Abstract
Procedures for diagnosis of mammary candidosis, including laboratory confirmation, are not well defined. Lactoferrin present in human milk can inhibit growth of Candida albicans, thereby limiting the ability to detect yeast infections. The inhibitory effect of various lactoferrin concentrations on the growth of C. albicans in whole human milk was studied. The addition of iron to the milk led to a two- to threefold increase in cell counts when milk contained 3.0 mg of lactoferrin/ml and markedly reduced the likelihood of false-negative culture results. This method may provide the necessary objective support needed for diagnosis of mammary candidosis.
Candida albicans is an opportunistic pathogen that can cause superficial, localized, and/or systemic infection (4). Superficial and localized breast yeast infections (mammary candidosis) in lactating women have been reported (1, 2). The diagnosis and management of candidosis in the breastfeeding mother and infant are challenging (5). Mammary candidosis is most often presumptively diagnosed by signs or symptoms such as burning or shooting pain in the breast during and after feedings and persistently sore or cracked nipples that do not heal (11). In a recent survey of 312 physicians in the Academy of Breastfeeding Medicine, 95% of family practitioners and 87% of obstetricians/gynecologists did not use laboratory tests to diagnose mammary candidosis (5). However, diagnosis of mammary candidosis based on symptoms alone may be erroneous, as it has been shown that breast pain is frequently associated with Staphylococcus aureus infection among breastfeeding women (1, 12). On the other hand, laboratory culturing of human milk for mammary candidosis is not well defined. Constituents of milk (9), such as lactoferrin (10), may interfere with recovery of Candida by culture. Iron-free human lactoferrin kills C. albicans in a dose-dependent manner (13), whereas iron-saturated lactoferrin does not inhibit C. albicans growth (7). Andersson et al. found that dilute, skim human milk (diluted with RPMI 1640 medium) had fungistatic effects on C. albicans that were reversed by the addition of iron (3). Therefore, we studied the effects of lactoferrin, with and without added iron, on the growth of C. albicans in undiluted whole human milk.
The objectives of this study were (i) to determine how various concentrations of lactoferrin in whole human milk affect the growth of C. albicans and (ii) to quantitate the effect of added iron in cultural recovery of C. albicans from human milk containing lactoferrin. The ultimate goal was to develop a culture technique that minimizes the likelihood of false-negative cultures.
Lactoferrin-free human milk was used as the culture medium. Lactoferrin was removed by treatment with heparin-Sepharose (6). Lactoferrin purchased from Sigma-Aldrich (St. Louis, Mo.) was then added to the milk to obtain three concentrations: 0.1, 1.0, and 3.0 mg per ml. Lactoferrin-free milk and phosphate-buffered saline (PBS) served as control media. C. albicans EK2001 isolated from human milk and maintained on Sabouraud's dextrose agar (SDA) was used as the inoculum. The organism was grown on SDA for 24 h at 37°C. Cells were examined with a microscope to verify cell growth and blastospore phase and then counted in a hemacytometer. Cells were inoculated into milk containing or lacking lactoferrin and PBS to provide 10, 100, or 1,000 cells per ml in duplicate samples and then incubated for 24 h at 37°C to verify cell growth. After incubation for 24 h, iron (300 μg/ml as ferrous sulfate) was added to one set of samples, containing 0 to 3.0 mg of lactoferrin/ml. Another set of samples had no added iron. All samples were incubated at 37°C. Cell concentrations were determined with a hemacytometer at 24-h intervals. To verify the cell counts, samples were also cultured on SDA and the number of CFU was counted at 24-h intervals. The entire procedure was completed in duplicate, and the cell counts and CFU counts were averaged. The coefficient of variation in cell counts for duplicate samples was <10% in all cases and <5% in 85% of the cases. Inhibition of growth by lactoferrin was determined by comparing the number of C. albicans cells (or CFU per milliliter) after 96 h of incubation to the values in the control milk with no lactoferrin and no added iron. Analysis of variance was used to analyze the main effects of (i) inoculum size, (ii) lactoferrin concentration, and (iii) no added iron or added iron.
