Abstract
Neonatal diarrhea induced by bovine group A rotavirus causes significant economic loss in the dairy and beef industry due to increased morbidity and mortality, treatment costs, and reduced growth rates. The objective of this study was to evaluate a human group A rotavirus assay (ImmunoCardSTAT Rotavirus [ICS-RV]) as an on-site diagnostic test for bovine rotavirus. When used with a collection of bovine diarrhea samples submitted to the Virology Section of the Diagnostic Center for Population and Animal Health at Michigan State University and compared to a bovine group A rotavirus-specific reverse transcription-PCR (RT-PCR), the ICS-RV assay had a sensitivity and specificity of 87.0 and 93.6%, respectively. A commercially available group A rotavirus enzyme-linked immunosorbent assay (ELISA) (Pathfinder; Sanofi Diagnostics, Redmond, Wash.), when used with the same fecal sample collection and compared to the same RT-PCR, had a sensitivity and specificity of 78.3 and 67.7%, respectively. Subsequently, the ICS-RV assay, RT-PCR, and a different commercially available group A rotavirus ELISA (Rotaclone; Meridian Diagnostics, Cincinnati, Ohio) were used to evaluate fecal samples collected from neonatal calves experimentally infected with bovine rotavirus. When diarrheic fecal samples that were positive for bovine rotavirus by RT-PCR were evaluated, the ICS-RV assay and the Rotaclone assay detected bovine rotavirus 85 and 95% of the time, respectively. Based on these studies, the ICS-RV assay appears to be an excellent test for detecting group A bovine rotaviruses. This assay may be useful as an on-site diagnostic test for veterinarians as an aid in the management of bovine neonatal diarrhea.
Diarrhea is one of the most important diseases of neonatal dairy and beef calves. Substantial economic loss occurs due to increased morbidity and mortality, treatment costs, and reduced growth rates (13). Rotavirus, coronavirus, Escherichia coli strain K99, coccidia, and Cryptosporidium parvum are the main infectious agents inducing enteric infections in neonatal calves less than 2 months of age. Rotavirus itself is responsible for a significant proportion of these (17). In U.S. beef calves, rotavirus and coronavirus together were found in 35% of the diarrhea cases (25). Rotavirus by itself was found to be responsible for 46% of the scours cases in dairy calves (22). The significance of rotavirus as a major cause of neonatal bovine diarrhea has also been documented worldwide (2, 3, 7, 20). Although the clinical course following rotavirus infection is typically short, virus has been detected in feces for up to 3 weeks following infection (17). Most bovine rotaviruses belong to group A and subgroup I, although isolates belonging to groups B and C have been described (16).
Laboratory diagnosis of rotavirus infection in calves has been based on the identification of viral particles, antigens, or nucleic acids in fecal samples. Fluorescent antibody staining of small-intestine tissue sections is a rapid and convenient method to diagnose rotavirus infections in calves. However, because rotavirus infection is not associated with high mortality, live calves are not commonly submitted for necropsy. A variety of methodologies have been developed to diagnose the presence of rotavirus in diarrheic fecal samples. Virus isolation is used in some diagnostic laboratories (1) and is considered to be a very sensitive detection method when combined with fluorescent antibody staining. Negative-staining electron microscopy has been the main method for detecting rotavirus in fecal specimens. Electron microscopy has a relatively low detection limit (105 to 106 viral particles per gram of feces). However, the quantity of rotavirus shed during the clinical phase of the disease is high enough for this not to be a limiting factor.
Antigen capture enzyme-linked immunosorbent assay (ELISA), latex agglutination, and reverse transcription-PCR (RT-PCR) have become more standard methods for the diagnosis of bovine rotavirus infections. Most of the available antigen detection ELISAs were designed and licensed for use in detecting human rotavirus. Their use for the detection of bovine rotavirus is based upon shared group-specific antigenic determinants between bovine and human group A rotaviruses (4, 6, 10, 12). Latex agglutination tests are more rapid than standard ELISAs and are easy to perform. Recent reports show that commercially available latex agglutination tests compare favorably to virus isolation and an ELISA (1, 18). PCR-based detection of group A bovine rotavirus has been described for detection and/or typing of rotavirus isolates (5, 14, 15, 19, 23). In a study by Chinsangaram et al. (5), combining RT-PCR with a post-PCR chemiluminescent hybridization assay resulted in a detection limit of 6 × 102 particles per ml of feces. The results obtained by RT-PCR and ELISA, or a combination of ELISA and electron microscopy, were in complete agreement.
