Abstract
PEA-15 is a small, death effector-domain (DED)–containing protein that was recently demonstrated to inhibit tumor necrosis factor-α–induced apoptosis and to reverse the inhibition of integrin activation due to H-Ras. This led us to investigate the involvement of PEA-15 in Ras signaling. Surprisingly, PEA-15 activates the extracellular signal receptor-activated kinase (ERK) mitogen-activated protein kinase pathway in a Ras-dependent manner. PEA-15 expression in Chinese hamster ovary cells resulted in an increased mitogen-activated protein kinase kinase and ERK activity. Furthermore, PEA-15 expression leads to an increase in Ras guanosine 5′-triphosphate loading. PEA-15 bypasses the anchorage dependence of ERK activation. Finally, the effects of PEA-15 on integrin signaling are separate from those on ERK activation. Heretofore, all known DEDs functioned in the regulation of apoptosis. In contrast, the DED of PEA-15 is essential for its capacity to activate ERK. The ability of PEA-15 to simultaneously inhibit apoptosis and potentiate Ras-to-Erk signaling may be of importance for oncogenic processes.
INTRODUCTION
PEA-15 is a 15-kDa protein that was originally identified as a major astrocytic phosphoprotein (Araujo et al., 1993; Estelles et al., 1996). The first 80 amino acids of PEA-15 correspond to the canonical death effector domain (DED) sequence found in proteins that regulate apoptotic-signaling pathways (Boldin et al., 1995; Chinnaiyan et al., 1995; Chinnaiyan et al., 1996). The DED of PEA-15 can bind to the DEDs of both Fas associated death domian (FADD) and caspase 8 (Condorelli et al., 1999; Kitsberg et al., 1999). The remaining 51 amino acids contain a serine (S104) that is phosphorylated by protein kinase C (Araujo et al., 1993) and a serine (S116) phosphorylated by calcium calmodulin kinase II (Estelles et al., 1996). The 3′-untranslated region of PEA-15 also was independently cloned as mammary-transforming gene 1 (Bera et al., 1994). PEA-15 mRNA is widely expressed in several tissues in addition to astrocytes, including lung, heart, spleen, kidney, thymus, and muscle, whereas the protein has been detected in astrocytes, lung, eye, and fibroblasts (Danziger et al., 1995; Estelles et al., 1996).
We previously isolated PEA-15 in an expression-cloning strategy in which we looked for proteins that block an H-Ras-to-integrin signal (Ramos et al., 1998). Activation of the small guanosine 5′-triphosphate (GTP)–binding protein H-Ras, or its effector kinase c-Raf-1, initiates a signaling pathway that suppresses integrin–ligand binding (activation) (Hughes et al., 1997). This pathway can regulate cell shape and fibronectin matrix assembly (Hughes et al., 1997). Conversely, expression of a constitutively active form of the small GTPase R-Ras enhances integrin–ligand binding in the normally nonadherent cell lines U937 and 32D.3. R-Ras also is reported to regulate integrin–ligand binding in Chinese hamster ovary (CHO) cell lines (Zhang et al., 1996). In addition, expression of activated R-Ras in CHO cells blocks the H-Ras-to-integrin–signaling pathway (Sethi et al., 1999). R-Ras is homologous to H-Ras but has an extra 26 amino acid residues at the amino terminus (Lowe et al., 1987). Furthermore, R-Ras is regulated by activators and effectors distinct from those that regulate H-Ras function (Huff et al., 1997). Thus, two Ras proteins can have opposing effects on integrin–ligand binding. We reported that PEA-15 blocks the H-Ras-to-integrin signal by activating a pathway dependent on R-Ras (Ramos et al., 1998).
PEA-15 also affects glucose transport in skeletal muscle cells (Condorelli et al., 1998). Overexpression of PEA-15 in L6 skeletal muscle cells increases the number of glucose transporter (Glut)-1 transporters on the plasma membrane and inhibits insulin-stimulated glucose transport and cell-surface recruitment of glucose transporter (Glut)-4. Furthermore, PEA-15 expression is elevated in the skeletal muscle and adipose tissue of patients with type II diabetes (Condorelli et al., 1998). The molecular mechanisms of PEA-15 function in these systems have not been characterized.
The connections between integrin affinity and the mitogen-activated protein (MAP) kinase pathway led us to examine the effects of PEA-15 on MAP kinases. We report that PEA-15 activates the extracellular signal receptor-activated kinase (ERK) MAP kinase pathway in a Ras-dependent manner. Moreover, PEA-15 activation of ERK was independent of cell adhesion. The DED of PEA-15 was necessary for ERK activation. Thus, our data provide evidence for a new role of DEDs in the activation of MAP kinase cascades. Hence, PEA-15 may serve to connect cell death pathways and the ERK MAP kinase pathway.
MATERIALS AND METHODS
Cell Culture
αβpy-Cells are a CHO cell line that expresses the polyoma large T antigen and a constitutively active recombinant chimeric integrin (αIIbα6Aβ3β1) (Baker et al., 1997). NIH3T3, BT20, MDA-MB-231, and MCF-7 cells were obtained from the American Type Tissue Culture Collection (Rockville, MD). αβpy-Cells were maintained in DMEM (Biowhittaker, Walkersville, MD.) supplemented with 10% fetal calf serum (Biowhittaker, Walkersville, MD.), 1% nonessential amino acids (NEAA; Life Technologies, Gaithersburg, MD), 1% glutamine (Sigma, St. Louis, MO), 1% penicillin and streptomycin (Sigma), and 700 μg/ml G418 (LifeTechnologies). NIH3T3 cells were maintained in DMEM supplemented with 10% calf serum, 1% NEAA, 1% glutamine, and 1% penicillin and streptomycin. Jurkat cells were maintained in RPMI (Biowhittaker) supplemented with 10% calf serum, 1% NEAA, 1% glutamine, and 1% penicillin and streptomycin.
