones that were selected on medium with 64 μg of fluconazole per ml. •, 20.021.051-64; ▪, 20.067.025-64; ×, 20.067.061-64.
Effect of temperature on expression of resistance.
The expression of resistance was found to be affected by temperature. Expression of heteroresistance was completely absent at 35°C, that is, no growth was seen for any of the three isolates on agar with 64 μg of fluconazole per ml at 35°C. Heteroresistance was only demonstrable at 30°C using agar-based tests.
Strain typing.
Although the karyotypes of the five isolates differed from each other, each parent and resistant clone had the identical karyotype, confirming the absence of cross-contamination between isolates during the experimentation process (data not shown).
DISCUSSION
Recently, a heterogeneous phenotype in clinical isolates of C. neoformans isolated from AIDS patients with persistent meningitis was described (18). Mondon et al. (18) reported seven isolates of C. neoformans containing fluconazole-heteroresistant subpopulations, of which one isolate was from Israel and six were serial isolates from an Italian patient with AIDS. Single colonies in each of the seven isolates were shown to contain mixed populations of C. neoformans with different susceptibilities to fluconazole. Highly resistant clones for which the MICs exceeded 64 μg/ml were observed in these isolates at frequencies ranging from 0.7 to 4.6%. Homogeneous, highly resistant clones were selectable on medium with a high drug concentration after a single passage (18). The prevalence of such heteroresistant phenotypes in clinical isolates of C. neoformans was previously unknown. In this study, we found fluconazole heteroresistance in C. neoformans at a rate of 4.7% (5 of 107 isolates) among clinical isolates obtained from a broad geographic distribution. Analysis of clonal subpopulations of these five isolates revealed that they exhibited heterogeneous populations which showed varied susceptibility to fluconazole, similar to the results reported by Mondon et al. (18). The highest concentration at which resistant clonal populations were able to grow differed among the five isolates. Two isolates already had highly resistant subpopulations which were able to grow on medium containing 64 μg of fluconazole per ml. Although the other three isolates did not grow on medium containing 64 μg of fluconazole per ml in the original culture, heteroresistant clones derived from these three isolates could grow at fluconazole concentrations that were at least fourfold higher than the original MIC. Furthermore, these clones were subcultured successfully at fluconazole concentrations up to 64 μg/ml upon exposure to stepwise increasing concentrations of fluconazole.
The heteroresistant phenotype has also been observed in Candida albicans (14, 15). The recent study by Marr et al. (15) identified serial isolates from two bone marrow transplant patients and demonstrated that initial isolates from these patients produced cultures with heterogeneous compositions in fluconazole susceptibility. Rapid induction of highly resistant isolates, which subsequently caused disseminated infection, during fluconazole therapy was found to be associated with this heterogeneous phenotype (14, 15). Furthermore, this rapid selection process was reproduced in vitro (15). These facts suggest the clinical significance of the heteroresistant phenotype as a potential cause of the failure of fluconazole treatment in immunosuppressed patients.
Whether the heteroresistant phenotype in C. neoformans accounts for the clinical failures of fluconazole therapy is currently unknown. Although the fluconazole heteroresistance reported in the study by Mondon et al. (18) was associated with recurrent cryptococcal meningitis, the patient was not treated with fluconazole.
Heteroresistance to methicillin among staphylococci is well known (10, 16), and the degrees of the heterogeneity and stability of the resistant clones have been shown to be influenced by environmental factors such as temperature, osmolarity, pH, light, anaerobiosis, chelating agents, and metal ions (16). In the present study, the heteroresistant phenotype of C. neoformans was found to be affected by incubation temperature and growth on agar medium, which supports and extends findings reported previously (18). These characteristics may be of interest with regard to clinically resistant isolates. Since in vitro resistance to fluconazole remains uncommon among C. neoformans isolates despite extensive use of this agent for maintenance therapy or prophylaxis (5, 6, 22, 30), a 4.7% rate of heteroresistance seems quite high. Brandt et al. (6) compared the antifungal susceptibilities of two collections of C. neoformans isolates obtained through active laboratory-based surveillance from 1992 to 1994 (368 isolates) and from 1996 to 1998 (364 isolates). They found that resistance to fluconazole in C. neoformans was uncommon and had not changed significantly with time over the past decade. Those authors also analyzed 172 serial isolates from 71 AIDS patients and found that there was little change in the fluconazole MIC in the majority of cases (6). Although we were not able to clearly assess the relationship between the heteroresistance phenotype and clinical resistance in vivo, it seems that fluconazole heteroresistance might not be observable in the majority of clinical cases because of the temperature dependence.