The availability of an on-site diagnostic test would be extremely useful to veterinarians and cattle producers in helping to make control and prevention decisions with regard to neonatal bovine diarrhea. A rapid immunochromatographic test for detection of bovine rotavirus was first described by de Verdier Klingenberg and Esfandiari (9). Compared to ELISA, this test had a sensitivity of 89%, a specificity of 99%, and a reproducibility of 100%. This test is not presently available in the U.S. The ImmunoCardSTAT Rotavirus (ICS-RV) assay (Meridian Diagnostics, Cincinnati, Ohio) is a patient side assay designed to detect group A rotavirus in humans (8). This assay is an antigen capture ELISA based upon lateral-flow rapid immunomigration. This technology allows for packaging of all reagents into a single compact system that can then be used on site. The objective of this study was to evaluate the ICS-RV assay for its usefulness as an on-site diagnostic test for bovine rotavirus.
MATERIALS AND METHODS
Pilot testing and determination of detection limit.
A reference strain of bovine rotavirus with a known viral titer was obtained from the National Veterinary Services Laboratory (Ames, Iowa). Serial dilutions were tested by an antigen detection ELISA (Pathfinder; Sanofi Diagnostics, Redmond, Wash.), by the ICS-RV assay, and by RT-PCR.
Screening of diagnostic submissions.
To determine the accuracy of the ICS-RV assay for detecting bovine rotavirus, a random collection of bovine fecal samples submitted to the Virology Section of the Diagnostic Center for Population and Animal Health at Michigan State University was tested. Samples were tested by the ICS-RV assay, and the results were compared to those obtained by antigen detection ELISA (Pathfinder) and RT-PCR.
Calf inoculation.
Seven newborn colostrum-deprived male Holstein calves were obtained immediately after birth from a local dairy farm that had not had a recent history of rotavirus infection in neonatal calves. On arrival, the calves were given an injection of selenium-vitamin E and vitamins A and D, and their navels were dipped in 7% tincture of iodine. In addition, all calves received 400 mg of enrofloxacin (Baytril; Bayer Animal Health, Shawnee Mission, Kans.) once daily for 7 days. The calves were fed a commercial milk replacer in accordance with the manufacturer's directions. Calves were randomly assigned into an experimental infection group (n = 6) and a control group (n = 1). The primary purpose of the control calf was to verify containment conditions. Infected and control calves were housed in separate rooms at the Michigan State University College of Veterinary Medicine containment facility (one to three calves per room). Within rooms, each calf was housed separately in an individual raised calf cage. Experimentally infected calves (n = 6) were orally inoculated at 48 h of age with 1 ml of rotavirus strain IND (G6P5) (titer, 107 focus-forming units/ml) diluted in 10 ml of 0.01 M phosphate-buffered saline (PBS) (pH 7.2) by using an esophageal tube. The control calf was sham inoculated with 10 ml of PBS.
Calves were examined daily, and health status was recorded. Fecal consistency was recorded on a scale of 0 to 3, with 0 being normal, 1 being pasty, 2 being semisolid, and 3 being watery in consistency. Fecal samples were collected daily for 14 to 21 days, starting 1 day prior to inoculation. At the end of the study, all calves were humanely euthanized. Fecal samples were tested for the presence of rotavirus by the ICS-RV assay, an antigen detection ELISA (Rotaclone; Meridian Diagnostics), and RT-PCR.
Rotavirus detection. (i) ICS-RV.
The ICS-RV assay is a commercially available immunomigration assay designed to detect human group A rotavirus. The assay was adapted to use with bovine feces by using the following method. Fecal matter was diluted 1:1 in PBS and then allowed to settle for 5 min. A 25-μl aliquot of the supernatant was then added to 350 μl of sample diluent buffer provided by the manufacturer. After thorough mixing, 150 μl of diluted sample was added to the sample port of the device. The test is read after a 10-min incubation period. Rotavirus, if present, combines with an anti-rotavirus monoclonal antibody immobilized onto red-purple colloidal gold particles. The mixture migrates by lateral flow along a nitrocellulose membrane towards the result and control windows. In the result window, immobilized anti-rotavirus antibody combines with rotavirus antibody-gold complexes to form a red-purple line. The control window contains immobilized goat anti-mouse antibody. A reddish purple line has to be observed in this window to conclude that the sample migrated properly along the membrane.