Antibodies, Reagents, and cDNA Constructs
The anti-PEA-15 polyclonal antibody (3099) was raised in rabbits against the thyroglobulin (Sigma)-conjugated peptide EEEIIKLAPPPKKA. Rabbits were immunized with peptide conjugate (100 μg) in Freund's complete adjuvant and then received two subsequent injections of conjugate in Freund's incomplete adjuvant at 3-wk intervals. Sera were tested for reactivity with the peptide by using a direct enzyme-linked immunosorbent assay. Reactivity with transfected and endogenous full-length hamster PEA-15 was verified by Western blotting. The activation-specific anti-αIIbβ3 monoclonal antibody PAC1 (Shattil et al., 1985) was generously provided by Dr. S. Shattil (The Scripps Research Institute, La Jolla, CA). The anti-αIIbβ3 monoclonal antibody anti-LIBS6 has been described previously (Frelinger et al., 1991). The anti-Tac antibody 7G7B6 was obtained from the American Tissue Culture Collection. The 7G7B6 was biotinylated with biotin-N-hydroxy-succinimide (Sigma) according to the manufacturer's instructions. The polyclonal antibodies anti-c-Jun NH2 terminal kinase (JNK), anti-p38, anti-phospho-Erk1/2, anti-phospho-JNK, and anti-phospho-p38 were purchased from Promega (Madison, WI). The polyclonal anti-Erk1/2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal anti-hemagglutinin (HA) antibody (12CA5) was produced and purified in our laboratory (Field et al., 1988). The αIIbβ3-specific peptide inhibitor Ro43-5054 (Alig et al., 1992) was a generous gift of B. Steiner (F. Hoffman, La Roche, Basel, Switzerland).
The cDNA encoding PEA-15 was cloned from a CHO cDNA library as previously described (Ramos et al., 1998). The PEA-15 mutants PEA-15-DED, PEA-15-CTERM, PEA-15-D74A, and DD-PEA-15 were previously described (Ramos et al., 1998). Tac-α5 (LaFlamme et al., 1994) was generously provided by Drs. S. LaFlamme and K. Yamada (National Institutes of Health, Bethesda, MD). pCGN-RafN4(23-284) (Brtva et al., 1995) and HA-ERK (Renshaw et al., 1996) were generous gifts from Dr. C. J. Der (University of North Carolina, Chapel Hill, NC) and Dr. Mark Renshaw (The Scripps Research Institute), respectively. Dr. G. Bokoch (The Scripps Research Institute, La Jolla, CA.) kindly provided both pCMV5-Cdc42(Q61L) and pCMV5-H-RasT17N. pcDNA3-R-Ras(G38V) and pcDNA3-R-Ras(T43N) (Zhang et al., 1996) were gifts from Dr. E. Ruoslahti (The Burnham Institute, La Jolla, CA) with permission from Dr. A. Hall (University of London, London, England). Dr. V. Dixit (Genentech, South San Francisco, CA) kindly provided the pcDNA3-E8 construct.
Measurement of ERK, Mitogen-activated Protein Kinase Kinase (MEK), JNK, and p38 Activity
For ERK kinase assays, αβpy-cells were transfected with HA-ERK2 (2 μg) along with test cDNA such as pcDNA3-PEA15 (3 μg) by using Lipofectamine (20 μl/plate; LifeTechnologies). In instances where more than one test plasmid was used, the amount of DNA transfected was standardized by addition of pcDNA1 control vector. In some experiments, transfections were done in duplicate to allow analysis of both PAC1 binding and kinase activity. Cells were lysed 48 h after transfection in ice-cold M2 buffer (0.5% NP-40, 20 mM Tris, pH 7.6, 250 mM NaCl, 5 mM EDTA, 3 mM ethylene glycol-bis(β-aminoethyl ester)-N,N,N′,N′-tetraacetic acid, 20 mM sodium phosphate, 20 mM sodium pyrophosphate, 3 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mM NaF, and 10 μg/ml each of leupeptin and aprotinin). ERK2 activity was measured by an immune-complex kinase assay (from 100 μg of cell lysate protein) by using myelin basic protein as a substrate (Renshaw et al., 1996). ERK2 activity was determined by autoradiography followed by scanning densitometry. Alternatively, ERK1/2 activity was determined by Western blot of 20 μg of cell lysate protein by using antibodies specific for phosphorylated ERK1/2 (Promega). MEK inhibitor U0126 (Promega) was used at 50 μM for 24 h to inhibit MEK activity.
Activity of MEK2 was assessed by cotransfection of HA-MEK2 with test plasmids, followed by cell lysis in M2 buffer, and anti-HA immunoprecipitation. MEK2 activity was measured by an immune-complex kinase assay (from 100 μg of cell lysate protein) with glutathione-S-transferase (GST)-ERK2 as the substrate. Alternatively, the same lysates (20 μg) were Western blotted with antibodies specific for phosphorylated MEK (no. 9121S; New England Biolabs, Beverly, MA). For measurement of JNK and p38 activity, αβpy-cells were transfected and lysed as described above. Lysates (20 μg) were Western blotted with antibodies specific for phosphorylated JNK or p38 (Promega). Western blots were developed by enhanced chemiluminescence.
ERK activity in attached versus suspended NIH3T3 cells was measured with an in-gel kinase assay as previously described (Renshaw et al., 1996). For attached cells, NIH3T3 cells in DMEM with 0.4% calf serum were plated in 60-mm tissue culture dishes at ∼80% confluence. For suspended cells, an equal number of NIH3T3 cells in DMEM with 0.4% calf serum and 0.5% methylcellulose was plated in 10-cm dishes coated with 1 ml of 1% agarose equilibrated with DMEM. In each instance, cells were then incubated for 24 h and lysed as described above. Some cells were serum stimulated for 10 min with 10% serum just before lysis as indicated in the text.