On the other hand, this study confirmed that fluconazole resistance can be developed rapidly through stepwise induction by fluconazole exposure. Three clones were subcultured successfully at fluconazole concentrations up to 64 μg/ml by exposure to increasing concentrations of the drug. Inducible azole resistance has been documented previously for other yeasts such as C. albicans (7, 15), Candida glabrata (3), and Candida dubliniensis (19). In the clinical situation, serial isolates of C. neoformans from AIDS patients generally show no increase in fluconazole resistance (6). However, the emergence of clinically significant resistance to fluconazole during therapy has been reported in individual cases of recurrent cryptococcosis (1, 4, 24; Viard et al., letter). Brandt et al. (6) reported that greater-than-fourfold changes in MICs were seen in eight patients and documented an intriguing case in which fluconazole MICs for serial isolates increased stepwise from 4.0 to 64 μg/ml over 18 months of fluconazole maintenance therapy without subtype changes analyzed by genotype (5, 6). Thus, the possibility that stepwise development of fluconazole resistance in vivo may occur during fluconazole therapy clearly exists. Our findings in vitro complement these reports.
The molecular mechanism of fluconazole resistance in C. neoformans has not yet been clearly elucidated. Changes in the affinity of the target enzyme (sterol 14α-demethylase) and decreases in the cellular content of fluconazole have been found to be responsible for the resistance in isolates with low-level and high-level resistance, respectively (27). It is likely that multiple mechanisms, such as decreased target affinity, drug uptake defects, induction of resistance genes that encode multidrug efflux pump proteins, or overexpression of the target enzymes, all play a role (9, 28). In C. albicans, induction of azole resistance was associated with an increase in mRNA specific for the CDR ATP-binding cassette transporter efflux pump (15). A similar gene encoding a protein related to several eukaryotic multidrug resistance proteins, CneMDR1, has been identified in a clinical isolate of C. neoformans (26). Although we did not examine the molecular mechanisms of resistance, gene expression and transcriptional regulation of such genes may play an important role in the heteroresistant phenotype. In this study, with three isolates, clones selected on PDA with 64 μg of fluconazole per ml demonstrated a homogenous population of highly resistant cells. In contrast, the resistant clone of strain 20.074.013 failed to grow on agar with 64 μg of fluconazole per ml. Although the resistant clone of strain 20.074.039 grew on plates with 64 μg of fluconazole per ml, the growth represented only 3.4% of the starting inoculum, similar to that of the parent strain. We also found that the high-level resistance was reversible after serial passage in drug-free medium. This transient resistance was also seen among heteroresistant isolates of C. neoformans (18) and C. albicans (14, 15).
The process of development of fluconazole resistance in C. neoformans may be quite varied. Recently, Xu et al. (29) investigated patterns of mutation leading to fluconazole resistance among 21 clinical isolates of C. neoformans for which fluconazole MICs ranged from 0.25 to 4 μg/ml. They observed the growth pattern of the isolates on medium with 8 μg of fluconazole per ml and found that the growth rates of putative resistant mutants on the fluconazole plates were significantly different among the isolates. Furthermore, the MICs of fluconazole for these mutants differed significantly among the isolates as well as among replicates of the same isolate, with values ranging from 2- to 64-fold higher than those for the original isolates. Xu et al. concluded that the mutation leading to fluconazole resistance in C. neoformans in vitro is a dynamic and heterogeneous process and speculated that there exist multiple mechanisms for acquisition of drug resistance in this species (29). Interestingly, the MICs for the mutants developed by Xu et al. (29) were stable after subculture in drug-free medium, suggesting that the mechanisms of resistance may be different from that observed in the present study.
