Abstract
Cryptococcus neoformans is an important zoopathogen, and it is one of the most prevalent lethal mycotic agents. Its polysaccharide capsule, synthesized in vivo and in vitro, is a virulence factor, contains predominantly glucuronoxylomannan, and is responsible for the antigenic differentiation of serotypes A, B, C, D, and AD. A total of 467 isolates of C. neoformans obtained from clinical and environmental sources from Brazilian regions were studied serologically by using the Crypto Check Iatron RM 304-K kit. Serotyping of the clinical isolates showed the following prevalences of the serotypes: A (77.95%), followed by B (18.2%), AD (1.3%), D (0.4%), C (0.2%), and untypeable (1.93%). The epidemiology of serotype A in the Brazilian southern and southeastern regions reproduces the picture observed worldwide. In contrast, serotype B was the most frequent agent of cryptococcosis in the northeastern region, occurring nearly equally in male and female healthy hosts. Among the isolates from environmental sources, serotypes A and B were found to occur in the hollows of tropical trees of the genera Cassia, Ficus, and Moquillea. The few isolates from Eucalyptus camaldulensis debris were serotypes A and B and untypeable. Overall, no association with a specific host tree was identified for these serotypes, denoting a distinct ecoepidemiological regional pattern. The one serotype C isolate was recovered from a human immunodeficiency virus-negative host. Serotype AD predominated over serotype D among both clinical and environmental isolates.
Cryptococcus neoformans is a cosmopolitan zoopathogenic fungus. In its asexual form, this heterobasidiomycete appears as round or oval encapsulated yeast cells, singly or with buds, and is capable of synthesizing melanine through a diphenoloxidase identified as a laccase (19, 37). More than 90% of the yeast cell envelope is carbohydrate, and a glucuronoxylomannan is the main capsular polysaccharide produced in vitro and in vivo. The differences in the glucuronoxylomannan structure among strains is responsible for the antigenic differentiation of C. neoformans strains, resulting in the separation of C. neoformans into five serotypes known as A, B, C, D, and AD (2, 8, 16).
Two varieties of C. neoformans have traditionally been recognized on the basis of ecological, epidemiological, physiological, and genetic variabilities. C. neoformans var. neoformans (serotypes A, D, and AD) is the opportunistic agent of cryptococcosis in immunodepressed hosts. C. neoformans var. gattii (serotypes B and C) causes cryptococcosis in apparently healthy hosts in tropical and subtropical regions and behaves as a true pathogen (19). Recently, a third variety, C. neoformans var. grubii, has been described among serotype A isolates (15); however, under this classification the correct designations for serotype AD and untypeable (UT) isolates are not yet resolved. Moreover, other molecular studies suggested that serotype A should not be recognized as a separate variety (3, 10).
In Brazil, the study of small series of C. neoformans isolates, most of them from the southeast (SE) and southern (S) regions, showed that serotype A was the most prevalent, followed by serotypes B, D, and AD and UT isolates (17, 20, 30). Because there are the few data on the epidemiology and the ecology related to the various serotypes of C. neoformans in Brazil, we studied the prevalences and distributions of 467 isolates from clinical and environmental sources, detailing regional aspects.
MATERIALS AND METHODS
Microorganisms.
A total of 467 C. neoformans isolates preserved in the Culture Collection of the National Institute for Quality Control in Health and of the Evandro Chagas Institute of Clinical Research, Oswaldo Cruz Foundation (INCQS-IPEC/FIOCRUZ), and recovered from 1987 to 1998 were studied. They included 387 clinical isolates obtained from liquor (n = 231), blood (n = 39), respiratory tract secretions (n = 14), urine (n = 35), and other organic fluids (n = 16) and 80 environmental isolates obtained from bird excreta (n = 10), domestic dust (n = 25), decaying wood in hollow trees (n = 44), and bat guano (n = 1). Reference isolates from the American Type Culture Collection (ATCC) were included: C. neoformans serotypes A (INCQS 40168, ATCC 34872), B (INCQS 40113, ATCC 32269), C (INCQS 40143, ATCC 24066), D (INCQS 40142, ATCC 28958), and AD (INCQS 40150, ATCC 48184); Cryptococcus albidus (INCQS 40077, ATCC 10666); Cryptococcus laurentii (INCQS 40043, ATCC 18803); Candida albicans (INCQS 40006, ATCC 10231); and Saccharomyces cerevisiae (INCQS 40002, ATCC 9763).
