CASE REPORT
A previously healthy 35-year-old man presented with fever, coughs, and jaundice. After flu-like symptoms for 2 weeks, the patient developed mild jaundice and high fever. On admission to our hospital, the physical examination revealed scleral jaundice and was otherwise unremarkable. Body temperature was 39.5°C. Chest X ray showed a patchy dense infiltration of the right lower lobe. Abdominal ultrasound showed small bilateral pleural effusions and minimal ascites. Abnormal laboratory findings on admission included alanine aminotransferase (ALT), 79 U/liter (normal, <23 U/liter); bilirubin, 2.8 mg/dl (normal, <1.2 mg/dl); alkaline phosphatase, 753 U/liter (normal, <200 U/liter); serum albumin, 2.7 g/dl (normal, >3.5 g/dl); and international normalized ratio, 2.1 (normal, 1.0).
Known causes of acute hepatitis were excluded: anti-hepatitis A virus immunoglobulin M (IgM), negative; HBsAg and anti-HBc, negative; anti-HCV, negative; anti-nuclear antibodies, <1:10; antimitochondrial antibodies, negative; anti-smooth-muscle antibodies, negative; and no evidence for acute infection with herpes simplex virus, cytomegalovirus, or Epstein-Barr virus. Further, there was no evidence for hemochromatosis or Wilson′s disease. Alcoholic or drug-induced hepatitis was excluded by the patient′s history.
Further serologic workup revealed acute infection with Mycoplasma pneumoniae (IgG enzyme immunoassay [EIA], 263 U/ml; IgA EIA, 162 U/ml; IgM EIA, 338 U/ml (titers of >64 U/ml are considered positive). Antibiotic therapy was started with cefuroxime given intravenously; 1 day later, therapy was changed to clarithromycin (500 mg orally twice daily for 3 weeks), resulting in a quick improvement of the patient′s clinical condition. Ten days after the initiation of the antibiotic therapy, the pulmonary infiltration had disappeared. Liver function tests also improved quickly (Fig. 1), except for the ALT elevation, which persisted about 6 months before returning to normal levels.
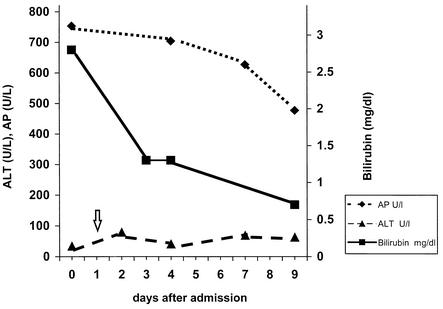
FIG. 1.
Time course of liver function parameters during the patient’s stay in our hospital. Arrow indicates start of macrolide therapy on day 1 following admission. Note rapid improvement of bilirubin level and alkaline phosphatase (AP), while the ALT level remains elevated.
Infection with M. pneumoniae primarily affects the respiratory tract. Symptoms range from mild fever and nonproductive cough to severe pneumonia. Extrapulmonary manifestations include the central nervous system as well as the cardiovascular, gastrointestinal, and hematological systems (1, 2, 5, 6). This case demonstrates that hepatobiliary involvement of M. pneumoniae can occur in the immunocompetent adult. Hepatobiliary involvement of M. pneumoniae infection was confirmed by the close temporal association of impaired liver function test results with clinical and radiological findings typical for M. pneumoniae infection and the exclusion of other causes of cholestatic hepatitis. To date, M. pneumoniae-associated cholestatic hepatitis has been reported only for children (1-3). The pathogenesis of extrapulmonary disease in M. pneumoniae infection is poorly understood. Possible mechanisms include infection of epithelial cells as well as an immune-mediated damage by cross-reactive anti-M. pneumoniae antibodies. A well-described feature of mycoplasma infection is the development of auto-antibodies, such as cold agglutinins, that causehemolysis (4). It is not known whether M. pneumoniae can infect cells of the hepatobiliary system. The rapid improvement of liver function after institution of antibiotic therapy suggests an infection of hepatocytes or biliary cells. The longstanding ALT elevation may be mediated by immunological mechanisms, such as cross-reactive antibodies induced by M. pneumoniae interacting with sialo-oligosaccharides on hepatic cells (3).
Our clinical case suggests that the differential diagnosis of impaired liver function in patients with pneumonia should include cholestatic hepatitis associated with M. pneumoniae infection. The diagnosis is based on a positive M. pneumoniae serology (confirmed several weeks later in convalescence) and the rapid improvement after institution of the appropriate antibiotic therapy.
REFERENCES
- 1.Arav-Boger, R., A. Assia, Z. Spirer, Y. Bujanover, and S. Reif. 1995. Cholestatic hepatitis as a main manifestation of Mycoplasma pneumoniae infection. J. Pediatr. Gastroenterol. Nutr. 21:459-460. [DOI] [PubMed] [Google Scholar]
- 2.Hiew, T. M., A. M. Tan, E. K. Ong, and L. Ho. 1995. Unusual manifestations of Mycoplasma pneumoniae infection in children. Singapore Med. J. 36:293-298. [PubMed] [Google Scholar]
- 3.Roelcke, D., H. Kreft, H. Northoff, and E. Gallasch. 1991. Sia-b1 and I antigens recognized by Mycoplasma pneumoniae-induced human cold agglutinins. Transfusion 31:627-630. [DOI] [PubMed] [Google Scholar]
- 4.Rosengarten, R., C. Citti, M. Glew, A. Lischewski, M. Droesse, P. Much, F. Winner, M. Brank, and J. Spergser. 2000. Host-pathogen interactions in mycoplasma pathogenesis: virulence and survival strategies of minimalist prokaryotes. Int. J. Med. Microbiol. 290:15-25. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz Contreras, V., and V. Pascual Ruiz. 1986. Mycoplasma pneumoniae infection in childhood respiratory pathology. Respiratory, extrarespiratory manifestations and immunological findings. A 2-year study. An. Esp. Pediatr. 24:15-25. (In Spanish.) [PubMed] [Google Scholar]
- 6.Squadrini, F., G. Lami, F. Pellegrino, G. Pinelli, M. Bavieri, A. Fontana, and A. Bisetti. 1988. Acute hepatitis complicating Mycoplasma pneumoniae infection. J. Infect. 16:201-202. [DOI] [PubMed] [Google Scholar]