(ii) Rotaclone assay.
The Rotaclone assay is a commercially available ELISA designed to detect human group A rotavirus. The assay uses plastic microtiter wells coated with murine monoclonal antibodies directed against VP6, the human rotavirus group-specific antigen. The same monoclonal antibody conjugated to horseradish peroxidase is used as the detector antibody. The assay was run in accordance with the manufacturer's directions.
(iii) Pathfinder assay.
The Pathfinder assay is a commercially available ELISA for the detection of human group A rotavirus. The assay uses plastic tubes coated with a polyclonal antibody to simian SA-11 rotavirus as the capture antibody. The detection antibody is a peroxidase-labeled monoclonal antibody directed against a VP6 epitope of murine rotavirus. The assay conditions used were those specified by the manufacturer.
(iv) RT-PCR.
Primers amplifying a 294-bp fragment of the VP6 gene of type A rotavirus have been designed and previously tested. The sequence of the upstream primer was 5′-ACCACCAAATATGACACCAGC-3′. The sequence of the downstream primer was 5′-CATGCTTCTAATGGAAGC-3′. The suitability of these primers was verified by BLAST analysis and by their ability to also amplify porcine and equine type A rotavirus reference strains (data not shown). RNA from fecal samples and reference stock virus preparations were extracted by using a QIAGEN RNeasy kit (QIAGEN, Inc., Valencia, Calif.). RT-PCR was performed with the Superscript One-Step RT-PCR System (Life Technologies, Rockville, Md.). Five microliters of extracted RNA was mixed with the primers (0.4 μ M), and RNase-free water was added to a total volume of 24 μl. The mixture was heated at 95°C for 4 min and then quickly cooled to 4°C. Superscript 2X-reaction mix (25 μl) and RT-Taq mix (1 μl) were then added. The RT-PCR conditions consisted of cDNA synthesis at 50°C for 30 min and denaturation at 94°C for 2 min, followed by 40 cycles of PCR amplification (94°C for 30 s, 52°C for 30 s, 72°C for 1 min) and a final extension at 72°C for 7 min. Amplification products were visualized in ethidium bromide-stained gels.
Test evaluation.
Sensitivity, specificity, positive predictive value, and negative predictive values were determined by using RT-PCR as the reference test. The ability of the assays to detect bovine rotavirus over time following experimental infection was determined by testing serial fecal samples. The correlation between the test results and the presence of clinical signs of rotavirus-induced diarrhea was determined.
RESULTS
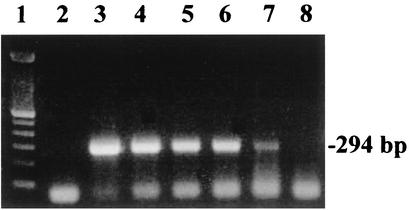
To determine the detection limit of the ICS-RV assay, serial dilutions of a reference strain of bovine rotavirus with a known virus concentration were assayed. Results from the ICS-RV assay were compared with those obtained by using the Pathfinder Rotavirus ELISA and RT-PCR. The detection limits were 3 × 104 50% tissue culture infective dose (TCID50) for the Pathfinder ELISA, 1 × 10 4 TCID50 for the ICS-RV assay, and 70 TCID50 for the RT-PCR (Fig. 1).
FIG. 1.
RT-PCR-based amplification of bovine group A rotavirus RNA. Lane 1, 100-bp DNA ladder; lane 2, negative control (distilled H2O used as a template); lanes 3 to 8, serial 10-fold dilutions of bovine rotavirus stock (107 TCID50/ml). The detection endpoint (lane 7) is equivalent to 70 TCID50.