In a parallel study to determine the prevalence of C. albicans among lactating women, samples of freshly expressed milk with and without added ferrous sulfate (300 μg of iron/ml) were cultured on SDA to determine the influence of added iron on recovery of Candida species.
Because the correlation between cell counts and CFU was very high (r = 0.96), we report herein only the results for the cell counts. Growth of C. albicans in lactoferrin-free human milk was significantly associated with the size of the inoculum (Table 1). The slope of the ascent for the number of cells per milliliter declined after 48 h except for the 103-cell inoculum.
TABLE 1.
Cell concentrations of C. albicans in human milka
| Inoculum and growth medium | Cell count (103)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No added iron
|
Added iron (300 μg/ml)
|
|||||||
| 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | |
| 10 cells/ml | ||||||||
| PBS | 0.004 | 0 | 0 | 0 | 0.005 | 0 | 0 | 0 |
| Lactoferrin-free milk | 400 | 2,490 | 3,075 | 3,143 | 400 | 3,600 | 3,665 | 3,767 |
| Milk + 0.1 mg of lactoferrin/ml | 375 | 1,780 | 2,200 | 2,240 | 375 | 3,225 | 3,380 | 3,538 |
| Milk + 1.0 mg of lactoferrin/ml | 100 | 1,080 | 1,175 | 1,200 | 100 | 2,375 | 2,418 | 2,474 |
| Milk + 3.0 mg of lactoferrin/ml | 75 | 380 | 500 | 510 | 75 | 1,275 | 1,308 | 1,345 |
| 100 cells/ml | ||||||||
| PBS | 0.05 | 0.02 | 0 | 0 | 0.07 | 0.03 | 0 | 0 |
| Lactoferrin-free milk | 625 | 6,421 | 7,525 | 7,765 | 625 | 8,850 | 9,093 | 9,353 |
| Milk + 0.1 mg of lactoferrin/ml | 525 | 4,132 | 4,800 | 4,886 | 525 | 7,900 | 8,142 | 8,246 |
| Milk + 1.0 mg of lactoferrin/ml | 250 | 1,776 | 2,400 | 2,533 | 250 | 5,375 | 5,570 | 5,717 |
| Milk + 3.0 mg of lactoferrin/ml | 200 | 1,562 | 1,775 | 1,807 | 200 | 2,975 | 3,029 | 3,113 |
| 1,000 cells/ml | ||||||||
| PBS | 0.64 | 0.31 | 0 | 0 | 0.72 | 0.34 | 0.12 | 0 |
| Lactoferrin-free milk | 800 | 11,991 | 14,275 | 17,986 | 800 | 56,980 | 67,250 | 80,020 |
| Milk + 0.1 mg of lactoferrin/ml | 750 | 7,056 | 8,400 | 8,551 | 750 | 30,150 | 36,750 | 38,672 |
| Milk + 1.0 mg of lactoferrin/ml | 725 | 3,977 | 5,375 | 5,577 | 725 | 20,250 | 27,500 | 28,847 |
| Milk + 3.0 mg of lactoferrin/ml | 425 | 2,386 | 2,775 | 2,924 | 425 | 6,975 | 7,155 | 7,357 |
The main effects of inoculum size (P < 0.001), lactoferrin concentration (P < 0.001), and addition of iron (P < 0.001) were all highly significant. In addition, the interaction of iron with size of inoculum was significant (P = 0.01): the effect of iron on growth of C. albicans was greatest when the inoculum contained 1,000 cells/ml.