Identification.
Organism identification was based on colonial and cellular morphologies and physiological and biochemical characteristics (18).
Serotyping.
A slide agglutination test (Crypto Check Iatron RM 304-K kit; Iatron Laboratories, Tokyo, Japan) was used for serotyping. The isolates were cultivated in YMA (yeast extract-malt extract-agar; Difco) at 25°C. After 48 h of incubation the culture was suspended in sterile physiological saline solution at McFarland scale pattern 2 (about 6 × 108 CFU/ml). A drop of each seric factor (F1, F5, F6, F7, and F8) was placed in the corresponding cycle of the agglutination glass slides, and 50 μl of the C. neoformans suspension was added over each seric factor. The glass slides were homogenized with an agitator with rotational movement at 25 rpm for 2 min. The slides were read by direct observation of the small clots that formed, as follows: serotype A, F1 and F7; serotype B, F1 and F5; serotype C, F1 and F6; serotype D, F1 and F8; and serotype AD, F1, F7, and F8.
Statistical analysis.
Data were analyzed by the χ2 test with Yates' correction. The Fisher test was used when the values were less than 5, and the results were considered statistically significant when a 5% level was achieved. The analysis was conducted with the Epi-info (version 6.3 C) software and the Statistical Package for the Social Sciences Program (SPSS-PC).
RESULTS
The distribution of serotypes by Brazilian geographic region and the sources of isolation for the 467 C. neoformans isolates are shown in Table 1. Serotype A prevails in clinical and environmental isolates from the S, SE, and central-western (CW) regions, whereas serotype B predominates in clinical and environmental isolates from the north (N) and northeastern (NE) regions. There were no statistically significant differences when the serotypes of isolates from clinical and environmental sources in the same geographic region were compared. Among the clinical isolates, serotype A predominated (285 of 308 isolates) in the S, SE, and CW regions, while serotype B predominated (42 of 66 isolates) in the N and NE regions (χ2 test with Yates' correction = 115.54 [P < 0.000001]). Among the environmental isolates, serotype A also predominated (46 of 56 isolates) in the S, SE, and CW regions, while serotype B was strikingly more prevalent (18 of 19 isolates) in the N and NE regions (χ2 test with the Fisher test = 32.63 [P < 0.000001]). UT isolates were canavanine-glycine-bromothymol blue (CGB) test-negative C. neoformans var. neoformans.
TABLE 1.
Distribution of C. neoformans serotypes in Brazil by geographic region and source of isolation
| Geographic region | Source of isolation | No. (%) of isolates of indicated serotype
|
||||||
|---|---|---|---|---|---|---|---|---|
| A | B | AD | D | C | UT | Total | ||
| S, SE, CW | Clinical | 285 (89) | 24 (7.5) | 4 (1.25) | 1 (0.3) | 1 (0.3) | 5 (1.65) | 320 (100) |
| Environment | 46 (90.2) | 1 (1.96) | 1 (1.96) | 1 (1.96) | 0 | 2 (3.9) | 51 (100) | |
| N, NE | Clinical | 23 (34.3) | 42 (62.7) | 1 (1.5) | 0 | 0 | 1 (1.5) | 67 (100) |
| Environment | 10 (34.5) | 18 (62) | 0 | 0 | 0 | 1 (3.45) | 29 (100) | |
| Total | 364 (77.95) | 85 (18.2) | 6 (1.3) | 2 (0.4) | 1 (0.2) | 9 (1.93) | 467 (100) | |
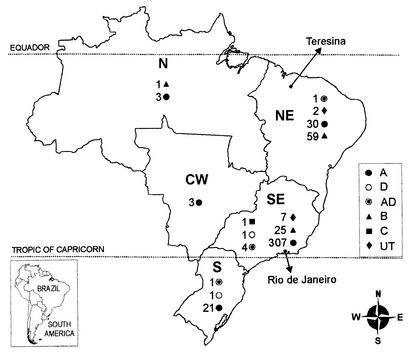
Figure 1 presents the distributions of the serotypes of the 467 C. neoformans isolates by Brazilian geographic region.