Ras GTP Loading
Ras GTP loading was measured as previously reported (de Rooij and Bos, 1997; Marais et al., 1998). Cell lysates were prepared at 4°C. Cells were washed twice with cold phosphate-buffered saline and each 100-mm dish was extracted in 300 μl of extraction buffer (20 mM Tris, pH 7.5, 1 mM EDTA, 10% glycerol, 1% Triton X-100, 100 mM KCl, 5 mM MgCl2, 0.05% 2-mercaptoethanol, and protease inhibitors). DNA was sheared and extracts clarified by centrifugation at 13,000 × g for 2 min. Extracts were then incubated with Sepharose beads coated with a bacterially expressed Ras-binding domain of Raf (GSTRBD) to pulldown GTP-loaded Ras. GTP-loaded Ras was then revealed by immunoblotting with a pan Ras antibody (no. R02120; Transduction Laboratories, Lexington, KY). Lysate (10%) also was blotted to determine endogenous levels of Ras expression.
Flow Cytometry
Analytical two-color flow cytometry was done as previously described (O'Toole et al., 1994). In transiently transfected αβpy-cells, PAC1 binding was determined for transfected cells (cells positive for the cotransfected Tac-α5 as measured by 7G7B6 binding). Integrin activation was quantitated in the form of an activation index defined as 100 × (F − Fr)/(FLIBS6 − Fr); in which F is the median fluorescence intensity (MFI) of PAC1 binding; Fr is the MFI of PAC1 binding in the presence of competitive inhibitor (Ro43-5054, 1 μM); and FLIBS6 is the MFI in the presence of anti-LIBS6 (2 μM).
RESULTS
PEA-15 Activates ERK MAP Kinase through a Ras-dependent Pathway
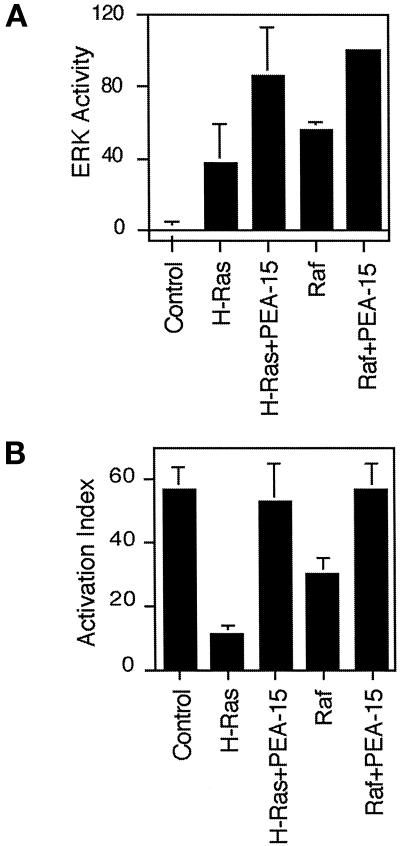
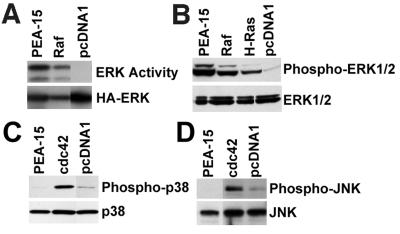
Activated Ras suppresses integrin–ligand binding (activation) via an MAP kinase-dependent pathway (Hughes et al., 1997) and PEA-15 blocks this suppression in an R-Ras–dependent manner (Ramos et al., 1998). One way in which PEA-15 might block the Ras-to-integrin pathway is by blocking MAP kinase activation. However, we found that PEA-15 did not block ERK MAP kinase activation but rather augmented activation of ERK by H-Ras (Figure 1A) or Raf (Figure 1A). The augmentation of Raf activation of ERK was statistically significant (p < 0.00), whereas the augmentation of Ras activation of ERK approached significance (p = 0.07). As a control, we found that in the same experiments, PEA-15 reversed suppression of integrin activation (Figure 1B). Furthermore, transfection of PEA-15 in the absence of Ras or Raf also activated cotransfected ERK kinase activity (Figure 2A). It also caused the phosphorylation of endogenous ERK1 and ERK2 as assessed by blotting with a phosphorylation-specific antibody (Figure 2B). The degree of activation of MAP kinase by PEA-15 was comparable to that induced by activated forms of Ras (H-RasG12V) or Raf (RafCAAX) (Figure 2, A and B). Thus, transfection with PEA-15 augments the activity of the ERK MAP kinase pathway.
Figure 1.
PEA-15 augments Ras and Raf activation of ERK2. (A) CHO cells were cotransfected with HA-ERK2 (2 μg) and 3 μg of expression vectors encoding H-RasG12V (H-Ras) or RafCAAX (Raf) in combination with PEA-15 (3 μg) or vector lacking an insert (3 μg). Cells transfected with 6 μg of vector cDNA also were assayed (Control). Transfected ERK2 was immunoprecipitated and its activity was assayed by its ability to phosphorylate myelin basic protein. Autoradiographs of three independent experiments were analyzed by spot densitometry as described under MATERIALS AND METHODS. Cotransfection of Raf and PEA-15 activates ERK significantly better than transfection of Raf alone (p = 0.0), whereas cotransfection of Ras and PEA-15 does not clearly activate ERK significantly better than Ras alone (p = 0.05). Depicted is the ERK2 activity relative to maximal activation (Raf + PEA-15). Note that PEA-15 augmented activation by both RafCAAX and RasG12V. Mean ± SD (n = 3). (B) αβpy-cells were cotransfected with expression vectors encoding 3 μg of RafCAAX or H-RasG12V in combination with PEA-15 (4 μg) or vector lacking an insert (4 μg). After 48 h, integrin activation was assayed by PAC1 binding as described under MATERIALS AND METHODS. Shown is the mean activation index ± SD of three independent experiments.