The effect of added lactoferrin is indicated in Table 1 (absolute cell counts) and Fig. 1, 2, and 3 (percent of lactoferrin-free control). At all three levels of inoculation and at each time of observation, the replication of C. albicans was inhibited by lactoferrin. The inhibition of replication was significantly related to the concentration of lactoferrin (P = 0.001). Milk samples containing 1.0 mg of lactoferrin/ml yielded less than 50% of the growth produced in the absence of lactoferrin. At 3.0 mg/ml, lactoferrin also had a notable effect on the morphology of C. albicans; blastospores were smaller (1.5 to 2.0 μm compared to 4 to 5 μm in the lactoferrin-free milk). The addition of iron significantly increased growth of C. albicans (P = 0.001) in the absence or presence of lactoferrin. This stimulation of growth by the presence of iron was greatest in lactoferrin-free milk inoculated with 103 cells/ml but was also evident with the smaller inocula. The addition of iron (300 μg/ml) restored growth to at least 100% of that observed in the lactoferrin-free controls (without added iron) at the 0.1-mg/ml lactoferrin concentration, to at least 75% of the control value at the 1.0-mg/ml concentration, and to at least 40% of the control value at the 3.0-mg/ml concentration.
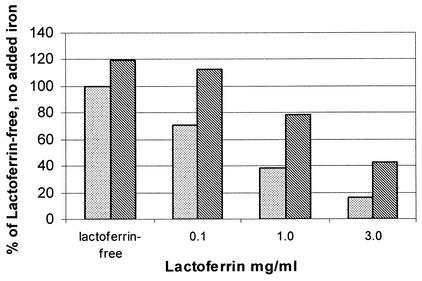
FIG. 1.
Effect of added iron (300 μg/ml) on growth of C. albicans in whole human milk with added lactoferrin at an inoculum size of 10 cells/ml. Cells were incubated for a total of 96 h at 37°C. Cell counts are shown as a percentage of the lactoferrin-free control with no added iron. Lactoferrin significantly inhibited the growth of C. albicans (P < 0.01), and adding iron reversed most of the inhibition (P < 0.001). Stippled bars, no added iron; hatched bars, added iron.
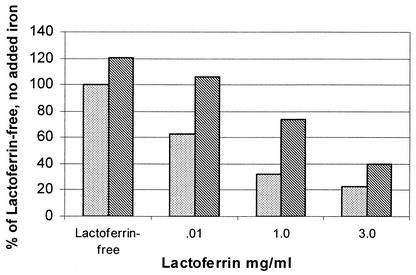
FIG. 2.
Effect of added iron (300 μg/ml) on growth of C. albicans in whole human milk with added lactoferrin at an inoculum size of 100 cells/ml. Cells were incubated for a total of 96 h at 37°C. Cell counts are shown as a percentage of the lactoferrin-free control with no added iron. Lactoferrin significantly inhibited the growth of C. albicans (P < 0.01), and adding iron reversed most of the inhibition (P < 0.001). Stippled bars, no added iron; hatched bars, added iron.
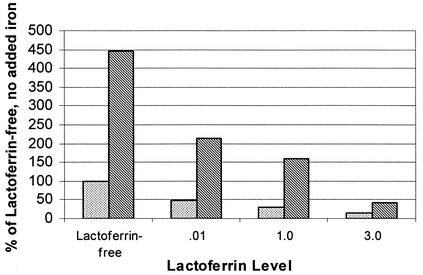
FIG. 3.
Effect of added iron (300 μg/ml) on growth of C. albicans in whole human milk with added lactoferrin at an inoculum size of 1,000 cells/ml. Cells were incubated for a total of 96 h at 37°C. Cell counts are shown as a percentage of the lactoferrin-free control with no added iron. Lactoferrin significantly inhibited the growth of C. albicans (P < 0.001), and adding iron reversed most of the inhibition (P < 0.001). Stippled bars, no added iron; hatched bars, added iron.
When the rates of recovery of C. albicans directly from samples of milk with and without added iron in the parallel prevalence study of 100 women were compared, the addition of iron to the milk prior to inoculation yielded positive cultures in 28 samples, compared with 5 positive (23 negative) without the addition of iron.
The present findings provide evidence that the size of the inoculum (concentration of C. albicans cells) influences the rate of growth of this yeast in whole human milk in vitro, that lactoferrin can inhibit the growth of C. albicans in human milk, and that this inhibition can be reversed by the addition of ferrous iron.
The concentration of lactoferrin is 3.1 mg/ml in transitional milk (6 to 14 days postpartum), 2.0 mg/ml in early mature milk (15 to 28 days postpartum), and 0.5 to 1.5 mg/ml at 30 days postpartum (7, 8). At all these stages, particularly early postpartum, lactoferrin is present at concentrations which were significantly inhibitory to C. albicans in vitro.