FIG. 1.
Distribution of serotypes (serotypes A, D, AD, B, and C and UT) of 467 C. neoformans isolates by geographic region in Brazil.
Clinical isolates. (i) HIV infection status and sex.
Information about the human immunodeficiency virus (HIV) infection status and sex was available for 246 patients from whom C. neoformans isolates were obtained. Among the 198 HIV-positive patients, serotype A isolates occurred in 186 patients, followed by serotype B isolates in 5 patients and serotype AD isolates in 3 patients; 4 patients were infected with UT C. neoformans var. neoformans isolates. Among the 48 HIV-negative patients, serotype B isolates occurred in 35 patients, followed by serotype A isolates in 11 patients, serotype AD isolates in 1 patient, and a serotype C isolate in 1 male patient (Table 2).
TABLE 2.
Distribution of C. neoformans serotypes A and B by HIV status and sex of patients
| HIV status | No. of patients | Serotype (no. of isolates/%) | No. of patients by sex
|
Male:female ratio | |
|---|---|---|---|---|---|
| Male | Female | ||||
| Positive | 191 | A (186/94) | 161 | 25 | 6.4:1 |
| B (5/2.5) | 3 | 2 | 1.5:1 | ||
| Negative | 46 | A (11/23) | 8 | 3 | 2.6:1 |
| B (35/73) | 21 | 14 | 1.5:1 | ||
Serotype A was associated with male HIV-positive patients (P < 0.00001), and serotype B was associated with male HIV-negative patients (P < 0.00001). Similarly, serotype A was associated with female HIV-positive patients, and serotype B was associated with female HIV-negative patients. However, serotype B occurred nearly equally in HIV-negative and HIV-positive patients of both genders (Table 2).
(ii) Geographic region.
The data for the two geographic regions from which most data were obtained were compared. Infection with C. neoformans serotype A was associated with AIDS in the SE region (98.3% [172 of 175 patients]) and the NE region (87.5% [28 of 31 patients]) and occurred in about 53% of the non-HIV-infected patients (8 of 15) in the SE region and 9.75% of the non-HIV-infected patients (3 of 31) in NE region. Serotype B was associated with AIDS in 12.5% of the patients (2 of 16) in the NE region and 1.7% of the patients (3 of 175) in the SE region, resulting in a 10-fold increase in the rate of infection with this serotype in the HIV-infected group from the NE region (P = 0.06). Serotype B clearly predominated in the NE region, with 87.5% (28 of 31) of HIV-negative patients being infected with isolates of this serotype. However, nearly half of the isolates (47% [7 of 15 patients]) from non-HIV-infected patients in the SE region were also serotype B (P < 0.002).
The only serotype C isolate identified in this study was a transient colonizer of the lower respiratory tract of a heavy smoker and represents, to our knowledge, the first serotype C isolate disclosed in Rio de Janeiro, Brazil.
Environmental isolates.
The distributions of the serotypes among the 80 C. neoformans isolates from environmental sources are presented in Table 3. When the distributions are considered by geographic region, all isolates from the hollows of trees in the SE region were serotype A, whereas both serotype A and B isolates were found in the hollows of trees in the N and NE regions, as shown in Table 4. The only environmental isolate of serotype B from the SE region was isolated from bat guano in the city of Rio de Janeiro.
TABLE 3.
Distribution of serotypes of environmental C. neoformans isolates in Brazil by substratum
| Serotype | No. of isolates by substratum
|
|||
|---|---|---|---|---|
| Bird excreta (n = 10) | Domestic dust (n = 25) | Decaying wood (n = 44) | Bat guano (n = 1) | |
| A | 8 | 23 | 25 | 0 |
| D | 1 | 0 | 0 | 0 |
| AD | 1 | 0 | 0 | 0 |
| B | 0 | 0 | 18 | 1 |
| UT | 1 | 1 | 1 | 0 |
TABLE 4.