Figure 2.
PEA-15 activates the MAP kinase pathway. (A) αβpy-Cells were cotransfected with HA-ERK2 (2 μg) in combination with vector lacking an insert (3 μg) or encoding PEA-15 or RafCAAX (Raf). The transfected ERK2 was immunoprecipitated and its activity determined by its phosphorylation of myelin basic protein. Top, activity of transfected HA-ERK2. Bottom, immunoblots with anti-HA antibody 12CA5 indicate PEA-15 increases HA-ERK2 activity rather than its abundance. (B) CHO cells were transfected with 3 μg of PEA-15, RafCAAX (Raf), H-RasG12V (H-Ras), or pcDNA1. Cell lysates were analyzed by SDS-PAGE followed by immunoblotting. Top, immunoblot with a polyclonal antibody specific for phosphorylated active ERK1 and ERK2. Note that PEA-15 activates endogenous ERK1 and ERK2 to a comparable extent as RafCAAX and H-RasG12V. Bottom, immunoblot with polyclonal antibodies specific for ERK1 and ERK2. Note that endogenous ERK1/2 expression levels were similar in each transfection. (C and D) CHO cells were transfected with 3 μg of PEA-15, Cdc42, or control vector lacking an insert (pcDNA1). Cell lysates were immunoblotted by using polyclonal antibodies specific for phosphorylated p38 (C, top) or phosphorylated JNK (D, top). Alternatively, lysates were blotted with antibodies specific for p38 (C, bottom) or JNK (D, bottom) to verify similar expression levels. Note that PEA-15 does not cause phosphorylation of p38 or JNK. As a control, Cdc42 activates both p38 and JNK.
To assess the specificity of PEA-15 activation of ERK, we examined its capacity to activate the related MAP kinases p38 and JNK. Transfection of PEA-15 did not activate either JNK or p38 as measured by phosphorylation-specific antibodies (Figure 2, C and D). In contrast, Cdc42 activated both p38 and JNK as previously reported (Bagrodia et al., 1995; Coso et al., 1995; Olson et al., 1995) (Figure 2, C and D). In the same experiments, PEA-15 activated ERK1 and ERK2 (our unpublished results). Therefore, PEA-15 activation of MAP kinase is relatively specific for the ERK pathway.
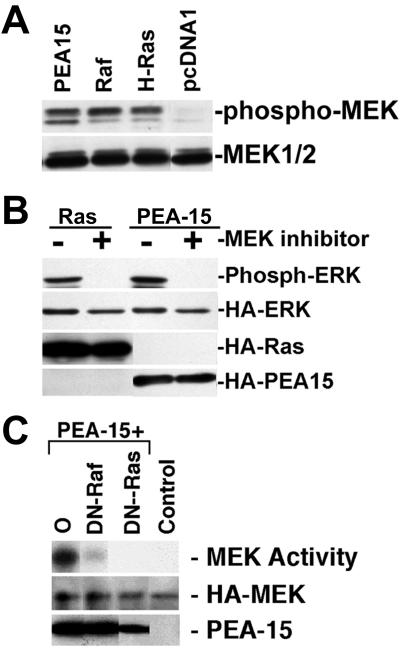
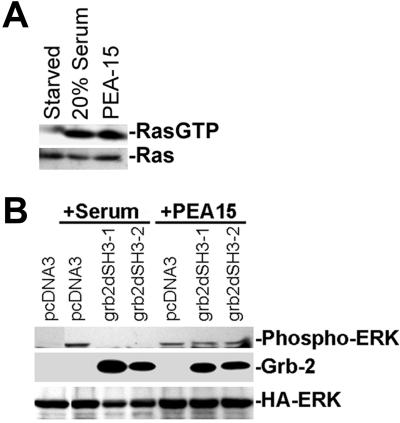
We next investigated the locus in the pathway at which PEA-15 causes increased ERK activation. Consistent with the increased activation of the ERK MAP kinase pathway, PEA-15 activated MEK, the kinase immediately upstream of ERK (Figure 3, A and C). MEK activation was accompanied by its phosphorylation on serine 217 and serine 221 as measured by a phospho-MEK antibody (Figure 3A). Furthermore, the MEK inhibitor U0126 inhibited PEA-15 activation of ERK1 and 2 (Figure 3B). Dominant-negative forms of Ras (H-RasT17N) or Raf (RafN4) blocked PEA-15–induced activation of MEK1 (Figure 3C). Similar effects also were seen on ERK activation (our unpublished results; but see Figure 7). Finally, expression of PEA-15 caused GTP loading of H-Ras (Figure 4A). Therefore, PEA-15 induces increased activity of the classical ERK MAP kinase pathway in a manner dependent on Ras activity.
Figure 3.