Fecal samples from 54 calves submitted to the Diagnostic Center for Population and Animal Health at Michigan State University were tested by the ICS-RV assay, the Pathfinder assay, and a group A rotavirus-specific RT-PCR. Of these samples, 22 (41%) were positive by the ICS-RV assay, 28 (52%) were positive by the Pathfinder assay, and 22 (41%) were positive by RT-PCR. Two samples that were positive by the ICS-RV assay were negative by RT-PCR, while three samples that were positive for bovine rotavirus by RT-PCR were negative by the ICS-RV assay. Ten samples yielded a positive rotavirus signal when tested by the Pathfinder assay but were found to be negative when tested by RT-PCR. Five samples were positive by RT-PCR and negative by the Pathfinder assay. Nineteen samples were positive for rotavirus by all three tests. The sensitivities of the ICS-RV assay and the Pathfinder assay were calculated by using RT-PCR as the “gold standard” (Table 1).
TABLE 1.
Sensitivity and specificity of the ICS-RV assay and the Pathfinder ELISA compared to RT-PCR for detecting bovine rotavirus in 54 diarrheic fecal samplesa
| Sensitivity (lower CI)b | Specificity (lower CI)b | Positive predictive value (lower CI)b | Negative predictive value (lower CI)b | Kappa (lower CI) | |
|---|---|---|---|---|---|
| Pathfinder | 78.3 (61.4) | 67.7 (51.3) | 64.3 (46.5) | 80.8 (65.6) | 0.45 (0.19) |
| ICS-RV | 87.0 (73.2) | 93.6 (84.9) | 90.9 (78.9) | 90.6 (80.5) | 0.81 (0.54) |
CI, confidence interval.
Values are percentages.
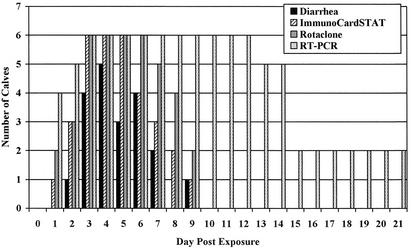
To establish the detection window for diagnosing bovine rotavirus-induced diarrhea, serial fecal samples were taken from calves experimentally infected with group A bovine rotavirus and tested with the ICS-RV assay, the Rotaclone ELISA rotavirus assay, and RT-PCR. The Rotaclone ELISA was substituted for the previously used Pathfinder assay because positive Pathfinder ELISA results on six diagnostic samples could not be substantiated by either the ICS-RV test or RT-PCR. All infected calves developed diarrhea within 4 days of exposure to rotavirus. Diarrhea started an average of 3 days (range, 2 to 4 days) following inoculation and lasted an average of 3.3 days (range, 2 to 5 days) (Fig. 2). The ICS-RV assay first detected rotavirus on average starting 2.2 days (range, 1 to 3 days) postinoculation. The Rotaclone assay performed similarly, first detecting rotavirus starting an average of 2.2 days following infection, while the RT-PCR assay was able to first detect virus starting, on average, 1.5 days (range, 1 to 3 days) following infection. On average, the latest times postinfection that the ICS-RV and Rotaclone assays detected rotavirus were 7 days (range, 5 to 9 days) and 7.8 days (range, 6 to 9 days), respectively. The RT-PCR assay was positive for rotavirus for an average of 13 and 16 days (range, 12 to 18 days) postinfection in calves monitored for 14 and 21 days, respectively.
FIG. 2.
Number of calves for which rotavirus was detected in fecal samples following experimental infection with bovine rotavirus on day 0. From day 0 to 14, n = 6. From day 15 to 21, n = 3.
The usefulness of the ICS-RV and Rotaclone tests was separately assessed also for samples collected during the diarrheic phase resulting from experimental infection. The RT-PCR assay detected rotavirus in 20 (100%) of the diarrheic samples, while 19 (95%) and 17 (85%) of the samples were positive by the Rotaclone and ICS-RV assays, respectively. No diarrhea was recorded in the control calf, and all fecal samples from the control calf were negative for rotavirus by each of the assays.
DISCUSSION
In this study, we evaluated the ICS-RV assay as a potential diagnostic test for bovine rotavirus. We found that the sensitivity and specificity of the test were comparable to those of commercially available enzyme immunoassays and RT-PCR when used to evaluate fecal samples from calves with neonatal diarrhea.