The addition of iron (ferrous) countered the inhibitory effect of lactoferrin. For example, 300 μg of iron/ml (a 5.35 M solution) led to approximately a two- to threefold increase in cell counts in the presence of 3.0 mg of lactoferrin/ml (a 38.4 μM solution).
The addition of iron demonstrably increased the rate of recovery of C. albicans from whole human milk. Our results suggest that the addition of iron to whole human milk samples reduces the likelihood of a false-negative result in laboratory tests. Additional clinical trials are needed to determine the extent to which this method of culturing milk will be applicable in enhancing detection of C. albicans in human milk.
Acknowledgments
This project was funded by Supplemental Hatch Funds from the University of California, Davis.
REFERENCES
- 1.Amir, L., S. Garland, L. Dennerstein, and S. Farish. 1996. Candida albicans: is it associated with nipple pain in lactating women? Gynecol. Obstet. Investig. 41:30-34. [DOI] [PubMed] [Google Scholar]
- 2.Amir, L., and S. Pakula. 1991. Nipple pain, mastalgia and candidiasis in the lactating breast. Aust. N. Z. J. Obstet. Gynaecol. 31:378-380. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, Y., S. Lindquist, C. Lagerqvist, and O. Hernell. 2000. Lactoferrin is responsible for the fungistatic effect of human milk. Early Hum. Dev. 59:95-105. [DOI] [PubMed] [Google Scholar]
- 4.Bodey, G. P. 1993. Candidiasis: pathogenesis, diagnosis, and treatment, 2nd ed., p. 371. Raven Press, New York, N.Y.
- 5.Brent, N. B. 2001. Thrush in the breastfeeding dyad: results of a survey on diagnosis and treatment. Clin. Pediatr. 40:503-506. [DOI] [PubMed] [Google Scholar]
- 6.Davidsson, L., P. Kastenmayer, M. Yuen, B. Lönnerdal, and R. F. Hurrell. 1994. Influence of lactoferrin on iron absorption from human milk in infants. Pediatr. Res. 35:117-124. [DOI] [PubMed] [Google Scholar]
- 7.Kirkpatrick, C. H., I. Green, R. R. Rich, and A. L. Schade. 1971. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infect. Dis. 124:539-544. [DOI] [PubMed] [Google Scholar]
- 8.Lönnerdal, B., E. Forsum, M. Gebre-Medhin, and L. Hambraeus. 1976. Breast milk composition in Ethiopian and Swedish mothers. II. Lactose, nitrogen, and protein contents. Am. J. Clin. Nutr. 29:1134-1141. [DOI] [PubMed] [Google Scholar]
- 9.Montagne, P., M. L. Cuilliere, C. Mole, M. C. Bene, and G. Faure. 2001. Changes in lactoferrin and lysozyme concentration in human milk during the first twelve weeks of lactation. Adv. Exp. Med. Biol. 501:241-247. [DOI] [PubMed] [Google Scholar]
- 10.Soukka, T., J. Tenovuo, and M. Lenander-Lumikari. 1992. Fungicidal effect of human lactoferrin against Candida albicans. FEMS Microbiol. Lett. 69:223-228. [DOI] [PubMed] [Google Scholar]
- 11.Tanguay, K. E., M. R. McBean, and E. Jain. 1994. Nipple candidiasis among breastfeeding mothers. Can. Fam. Physician 40:1407-1413. [PMC free article] [PubMed] [Google Scholar]
- 12.Thomassen, P., V. Johansson, C. Wassberg, and B. Petrini. 1998. Breastfeeding, pain and infection. Gynecol. Obstet. Investig. 46:73-74. [DOI] [PubMed] [Google Scholar]
- 13.Xu, Y. Y., Y. H. Samaranayake, L. P. Samaranayake, and H. Nikawa. 1999. In vitro susceptibility of Candida species to lactoferrin. Med. Mycol. 37:35-41. [DOI] [PubMed] [Google Scholar]