PEA-15 activates MEK in a Ras-dependent manner. (A) αβpy-Cells were cotransfected with HA-ERK (2 μg) and PEA-15 (3 μg) or RasG12V (3 μg). Twenty-four hours after transfection, 50 μM U0126 MEK inhibitor dissolved in dimethyl sulfoxide was added. Dimethyl sulfoxide alone was added to control plates. Cell lysates were analyzed by SDS-PAGE followed by immunoblotting. Top, immunoblot with a polyclonal antibody specific for phosphorylated active ERK1/2. Activity of transfected ERK is shown. Bottom three gels, immunoblots with monoclonal antibody 12CA5 to the HA epitope tag. Expression levels of HA-ERK, RasG12V, and PEA-15 were equal in treated versus untreated cells. (B) αβpy-Cells were transfected with HA-MEK2 (2 μg) in combination with pcDNA1 (6 μg) (Control). In separate plates, cells were transfected with HA-MEK2 (2 μg) and PEA-15 (3 μg) in combination with pcDNA1 (O; 2 μg), RafN4 (DN-Raf; 2 μg), or RasT17N (DN-Ras; 1 μg). Transfected HA-MEK2 was immunoprecipitated with anti-HA antibody, 12CA5, and MEK2 activity was determined by its capacity to phosphorylate GST-ERK2 as described under MATERIALS AND METHODS. Top, note that dominant-negative constructs of both Raf and Ras impair the capacity of PEA-15 to activate MEK2. Middle, immunoblot with anti-HA antibody 12CA5 indicates similar MEK2 expression levels in all transfections. Bottom, PEA-15 is overexpressed in each transfection as detected by immunoblotting.
Figure 7.
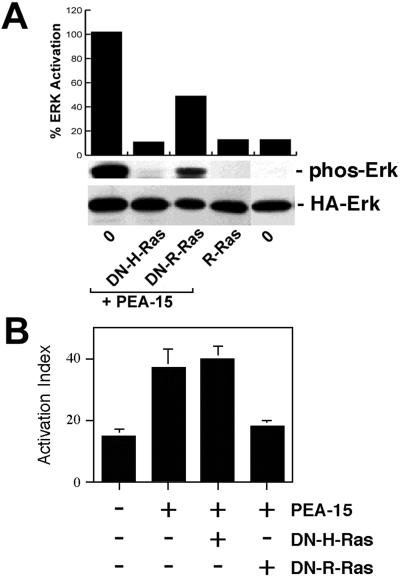
PEA-15 activation of ERK is independent of its effects on integrin activation. (A) Effects on ERK pathway. CHO cells were transfected with HA-ERK2 (2 μg). They also were transfected with vectors encoding PEA-15 (3 μg) in combination with H-RasT17N (DN-H-Ras; 1 μg), R-RasT43N (DN-R-Ras; 3 μg), or pcDNA1 lacking an insert (O; 8 μg). Other cells were transfected with activated R-RasV38 (R-Ras, 3 μg). Top, cell blot was quantitated by spot densitometry and corrected for ERK levels. These data are plotted above the blot. Middle, cell lysates were immunoblotted with antibodies specific for phosphorylated ERK. Note that dominant-negative H-Ras but not dominant-negative R-Ras blocked PEA-15 activation of ERK. H-Ras and PEA-15 expression levels were similar in all transfections. R-Ras does not activate ERK in these cells. Bottom, HA-ERK2 expression levels were comparable in all transfections. (B) Effects on integrin activation. αβpy-Cells were cotransfected with Tac-α5 (2 μg) and the indicated combinations of H-RasG12V (3 μg), PEA-15 (3 μg), R-RasT43N (DN-R-Ras, 3 μg), R-RasG38V (R-Ras, 2 μg), and H-RasT17N (DN-H-Ras, 2 μg). Total amounts of transfected plasmid were adjusted to 11 μg by addition of appropriate amounts of vector lacking an insert. After 48 h, integrin activation was determined by PAC1 binding to the Tac-positive subset of cells as described (Chen et al., 1994). Depicted is the mean activation index ± SD for three independent experiments. H-Ras and PEA-15 levels were similar in all transfections.
Figure 4.
PEA-15 activates GTP loading of Ras in a Grb-2-independent manner. (A) CHO cells were transfected with PEA-15 (2 μg). All plates were serum starved for 24 h. One plate of cells was stimulated with 20% serum for 15 min. Cell lysates were prepared and incubated with beads coated with the Ras-binding domain of Raf. The beads were washed twice with phosphate-buffered saline and the bound GTP-loaded Ras was visualized by immunoblotting with a pan Ras antibody (top). The amount of Ras in the lysates also was determined by immunoblotting (bottom). (B) CHO cells were cotransfected with HA-ERK (2 μg) and PEA-15 (3 μg) or control vector (pcDNA3; 3 μg) in combination with Grb2 mutants lacking the N-terminal SH3 domain (grb2dSH3–1; 2 μg) or the C-terminal SH3 domain (grb2dSH3–2; 2 μg) as indicated. Cells were serum stimulated (+serum) or serum starved (+PEA-15) for 24 h. Cell lysates were analyzed by SDS-PAGE followed by immunoblotting. Top, immunoblot with a polyclonal antibody specific for phosphorylated active ERK1/2. Activity of transfected ERK is shown. Bottom two gels, immunoblots with an antibody to the central SH2 domain of Grb2 or antibody 12CA5 to the HA epitope tag. Expression levels of HA-ERK, Grb-2 constructs, and PEA-15 were equal in treated versus untreated cells.
Ligated tyrosine kinase receptors activate Ras by recruiting the adapter protein Grb2. Grb2 binds SOS, which enhances Ras GTP exchange, resulting in Ras activation of Raf and the ERK pathway (Chardin et al., 1995). The N-terminal and C-terminal SH3 domains of Grb2 are required for complete binding to SOS (Simon and Schreiber, 1995; Xie et al., 1995). To determine whether PEA-15 activation of Ras, MEK, and ERK requires the formation of this Grb-2 and SOS complex, we expressed Grb-2 lacking either the N-terminal or C-terminal SH3 domains with PEA-15 or control vector. These Grb2 dominant negatives did not impair PEA-15 activation of ERK, although they blocked serum stimulation of ERK as expected (Figure 4B). This places the point of the PEA-15 effect on ERK activity downstream of Grb2 and upstream of Ras.