Rapid, simple diagnostic tests that can be used by clinicians to quickly identify the etiological agents associated with neonatal bovine diarrhea would facilitate the implementation of effective control measures. Most currently available methods for the detection of bovine rotavirus are not easily adapted to field settings. Electron microscopy and fluorescent antibody testing require specialized equipment and highly trained personnel to operate. Antigen detection ELISAs are now commonly used in many diagnostic laboratories. All of these have been developed for the detection of human rotavirus, but because the group A antigenic determinants are shared among rotavirus isolates affecting different species, these assays have been readily adapted for use in veterinary medicine (10, 11). The need for specialized laboratory equipment and the time it takes to complete them limit the use of these assays to laboratories or clinics. A drawback of this method is that virus-antibody complexes formed after the onset of the intestinal immune response cannot be detected. PCR is being used more commonly as a tool for the diagnosis of rotavirus. Its advantages are very high sensitivity and its ability to detect viral RNA as effectively in samples containing virus-antibody complexes as in those composed mainly of fully infectious virions. The disadvantages of RT-PCR are cost and the need for specialized equipment and technically proficient staff.
The ICS-RV assay is a simple and accurate patient side assay for the detection of human group A rotavirus. This assay is a rapid immunomigration test. Rotavirus particles present in clarified fecal samples are captured by antibody-coated colloidal gold particles. By horizontal flow, the captured antigen moves towards a linear zone of anti-rotavirus antibody. Binding of the antigen-colloidal gold complexes to the linearized antibody produces a visible line. This one-step assay can be completed in approximately 10 min. The usefulness of this test for detecting human group A rotavirus has been documented. Compared to electron microscopy in one study, the ICS-RV assay had a sensitivity and specificity of 94 and 100%, respectively, for detecting rotavirus in human diarrhea samples (5). These results were comparable with those for two widely used commercial laboratory-based ELISAs.
Initially, we utilized a pretitrated rotavirus preparation to determine the detection limit of the ICS-RV test relative to those of a commercial antigen detection ELISA and RT-PCR. The detection limits of the ICS-RV and ELISA were comparable but at least 100-fold lower than that of the RT-PCR. The absolute sensitivity of a rotavirus assay may not be overly critical as long as the samples tested are collected during the clinical phase of the disease. It has been determined that the viral load can be approximately 109 particles per g of feces during the early stages of the rotavirus-induced diarrhea (21, 24).
Following the initial evaluation, a collection of 54 clinical samples from diarrheic calves was used to evaluate the performance of the ICS-RV test versus ELISA and RT-PCR. The results of this comparison showed that the ICS-RV test had a sensitivity of 87% and a specificity of 93.6%. In contrast to our experience with the Pathfinder assay, it was noted that very weak positive ICS-RV assay results could be reliably scored as positive. Conversely, ICV-RV assay results that were scored negative remained negative well beyond the recommended incubation time of the test. The lower sensitivity of the ICS-RV assay compared to RT-PCR is likely accounted for by the ability of RT-PCR to detect much lower amounts of virus in clinical samples. Although the samples tested were presumably from calves with diarrhea, it is possible that some of these were from calves late in the course of disease when virus shedding may be below the detection limit of the ICS-RV assay. Confidence in test results is important when making disease control recommendations to producers because of the economic ramifications of those recommendations. A shortcoming of the commercial ELISA used was the relatively high rate of false positive tests. This led to a positive predictive value for the ELISA of only 64%. Conversely, the ICS-RV assay had a high positive predictive value of greater than 90%. Likewise, the negative predictive value of the ICS-RV assay was also relatively high.
A limitation of antibody-based detection tests for the detection of enteric pathogens is that the concentration of free antigen needed for the generation of a positive reaction in this test format can decrease significantly, even during the clinical phase. This is due to the complexing of antigen with intestinal mucosal antibody. To evaluate the overall usefulness of the ICS-RV assay in the field, we determined its detection window by orally inoculating colostrum-deprived calves with a reference strain of rotavirus and testing fecal samples daily. The data indicated that the ICS-RV and a commercial ELISA different from the one used in the first part of this study reliably detected rotavirus shedding during the diarrheic phase of the disease. The detection window of the RT-PCR went well beyond the clinical phase, underscoring its usefulness as a laboratory-based reference test for bovine rotavirus.