Regional distribution of C. neoformans serotypes in plant decay inside hollows of tropical trees and debris of eucalyptus trees in Brazil
| Brazilian region | Hollow tree | C. neoformans serotype |
|---|---|---|
| SE | Pink shower tree (Cassia grandis) | A |
| Fig tree (Ficus mucrocarpa) | A | |
| Java plum (Syzygium jambolanum) | A | |
| November shower (Senna multijuga) | A | |
| NE | Pottery tree (Moquilea tomentosa) | B |
| Pink shower tree (Cassia grandis) | B, A | |
| Fig tree (Ficus sp.) | B | |
| Eucalyptusa (Eucalyptus camaldulensis) | B, A, UTb | |
| N | Cocoa tree (Theobroma cacao) | A |
| Cabreuva (Myroxylon peruiferum) | A | |
| “Árvore táxi” | A | |
| Guettarda acreana | B |
Only debris, no hollow tree.
UT C. neoformans var. neoformans.
Only serotype A isolates and one UT, CGB-negative C. neoformans var. neoformans isolate were found in domestic dust from the NE and SE regions.
UT isolates.
In this study, all nine UT isolates were CGB-negative C. neoformans var. neoformans, occurring more frequently than isolates of serotypes AD, D, and C. Some of them agglutinated only with F1, and others reacted with all factors (F1, F5, F6, F7 and F8); these isolates were obtained from both clinical sources (HIV-positive patients) and environmental sources (domestic dust, bird excreta, and decay debris of E. camaldulensis).
DISCUSSION
Serotype A predominated in isolates from clinical sources, followed by serotype B, in agreement with previous studies with Brazilian isolates (4, 17, 20, 30). Environmental isolates of serotypes A and B showed regional distribution patterns similar to those of clinical isolates. Clinical and environmental isolates of serotype A prevailed in the S and SE regions, whereas serotype B clinical and environmental isolates prevailed in the NE region. The majority of the cases of cryptococcosis in immunodepressed hosts from the S and SE regions were caused by serotype A isolates, mainly male AIDS patients living in urbanized areas of large cities, where deforestation and anthropic action are evident. This picture is observed worldwide (22, 30).
Serotype B was the most frequent agent of cryptococcosis in the NE region of Brazil, occurring equally in male and female patients. Although the few cases of cryptococcosis associated with AIDS in the NE region were mainly caused by serotype A isolates, as was observed in the S and SE regions, the rate of occurrence of serotype B isolates in HIV-positive patients in this region was 10-fold higher than that in such patients in the SE region. The high frequency of serotype B infection in HIV-positive hosts may be related to its endemic status in the NE region, where most of the patients with cryptococcosis are natives of the region, coming from rural areas, suggesting that the mycosis is regionally acquired. Moreover, serotypes A and B may be isolated from the environment inside the hollows of trees in this region, as a result of which arises the possibility that the local population may be exposed to both serotypes (23, 24). Other indirect evidence of the presence of serotype B in the NE region is the recovery of isolates of this serotype from the spleen of an armadillo captured near the city of Teresina (NE region). Serotype B predominates in healthy hosts in the NE region, behaving as an endemic pathogen; on the other hand, serotype A behaves as an opportunistic agent, reproducing the cosmopolitan nature of the pathogen in urban areas (5, 33).
Noteworthy in the present study were the five cases of serotype B infection in AIDS patients, three from the SE region and two from the NE region, an uncommon association, as observed in the world literature (32, 35).
Our study differs from others in the sense that C. neoformans isolates from the urban environment not only are associated with pigeon and bird excreta but are also associated with wood. Moreover, the isolation of C. neoformans serotype A from trees along a trail of the Amazon forest demonstrates its occurrence in wild environments in association with jungle trees.
No association of serotypes A and B with a specific host tree was observed. In the SE region, only serotype A was found to be associated with different genera of tropical trees. However, in the NE region, serotypes A and B were found alone or together and were associated with the decay in the hollows of tropical trees (22, 23). The few C. neoformans isolates obtained from the debris of an E. camaldulensis plantation in the NE region were serotypes B and A and UT; this pattern is distinct from that described in studies performed in Australia (13), the United States (California) (29), Italy (26), India (6), Spain (25), and the Brazilian SE region (27), where only serotype B was isolated from this eucalyptus species. It seems that climatic conditions as well as the geographic locations and native or adapted vegetation could influence the growth of one serotype to the detriment of that of another, which other investigators have also suggested (7, 11, 23).