Detection of the heteroresistant phenotype in a clinical microbiology laboratory is difficult. Population analysis is not practical in the typical clinical laboratory, and a heteroresistant subpopulation might not be distinguishable from the trailing growth that is often observed with the reference broth microdilution method. From this standpoint, agar-based methods such as the E test and the disk diffusion test might have greater potential to detect the heteroresistant phenotype since the presence and size of the colonies within the zones of inhibition can be observed. The advantage of the E test in the detection of heteroresistance has been described in previous studies (15, 18). Although there were no distinct macrocolonies within the ellipse of the E test when we tested the five heteroresistant isolates, the observed ellipses were less distinct than those of fluconazole-susceptible isolates. Given the temperature-dependent characteristic of heteroresistance, conducting the E test at a lower temperature (30°C) might also enhance the detection of heteroresistant subpopulations.
In summary, we confirmed the existence of heteroresistant clones in a significant proportion (4.7%) of clinical isolates of C. neoformans. Notably, the degree of heteroresistance was strain dependent. The rapid selection of resistant phenotypes under exposure to fluconazole was also accomplished in vitro. Further studies are required to determine the clinical significance of fluconazole heteroresistance in C. neoformans and its role as a cause of clinical failure in fluconazole therapy. The development of appropriate methods to detect heteroresistant isolates and the elucidation of the molecular mechanisms of heteroresistance are also interesting areas for future investigation.
Acknowledgments
Toshiaki Yamazumi is partly supported by a grant from the Japan Clinical Pathology Foundation for International Exchanges. This study was supported by a grant from Pfizer Pharmaceuticals.
REFERENCES
- 1.Armengou, A., C. Porcar, J. Mascaro, and F. Garcia-Bragado. 1996. Possible development of resistance to fluconazole during suppressive therapy for AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 23:1337-1338. [DOI] [PubMed] [Google Scholar]
- 2.Barchiesi, F., R. J. Hollis, S. A. Messer, G. Scalise, M. G. Rinaldi, and M. A. Pfaller. 1995. Electrophoretic karyotype and in vitro antifungal susceptibility of Cryptococcus neoformans isolates from AIDS patients. Diagn. Microbiol. Infect. Dis. 23:99-103. [DOI] [PubMed] [Google Scholar]
- 3.Barchiesi, F., D. Calabrese, D. Sanglard, L. Falconi Di Francesco, F. Caselli, D. Giannini, A. Giacometti, S. Gavaudan, and G. Scalise. 2000. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750. Antimicrob. Agents Chemother. 44:1578-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, J., C. J. Clancy, and M. H. Nguyen. 1998. The hidden danger of primary fluconazole prophylaxis for patients with AIDS. Clin. Infect. Dis. 26:186-187. [DOI] [PubMed] [Google Scholar]
- 5.Brandt, M. E., M. A. Pfaller, R. A. Hajjeh, E. A. Graviss, J. Rees, E. D. Spitzer, R. W. Pinner, L. W. Mayer, et al. 1996. Molecular subtypes and antifungal susceptibilities of serial Cryptococcus neoformans isolates in human immunodeficiency virus associated cryptococcosis. J. Infect. Dis. 174:812-820. [DOI] [PubMed] [Google Scholar]
- 6.Brandt, M. E., M. A. Pfaller, R. A. Hajjeh, R. J. Hamill, P. G. Pappas, A. L. Reingold, D. Rimland, and D. W. Warnock for the Cryptococcal Disease Active Surveillance Group. 2001. Trends in antifungal drug susceptibility of Cryptococcus neoformans isolates in the United States: 1992 to 1994 and 1996 to 1998. Antimicrob. Agents Chemother. 45:3065-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvet, H. M., M. R. Yeaman, and S. G. Filler. 1997. Reversible fluconazole resistance in Candida albicans: a potential in vitro model. Antimicrob. Agents Chemother. 41:535-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall, A., and J. R. Perfect (ed.). 1998. Epidemiology, p. 351-380. In Cryptococcus neoformans. American Society for Microbiology, Washington, D.C.
- 9.Casadevall, A., and J. R. Perfect (ed.). 1998. Therapy of cryptococcosis, p. 457-518. In Cryptococcus neoformans. American Society for Microbiology, Washington, D.C.