Ideally, a field test would detect all rotavirus infections. Rotavirus isolates have been classified into groups A to G based upon the antigenic determinants present on the VP6 inner capsid protein. The majority of the characterized strains belong to group A, although group B and C rotaviruses have been described for cattle.
It is becoming clear that more than one infectious agent can be involved in cases of diarrhea in neonatal calves. We have found that simultaneous infections of neonatal calves with rotavirus and C. parvum are quite common. Consequently, it would be useful to develop diagnostic tests which can detect the major pathogens involved in calf diarrhea in a single assay.
An on-site test has to be practical and user friendly to be acceptable. The ICS-RV assay meets these requirements. The assay is a 10-min one-step test with all necessary reagents included in the kit and with no need for any laboratory equipment to perform the test. It is anticipated that it can play a significant role in timely diagnosis and management of bovine rotavirus infections.
Acknowledgments
This work was supported by the MSU Animal Initiative Industry through a grant from the Department of Large Animal Clinical Sciences and by the MSU Diagnostic Center for Population and Animal Health.
REFERENCES
- 1.Al-Yousif, Y., J. Anderson, C. Chard-Bergstrom, A. Bustamante, M. Muenzenberger, K. Austin, and S. Kapil. 2001. Evaluation of a latex agglutination kit (Virogen Rotatest) for detection of bovine rotavirus in fecal samples. Clin. Diagn. Lab. Immunol. 8:496-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinzoni, R. C., J. Blackhall, H. R. Terzolo, A. R. Moreira, N. Auza, N. Mattion, G. L. Micheo, J. L. La Torre, and E. A. Scodeller. 1990. Microbiology of diarrhea in young beef and dairy calves in Argentina. Rev. Argent. Microbiol. 22:130-136. [PubMed] [Google Scholar]
- 3.Bendali, F., H. Bichet, F. Schelcher, and M. Sanaa. 1999. Pattern of diarrhea in newborn beef calves in southwest France. Vet. Res. 30:61-74. [PubMed] [Google Scholar]
- 4.Benfield, D. A., I. J. Stotz, E. A. Nelson, and K. S. Groon. 1984. Comparison of a commercial enzyme-linked immunosorbent assay with electron microscopy, fluorescent antibody, and virus isolation for the detection of bovine and porcine rotavirus. Am. J. Vet. Res. 45:1998-2002. [PubMed] [Google Scholar]
- 5.Chinsangaram, J., G. Y. Akita, A. E. Castro, and B. I. Osburn. 1993. PCR detection of group A bovine rotaviruses in feces. J. Vet. Diagn. Investig. 5:516-521. [DOI] [PubMed] [Google Scholar]
- 6.de Beer, M., I. Peenze, V. M. da Costa Mendes, and A. D. Steele. 1997. Comparison of electron microscopy, enzyme-linked immunosorbent assay and latex agglutination for the detection of bovine rotavirus in faeces. J. S. Afr. Vet. Assoc. 68:93-96. [DOI] [PubMed] [Google Scholar]
- 7.de la Fuente, R., A. Garcia, J. A. Ruiz-Santa-Quiteria, M. Luzon, D. Cid, S. Garcia, J. A. Orden, and M. Gomez-Bautista. 1998. Proportional morbidity rates of enteropathogens among diarrheic dairy calves in central Spain. Prev. Vet. Med. 36:145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennehy, P. H., M. Hartin, S. M. Nelson, and S. F. Reising. 1999. Evaluation of the ImmunoCardSTAT! rotavirus assay for detection of group A rotavirus in fecal specimens. J. Clin. Microbiol. 37:1977-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Verdier Klingenberg, K., and J. Esfandiari. 1996. Evaluation of a one-step test for rapid, in practice detection of rotavirus in farm animals. Vet. Rec. 138:393-395. [DOI] [PubMed] [Google Scholar]