DED of PEA-15 Is Necessary but not Sufficient for ERK Activation
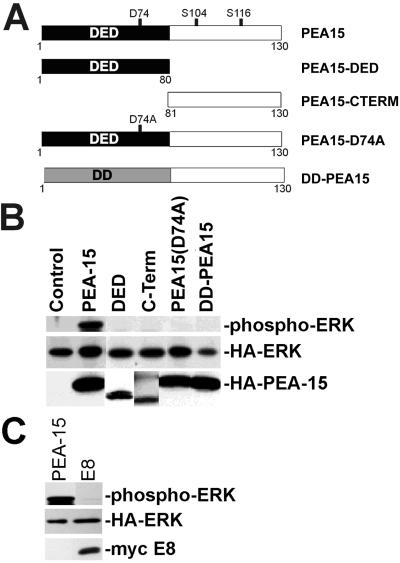
More than half of the PEA-15 protein consists of a conserved DED. This domain, to date, is associated exclusively with proteins involved in apoptosis (Goltsev et al., 1997; Hu et al., 1997; Irmler et al., 1997; Nagata, 1997). To determine whether the DED of PEA-15 is necessary or sufficient for PEA-15 activation of ERK, we overexpressed mutant forms of PEA-15 (Figure 5A) in CHO cells. Overexpression of only the DED of PEA-15 did not activate ERK (Figure 5B). It is therefore not sufficient to cause ERK activation. Mutants of PEA-15 lacking the DED (C-Term), containing a point mutation of a conserved DED aspartate (PEA-15-D74A), or containing the structurally related DD of FADD (DD-PEA-15) also were unable to activate ERK (Figure 5B). When expressed in tangent with full-length PEA-15, neither the DED nor the C-Term regions impaired activation of ERK (our unpublished results). Finally, the DED-containing viral protein E8 does not activate ERK in these assays (Figure 5C). Hence, the DED of PEA-15 is necessary but not sufficient for ERK activation, and ERK activation is not a general property of DED-containing proteins.
Figure 5.
The DED of PEA-15 is necessary but not sufficient for PEA-15 activation of ERK. (A) Depiction of the PEA-15 mutant constructs. The mutated aspartate (D74) is indicated, as are phoshphorylated serines (S104, S116). All recombinant proteins are fused to an HA tag. DD is structurally related to the DED. (B) αβpy-Cells were cotransfected with expression vectors encoding HA-ERK (2 μg) in combination with PEA-15 (4 μg), PEA-15-DED (DED; 8 μg), PEA-15-C-Term (C-Term; 8 μg), PEA-15(D74A) (4 μg), DD-PEA-15 (4 μg), or vector lacking insert (Control; 8 μg). Cell lysates were analyzed by SDS-PAGE followed by immunoblotting. Top, immunoblot with a polyclonal antibody specific for phosphorylated active ERK1. Note that only wild-type PEA-15 activates ERK. Middle and bottom, immunoblots with anti-HA antibody 12CA5. Note transfected HA-ERK expression levels are comparable in all transfections. Bottom, note the expression of all PEA-15 constructs. (C) αβpy-Cells were cotransfected with expression vectors encoding HA-ERK (2 μg) in combination with PEA-15 (3 μg) or E8 (3 μg). Cell lysates were analyzed by SDS-PAGE followed by immunoblotting. Top, immunoblot with a polyclonal antibody to phosphorylated ERK1/2 (phosphorylated HA-ERK is shown). Note that E8 does not activate ERK. Middle, immunoblot with anti-HA antibody. Bottom, immunoblot with antimyc antibody.
PEA-15 Activation of ERK Is Cell Adhesion Independent
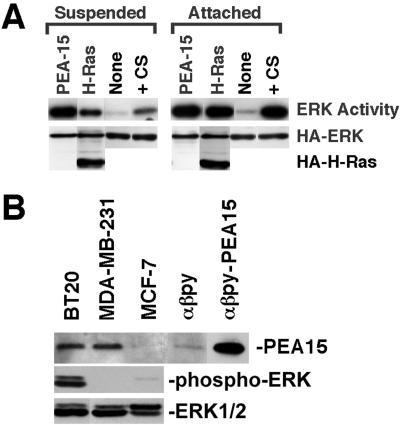
As noted above, we found that PEA-15 stimulated ERK in CHO cells. CHO cells are known to be transformed (Hsie and Puck, 1971; Leader et al., 1983; Esko et al., 1988). We therefore assessed the effects of transfection of mouse NIH3T3 fibroblasts with PEA-15. In these cells, PEA-15 activated ERK to levels comparable to those induced by either serum stimulation or activated Ras (Figure 6A, attached). Thus, PEA-15 activates ERK in nontransformed fibroblasts.
Figure 6.
PEA-15 activation of ERK2 does not require cell attachment. (A) NIH3T3 cells were cotransfected with vectors encoding HA-ERK2 (1 μg) and PEA-15 (1 μg), H-RasG12V (1 μg), or control vector lacking an insert (None; 1 μg). After 48 h, transfected cells were transferred to either agarose-coated tissue culture plates (suspended) or standard tissue culture plates (attached) and cultured an additional 24 h in low serum (0.4%). Control cells transfected with empty vector alone also were serum stimulated with 10% serum immediately before lysis (serum). Cells were lysed and recombinant ERK2 was immunoprecipitated with anti-HA antibody, 12CA5. ERK2 activity was determined by in-gel kinase assay with myelin basic protein as substrate. Top, relative ERK2 activity. Note that PEA-15 activates ERK2 to similar levels in both suspended and attached cells. Both Ras and serum stimulation are more effective in attached cells than in suspended cells. Bottom, immunoblot done with the anti-HA antibody 12CA5. Note comparable expression levels of HA-ERK and HA-tagged H-RasG12V in all transfections. (B) PEA-15 expression in mammary carcinoma cells. BT20, MDA-MB-231, MCF-7, αβpy, and PEA-15–transfected αβpy-cells were lysed in M2 buffer (under MATERIALS AND METHODS). The equivalent of 15 μg of each lysate was resolved by SDS-PAGE and transferred to a nitrocellulose membrane. PEA-15 was assayed by immunoblotting with polyclonal anti-PEA-15. ERK activity in the breast cell lines after serum starvation was measured by blotting with a phospho-ERK antibody. All blots were visualized by enhanced chemiluminescence.