In the SE region serotype A predominates among isolates from environmental sources such as bird excreta, the hollows of trees, and domestic dust, suggesting its widespread distribution in this area. Studies performed in Rio de Janeiro demonstrated the presence of serotype A in domestic dust in an average of 15% of the households whose dust was analyzed and in 28.5% of the decaying wood samples from the hollows of trees analyzed (22, 28). The only environmental serotype B isolate in this region was obtained from bat guano, collected from an old house in the city of Rio de Janeiro, thus showing that serotype B also occurs in this region and that humans may occasionally be exposed to isolates of this serotype (21). Moreover, a more comprehensive view of clinical C. neoformans isolates depends on the travel histories of patients, taking into account the possibility of a long-lasting latent infection with this agent. In Brazil, cryptococcosis caused by serotype B in Rio de Janeiro (SE region) mainly seems to be due to cases imported from the NE region, since 17 of 20 patients were natives of states located in the NE region, as observed previously (30).
In brief, the possibility of acquiring cryptococcosis caused by serotype B isolates in the S and SE regions of Brazil does exist, although isolates of this serotype seem to be scarce, since most of the patients infected with this serotype were from other regions of Brazil. These conclusions are reinforced by recent reports of cryptococcosis caused by serotype B isolates in native nonimmunosuppressed children in the N and NE regions of Brazil (5, 9, 31) and a report on the isolation of this serotype from a native jungle tree from the Amazonian rain forest (14). The few isolates from the CW region analyzed in our study did not allow an overall assessment of the serotypes in this region of Brazil.
The occurrence of serotype D is scarcely reported except in some European countries, such as Denmark (17), France (12), Italy (17), Germany, Austria, and Switzerland (17, 36), and Spain (1) and in areas of the United States (California and New York) (17, 34). In the present study the rate of occurrence of serotype D isolates was low, surpassed by that of serotype AD isolates, which were present in clinical sources as well as environmental sources related to pigeon excreta, but serotype D isolates were not found in association with wood decay in host trees. However, the finding of a CGB-negative UT isolate in association with E. camaldulensis reveals the need for further investigations.
Acknowledgments
We thank the National Institute for Quality Control in Health for support. The work was partially supported by grant 521141/98-2 from the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasília, Brazil, to Bodo Wanke.
We thank M. A. Salmito Cavalcanti, S. T. Fortes, L. F. C. Passoni, R. P. Igreja, G. P. Fernandes Bordignon, F. Queiroz-Telles, W. F. O. Filiu, M. R. Chang, N. Oliveira, and N. G. L. Gomes for providing C. neoformans isolates and the Laboratório de Produção e Tratamento de Imagens IOC/fIOCRUZ for image processing.
REFERENCES
- 1.Baró, T., J. M. Torres-Rodrigues, Y. Moreira, C. Alía, O. Lopes, and R. Méndez. 1999. Serotyping of Cryptococcus neoformans isolates from clinical and environmental sources in Spain. J. Clin. Microbiol. 37:1170-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharjee, A. K., J. E. Bennett, and C. P. J. Glaudemans. 1984. Capsular polysaccharides of Cryptococcus neoformans. Rev. Infect. Dis. 6:619-624. [DOI] [PubMed] [Google Scholar]
- 3.Boekhout, T., B. Theelan, M. Dias, J. W. Fell, W. C. J. Hop, C. A. Abeln, F. Dromer, and W. Meyer. 2001. Hybrid genotypes in the pathogenic yeast Cryptococcus neoformans. Microbiology 147:891-907. [DOI] [PubMed] [Google Scholar]
- 4.Calvo, B., O. Fischman, A. Pignatari, R. Del Bianco, and L. Zaror. 1990. Variedades y serotipos de Cryptococcus neoformans en pacientes con SIDA y neurocriptococosis en São Paulo, Brasil. Rev. Inst. Med. Trop. São Paulo 32:480-482. [PubMed] [Google Scholar]
- 5.Cavalcanti, M. A. S. 1997. Criptococose sistêmica endêmica pela variedade gattii no meio norte do Brasil. Ph.D. thesis. Fundação Oswaldo Cruz, Rio de Janeiro, Brazil.