- 10.Chambers, H. F. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 10:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A., M. Pfaller, M. E. Erwin, and R. N. Jones. 1996. Interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using Casitone agar and solidified RPMI 1640 medium with 2% glucose. J. Clin. Microbiol. 34:848-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajjeh, R. A., M. E. Brandt, and R. W. Pinner. 1995. Emergence of cryptococcal disease: epidemiologic perspectives 100 years after its discovery. Epidemiol. Rev. 17:303-320. [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick, W. R., R. K. McAtee, S. G. Revankar, A. W. Fothergill, D. I. McCarthy, M. G. Rinaldi, and T. F. Patterson. 1998. Comparative evaluation of National Committee for Clinical Laboratory Standards broth macrodilution and agar dilution screening methods for testing fluconazole susceptibility of Cryptococcus neoformans. J. Clin. Microbiol. 36:1330-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marr, K. A., C. N. Lyons, T. R. Rustad, R. A. Bowden, T. C. White, and T. Rustad. 1998. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob. Agents Chemother. 42:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marr, K. A., C. N. Lyons, K. Ha, T. R. Rustad, and T. C. White. 2001. Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob. Agents Chemother. 45:52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews, P. R., and P. R. Stewart. 1984. Resistance heterogeneity in methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 11:161-166. [Google Scholar]
- 17.Mitchell, T. G., and J. R. Perfect. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondon, P., R. Petter, G. Amalfitano, R. Luzzati, E. Concia, I. Polacheck, and K. J. Kwon-Chung. 1999. Heteroresistance to fluconazole and voriconazole in Cryptococcus neoformans. Antimicrob. Agents Chemother. 43:1856-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran, G. P., D. J. Sullivan, M. C. Henman, C. E. McCreary, B. J. Harrington, D. B. Shanley, and D. C. Coleman. 1997. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob. Agents Chemother. 41:617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Pfaller, M. A., J. Zhang, S. Messer, M. Tumberland, E. Mbidde, C. Jessup, and M. Ghannoum. 1998. Molecular epidemiology and antifungal susceptibility of Cryptococcus neoformans isolates from Ugandan AIDS patients. Diagn. Microb. Infect. Dis. 32:191-199. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., J. Zhang, S. A. Messer, M. E. Brandt, R. A. Hajjeh, C. J. Jessup, M. Tumberland, E. K. Mbidde, and M. A. Ghannoum. 1999. In vitro activities of voriconazole, fluconazole, and itraconazole against 566 clinical isolates of Cryptococcus neoformans from the United States and Africa. Antimicrob. Agents Chemother. 43:169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powderly, W. G., D. M. Finkelstein, J. Feinberg, P. T. Frame, W. He, C. M. van der Horst, S. L. Koletar, M. E. Eyster, J. Carey, H. A. Waskin, T. M. Hooton, N. E. Hyslop, S. A. Spector, and S. A. Bozzette. 1995. A randomized trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 332:700-705. [DOI] [PubMed] [Google Scholar]
- 24.Prugam, A., J. Dupouy-Camet, P. Blanche, J. P. Gangneux, C. Tourte-Schaefer, and D. Sicard. 1994. Increased fluconazole resistance of Cryptococcus neoformans isolated from a patient with AIDS and recurrent meningitis. Clin. Infect. Dis. 19:975-976. [DOI] [PubMed] [Google Scholar]
- 25.Sanati, H., S. A. Messer, M. A. Pfaller, M. Witt, R. Larsen, A. Espinel-Ingroff, and M. Ghannoum. 1996. Multicenter evaluation of broth microdilution method for susceptibility testing of Cryptococcus neoformans against fluconazole. J. Clin. Microbiol. 34:1280-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thornewell, S. J., R. B. Peery, and P. L. Skatrud. 1997. Cloning and characterization of CneMDR1: a Cryptococcus neoformans gene encoding a protein related to multidrug resistance proteins. Gene 201:21-29. [DOI] [PubMed] [Google Scholar]
- 27.Venkateswarlu, K., M. Taylor, N. J. Manning, M. G. Rinaldi, and S. L. Kelly. 1997. Fluconazole tolerance in clinical isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 41:748-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, J., C. Onyewu, H. J. Yoell, R. Y. Ali, R. J. Vilgalys, and T. G. Mitchell. 2001. Dynamic and heterogeneous mutations to fluconazole resistance in Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazumi, T., M. A. Pfaller, S. A. Messer, A. Houston, R. J. Hollis, and R. N. Jones. 2000. In vitro activities of ravuconazole (BMS-207147) against 541 clinical isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2883-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]