- 10.Ellis, G. R., and E. Daniels. 1988. Comparison of direct electron microscopy and enzyme immunoassay for the detection of rotaviruses in calves, lambs, piglets and foals. Aust. Vet. J. 65:133-135. [DOI] [PubMed] [Google Scholar]
- 11.Goyal, S. M., R. A. Rademacher, and K. A. Pomeroy. 1987. Comparison of electron microscopy with three commercial tests for detection of rotavirus in animal feces. Diagn. Microbiol. Infect. Dis. 6:249-254. [DOI] [PubMed] [Google Scholar]
- 12.Hammami, S., A. E. Castro, and B. I. Osburn. 1990. Comparison of polyacrylamide gel electrophoresis, an enzyme-linked-immunosorbent assay, and an agglutination test for the direct identification of bovine rotavirus from feces and coelectrophoresis of viral RNAs. J. Vet. Diagn. Investig. 2:184-190. [DOI] [PubMed] [Google Scholar]
- 13.House, J. A. 1978. Economic impact of rotavirus and other neonatal disease agents of animals. J. Am. Vet. Med. Assoc. 173:573-576. [PubMed] [Google Scholar]
- 14.Hussein, H. A., E. Frost, B. Talbot, M. Shalaby, E. Cornaglia, and Y. el-Azhary. 1996. Comparison of polymerase chain reaction and monoclonal antibodies for G-typing of group A bovine rotavirus directly from fecal material. Vet. Microbiol. 51:1-7. [DOI] [PubMed] [Google Scholar]
- 15.Isegawa, Y., O. Nakagomi, T. Nakagomi, S. Ishida, S. Uesugi, and S. Ueda. 1993. Determination of bovine rotavirus G and P serotypes by polymerase chain reaction. Mol. Cell. Probes 7:277-284. [DOI] [PubMed] [Google Scholar]
- 16.Lucchelli, A., S. E. Lance, P. B. Bartlett, G. Y. Miller, and L. J. Saif. 1992. Prevalence of bovine group A rotavirus shedding among dairy calves in Ohio. Am. J. Vet. Res. 53:169-174. [PubMed] [Google Scholar]
- 17.McNulty, M. S. 1978. Rotaviruses. J. Gen. Virol. 40:1-18. [DOI] [PubMed] [Google Scholar]
- 18.Nussbaum, D. J., J. R. Salord, and D. D. Rimmele. 1999. Evaluation of quantitative latex agglutination for detection of Cryptosporidium parvum, E. coli K99, and rotavirus in calf feces. J. Vet. Diagn. Investig. 11:314-318. [DOI] [PubMed] [Google Scholar]
- 19.Parwani, A. V., B. I. Rosen, J. Flores, M. A. McCrae, M. Gorziglia, and L. J. Saif. 1992. Detection and differentiation of bovine group rotavirus serotypes using polymerase chain reaction-generated probes to the VP7 gene. J. Vet. Diagn. Investig. 4:148-158. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds, D. J., J. H. Morgan, N. Chanter, P. W. Jones, G. C. Bridger, T. G. Debney, and K. J. Bunch. 1986. Microbiology of calf diarrhea in southern Britain. Vet. Rec. 119:34-39. [DOI] [PubMed] [Google Scholar]
- 21.Saif, L. J., B. I. Rosen, and A. V. Parwani. 1994. Animal rotaviruses, p. 279-367. In A. Z. Kapikian (ed.), Viral infections of the gastrointestinal tract. Marcel Dekker, Inc., New York, N.Y.
- 22.Snodgrass, D. R., H. R. Terzolo, D. Sherwood, I. Campbell, J. D. Menzies, B. A. Synge, et al. 1990. Etiology of diarrhea in young calves. Vet. Rec. 119:31-34. [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi, K., F. Wakasugi, Y. Pongsuwanna, T. Urasawa, S. Ukae, S. Chiba, and S. Urasawa. 1992. Identification of human and bovine rotavirus serotypes by polymerase chain reaction. Epidemiol. Infect. 109:303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theil, K. W. 1990. Group A rotaviruses, p. 35-72. In L. J. Saif and K. Thiel (ed.), Viral diarrheas of man and animals. CRC Press, Inc., Boca Raton, Fla.
- 25.Veterinary Services, Animal and Plant Health Inspection Service, U.S. Department of Agriculture. 1992. DX monitor: animal health report, fall. U.S. Department of Agriculture, Washington, D.C.