- 6.Chakrabarti, A., M. Jatana, P. Kumar, L. Chatha, A. Kaushal, and A. A. Padhye. 1997. Isolation of Cryptococcus neoformans var. gattii from Eucalyptus camaldulensis in India. J. Clin. Microbiol. 35:3340-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S., T. Sorrell, G. Nimmo, B. Seep, B. Currie, D. Ellis, D. Marriott, T. Pfeiffer, D. Parr, K. Byth, and the Australasian Crytpococcal Study Group. 2000. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Clin. Infect. Dis. 31:499-508. [DOI] [PubMed] [Google Scholar]
- 8.Cherniak, R., and J. B. Sundstrom. 1994. Polysaccharide antigens of Cryptococcus neoformans. Infect. Immun. 62:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrêa, M. P. S. C., E. C. Oliveira, R. R. B. S. Duarte, P. P. O. Pardal, F. M. Oliveira, and L. C. Severo. 1999. Criptococose em crianças no Estado do Pará, Brasil. Rev. Soc. Bras. Med. Trop. 32:505-508. [PubMed] [Google Scholar]
- 10.Diaz, M. R., T. Boekhout, B. Theelenn, and J. W. Fell. 2000. Molecular sequence analyses of the intergenic spacer (IGS) associated with rDNA of two varieties of the pathogenic yeast Cryptococcus neoformans. Syst. Appl. Microbiol. 23:535-545. [DOI] [PubMed] [Google Scholar]
- 11.Dromer, F., S. Mathoulin, B. Dupont, A. Laporte, and the French Cryptococcosis Study Group. 1996. Epidemiology of cryptococcosis in France: a 9-year survey (1985-1993). Clin. Infect. Dis. 23:82-90. [DOI] [PubMed] [Google Scholar]
- 12.Dromer, F., S. Mathoulin, L. Dupont, L. Letenneur, O. Ronin, and the French Cryptococcosis Study Group. 1996. Individual and environmental factors associated with infection due to Cryptococcus neoformans serotype D. Clin. Infect. Dis. 23:91-96. [DOI] [PubMed] [Google Scholar]
- 13.Ellis, D., and T. Pfeiffer. 1990. Natural habitat of Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 28:1642-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortes, S. T., M. S. Lazéra, M. M. Nishikawa, R. C. L. Macedo, and B. Wanke. 2001. First isolation of Cryptococcus neoformans var. gattii from a native jungle tree in the Brazilian Amazon rainforest. Mycoses 44:137-140. [DOI] [PubMed] [Google Scholar]
- 15.Franzot, S. P., I. F. Salkin, and A. Casadevall. 1999. Cryptococcus neoformans var. grubii: separate varietal status for Cryptococcus neoformans serotype A isolates. J. Clin. Microbiol. 37:838-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda, R., T. Shinoda, Y. Fukuzawa, and L. Kaufman. 1982. Antigenic characterization of Cryptococcus neoformans serotypes and its application to serotyping of clinical isolates. J. Clin. Microbiol. 36:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon-Chung, K. J., and J. E. Bennett. 1984. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am. J. Epidemiol. 120:123-130. [DOI] [PubMed] [Google Scholar]
- 18.Kwon-Chung, K. J., and J. W. Fell. 1987. Filobasidiella Kwon-Chung, p. 467-495. In Kreger-van Rij (ed.), The yeasts: a taxonomic study. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 19.Kwon-Chung, K. J., and J. E. Bennett. 1992. Cryptococcosis, p. 397-446. In Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 20.Lacaz, C. S., and M. C. Rodrigues. 1983. Sorotipagem de Cryptococcus neoformans. Rev. Bras. Med. 40:297-300. [Google Scholar]
- 21.Lazéra, M. S., B. Wanke, and M. M. Nishikawa. 1993. Isolation of both varieties of Cryptococcus neoformans from saprophytic sources in the city of Rio de Janeiro, Brazil. J. Med. Vet. Mycol. 31:449-454. [Google Scholar]