Optimal growth factor activation of the ERK MAP kinase pathway requires integrin-mediated cell adhesion to overcome blocks either in Raf or MEK activation (Lin et al., 1997; Renshaw et al., 1997). PEA-15 activated ERK equally well in suspended or adherent cells (Figure 6A). In sharp contrast, activation of ERK by serum stimulation or activated H-Ras transfection was markedly reduced in suspended cells (Figure 6A).
The capacity of PEA-15 to activate ERK in a cell anchorage-independent manner suggests that its overexpression could contribute to the transformed phenotype. We examined the expression levels of PEA-15 in metastatic and nonmetastatic breast cancer cell lines by immunoblotting with an anti-PEA-15 polyclonal antibody (3099). This rabbit antibody was raised against a peptide sequence that is completely conserved between mouse, human, and hamster proteins and so recognizes PEA-15 in all three species. PEA-15 was expressed at high levels in two metastatic cell lines (MDA-MB-231 and BT20; Figure 6B). Indeed, the expression levels approached those in PEA-15-transfected CHO cells. In contrast, PEA-15 was expressed only at very low levels in the nonmetastatic cell line (MCF-7; Figure 6B). We also determined the activity of ERK1/2 in these cells after serum starvation. In the two metastatic lines expressing PEA-15, one, BT20 cells, showed an increased level of ERK activity, whereas the other, MDA-MB-231, did not. The nonmetastatic cell line (MCF-7) had a low level of ERK activity (Figure 6B). Hence, PEA-15 expression is increased in some but not all transformed cells and thus may contribute to the transformed phenotype. However, increased PEA-15 expression does not alone determine the ERK activity in these cells, suggesting that there are other modulating factors.
PEA-15 Activation of ERK and Reversal of Integrin Suppression Are via Distinct Effector Pathways
As noted above, PEA-15 activation of ERK is blocked by dominant-negative H-Ras. We previously reported that a dominant-negative R-Ras blocked the capacity of PEA-15 to affect integrin activation (Ramos et al., 1998). H-Ras and R-Ras are similar in sequence and interact with some of the same effectors (Bos, 1997). The dominant-negative constructs we use work by binding up exchange factors in an inactive complex (Bos, 1997). Consequently, we sought to determine whether the effect of PEA-15 on integrins or on ERK used a common effector pathway. If PEA-15 affects ERK and integrins through a common effector pathway, then dominant-negative H-Ras and R-Ras would have similar effects in these two assays. PEA-15 activation of ERK was only partially blocked by dominant-negative R-Ras, whereas it was completely blocked by dominant-negative H-Ras (Figure 7A). In contrast, dominant-negative R-Ras abrogated PEA-15 reversal of Ras suppression of integrins, whereas dominant-negative H-Ras had no such effect (Figure 7B). Furthermore, activated R-Ras (R-RasV38) by itself did not activate ERK (Figure 7A). Consequently, the capacity of PEA-15 to stimulate ERK activity and to reverse suppression of integrin activation may be mediated by distinct effector pathways.
DISCUSSION
Until now, DEDs have been described as adapters contained only in proteins that regulate apoptotic pathways (Ashkenazi and Dixit, 1998). We have found that a DED-containing protein, PEA-15, activates the ERK MAP kinase pathway. PEA-15–induced ERK activation is cell adhesion independent, in marked contrast to activation by Ras or serum stimulation. The DED of PEA-15 is necessary but not sufficient for its capacity to activate ERK or to regulate integrin function (Ramos et al., 1998). However, these two activities of PEA-15 are mediated by distinct effector pathways. These data indicate that DEDs can participate in the regulation of the ERK MAP kinase pathway as well as in FADD-mediated apoptosis. Thus, DED-containing PEA-15 may serve to link ERK activation and apoptotic signaling pathways.
Transfection with PEA-15 stimulates the ERK MAP kinase pathway. ERK was phosphorylated and activated in PEA-15–expressing cells. MEK, the kinase immediately upstream of ERK, was phosphorylated and activated concomitantly with ERK. Furthermore, both ERK and MEK activation were blocked by dominant-negative constructs of Raf (RafN3) and Ras (RasN17). Dominant-negative Raf binds the effector domains of Ras but does not phosphorylate MEK (Brtva et al., 1995). RasN17 blocks Ras activation by titrating out guanine nucleotide exchange factors such as SOS (Boguski and McCormick, 1993). Although neither mode of inhibition is completely specific for Ras, both inhibit Ras function at two distinct sites. Finally, expression of PEA-15 led to GTP loading of H-Ras. Thus, these data strongly suggest that PEA-15 activation of the classical MAP kinase pathway requires Ras activity.