- 22.Lazéra, M. S., F. D. A. Pires, L. Camilo-Coura, M. M. Nishikawa, C. C. F. Bezerra, L. Trilles, and B. Wanke. 1996. Natural habitat of Cryptococcus neoformans var. neoformans in decaying wood forming hollows in living trees. J. Med. Vet. Mycol. 34:127-131. [PubMed] [Google Scholar]
- 23.Lazéra, M. S., M. A. S. Cavalcanti, L. Trilles, M. M. Nishikawa, and B. Wanke. 1998. Cryptococcus neoformans var. gattii—evidence for a natural habitat related to decaying wood in a pottery tree hollow. Med. Mycol. 36:119-122. [PubMed] [Google Scholar]
- 24.Lazéra, M. S., M. A. S. Cavalcanti, A. T. Londero, L. Trilles, M. M. Nishikawa, and B. Wanke. 2000. Possible primary ecological niche of Cryptococcus neoformans. Med. Mycol. 38:379-383. [DOI] [PubMed] [Google Scholar]
- 25.Licea, B. A., D. Z. Garza, and M. T. Zúñiga. 1996. Aislamiento de Cryptococcus neoformans var. gattii de Eucalyptus tereticornis. Rev. Iberoam. Micol. 13:27-28. [Google Scholar]
- 26.Montagna, M. T., M. A. Viviani, A. Pulito, C. Aralla, A. M. Tortorano, L. Fiore, and S. Barbuti. 1997. Cryptococcus neoformans var. gattii in Italy. Note II. Environmental investigation related to an autochthonous clinical case in Apulia. J. Mycol. Med 7:93-96. [Google Scholar]
- 27.Montenegro, H., and C. R. Paula. 2000. Environmental isolation of Cryptococcus neoformans var. neoformans in the city of São Paulo, Brazil. Med. Mycol. 38:385-390. [DOI] [PubMed] [Google Scholar]
- 28.Passoni, L. F. C., B. Wanke, M. M. Nishikawa, and M. S. Lazéra. 1998. Cryptococcus neoformans isolated from human dwellings in Rio de Janeiro, Brazil: an analysis of the domestic environment of AIDS patients with and without cryptococcosis. Med. Mycol. 36:305-311. [PubMed] [Google Scholar]
- 29.Pfeiffer, T., and D. Ellis. 1991. Environmental isolation of Cryptococcus neoformans var. gattii from California. J. Infect. Dis. 163:929-930. [DOI] [PubMed] [Google Scholar]
- 30.Rozenbaum, R., and A. J. R. Gonçalves. 1994. Clinical epidemiological study of 171 cases of cryptococcosis. Clin. Infect. Dis. 18:369-380. [DOI] [PubMed] [Google Scholar]
- 31.Santos, L. O. 2000.. Criptococose no estado do Amazonas: estudo de 75 casos diagnosticados na Fundação de Medicina Tropical/FMT/IMTM (1988-1998.) M.Sc. thesis. Fundação Oswaldo Cruz, Rio de Janeiro, Brazil.
- 32.Sorrell, T. C. 2001. Cryptococcus neoformans variety gattii. Med. Mycol. 39:155-168. [PubMed] [Google Scholar]
- 33.Speed, B., and D. Dunt. 1995. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin. Infect. Dis. 21:28-34. [DOI] [PubMed] [Google Scholar]
- 34.Steenbergen, J. N., and A. Casadevall. 2000. Prevalence of Cryptococcus neoformans var. neoformans (serotype D) and Cryptococcus neoformans var. grubii (serotype A) isolates in New York City. J. Clin. Microbiol. 38:1974-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swinne, D., M. Deppner, S. Maniratunga, R. Laroche, J. J. Floch, and P. Kadende. 1991. AIDS-associated cryptococcosis in Bujumbura, Burundi: an epidemiological study. J. Med. Vet. Mycol. 29:25-30. [DOI] [PubMed] [Google Scholar]
- 36.Tintelnot, K., G. Schär, and A. Polak. 2001. Epidemiological data of cryptococcosis in Austria, Germany and Switzerland: part of the ECMM survey in Europe. Mycoses 44:345-350. [DOI] [PubMed] [Google Scholar]
- 37.Williamson, P. R. 1994. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J. Bacteriol. 176:656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]