The DED of PEA-15 is necessary but not sufficient for PEA-15 activation of ERK. A PEA-15 mutant lacking the DED did not activate ERK. Likewise, PEA-15 mutants in which a conserved Asp74 in the DED is changed to Ala or where the DED is exchanged with the structurally related death domain (DD) of FADD (Eberstadt et al., 1998) did not activate ERK. Thus, the DED of PEA-15 is necessary for activation of ERK. Overexpression of the isolated DED of PEA-15 did not activate ERK, hence it is not sufficient for ERK activation. Overexpression of the isolated DED with wild-type PEA-15 did not affect PEA-15 activation of ERK (our unpublished results). It would be expected that if the DED is alone involved in binding some partner, then its overexpression might interfere with PEA-15 function. We have found that the DED alone does not interfere with PEA-15 activation of ERK or rescue of integrins. Therefore, it is likely that more than just the DED region is involved in PEA-15 interaction with other proteins. The (D74A) mutation interfered with the effect of PEA-15 on integrin activation (Ramos et al., 1998). The mutated conserved aspartate is present in a RxDLL sequence in the α6 helix of DEDs (Chinnaiyan et al., 1995; Boldin et al., 1996). It will be informative to see whether the homologous Asp in other DEDs also interferes with their functions in apoptosis. Additionally, substitution of the DED of PEA-15 with the DED of FADD resulted in a chimeric protein that induced apoptosis (our unpublished results). This indicates that the DED of PEA-15 is functionally distinct from that of FADD and contains primary sequence information required for PEA-15 function. Thus, our studies define a new function for a DED in activating the Ras-dependent ERK MAP kinase pathway.
The proteins that interact with PEA-15 to lead to ERK activation are not known. Because PEA-15 activation of ERK and MEK is Ras dependent, it is possible that PEA-15 interacts with Ras or a protein upstream of Ras in the signaling cascade. We have tested the ability of PEA-15 to bind Grb2, Ras, and Raf and have found no interaction with these molecules (our unpublished results). In addition, PEA-15 activation of ERK does not require Grb2 to bind SOS. Therefore, a possible mechanism for PEA-15 activation of ERK is that overexpression of PEA-15 leads to the activation of a Ras exchange factor other than SOS. Alternatively, PEA-15 may interfere with inactivation of the ERK pathway after stimulation. A number of mechanisms have been proposed to be involved in this down-regulation of the pathway, including ERK phosphorylation of SOS, which prevents SOS binding to Grb2 (Langlois et al., 1995; Corbalan-Garcia et al., 1996). Our data rule out this possibility because Grb2 dominant negatives do not interfere with PEA-15 activation of ERK. Another method of inactivation of the ERK pathway is the up-regulation of phosphatases such as MAP kinase phosphatase 1 (MKP1) (Keyse, 2000). We have analyzed MKP1 expression levels in PEA-15–transfected cells and saw no difference from controls (our unpublished results). However, there are a number of other phosphatases that could be affected and these remain to be investigated. Finally, other pathways can inactivate the ERK-signaling cascade through proteins, including Rap-1 and PKA (English et al., 1999). It remains to be determined whether PEA-15 can affect the activity of these molecules. Understanding the mechanism of PEA-15 activation of ERK will require the identification of PEA-15–binding proteins.
The presence of a DED on PEA-15 suggests that it might bind with another DED-containing molecule. In fact, PEA-15 has been reported to bind both FADD and caspase 8, and is suggested to inhibit tumor necrosis factor-α (TNF-α)–induced apoptosis this way (Condorelli et al., 1999; Kitsberg et al., 1999). In these experiments, overexpression of PEA-15 in fibroblasts was shown to decrease FADD-induced apoptosis. In addition, astrocytes from PEA-15 null mice were sensitive to TNF-α, whereas the wild-type astrocytes were not. Transfection of PEA-15 into the knockout astrocytes rendered them resistant to TNF-α–induced apoptosis. It may be that the anti-apoptotic effects of PEA-15 are in part due to the activation of MEK and ERK. Indeed, TNFR1 receptors can bind an intracellular molecule named MADD (MAP kinase-activating death domain) and signal this way to activate ERK MAP kinase and cPLA2 (Schievella et al., 1997). Thus, PEA-15 may tune TNF signaling by simultaneously blocking the apoptotic cascade and increasing the ERK-related survival pathways.
In addition to regulating cell death, DED adapter proteins such as FADD may promote activation of ERK and proliferation. For example, ligation of Fas receptor (CD95) activates H-Ras and ERK in Jurkat T cells (Gulbins et al., 1995) through an unknown mechanism. Moreover, T cells lacking FADD or expressing a dominant-negative form of FADD are defective in proliferation (Walsh et al., 1998), further implicating FADD signaling in pathways that control cell proliferation. Newton and colleagues postulated the existence of an adapter protein that contains a DED and stimulates T-cell proliferation (Newton et al., 1998). PEA-15 is a DED-containing protein that augments ERK activity in a Ras-dependent manner. The DED of PEA-15 also can bind to the DEDs of FADD and caspase 8 (Condorelli et al., 1999; Kitsberg et al., 1999). Consequently, our data suggest that PEA-15 is a potential link between apoptotic signaling and the MAP kinase pathway.
The affects of PEA-15 on ERK and integrin activation are via distinct pathways. We previously demonstrated that H-Ras blocks integrin signaling, but at the same time an expression of a constitutively active form of the small GTPase, R-Ras, enhances integrin–ligand binding in the normally nonadherent cell lines U937 and 32D.3. R-Ras also is reported to regulate integrin–ligand binding in CHO cell lines (Zhang et al., 1996). We reported that PEA-15 blocks the H-Ras-to-integrin signal by activating a pathway dependent on R-Ras (Ramos et al., 1998). Indeed, the effects of PEA-15 on integrin activation were blocked by dominant-negative R-Ras. R-Ras is homologous to H-Ras, but has an extra 26 amino acid residues at the amino terminus (Lowe et al., 1987). Furthermore, R-Ras can be regulated by activators and effectors distinct from those that regulate H-Ras function (Huff et al., 1997). PEA-15 activation of ERK was blocked by dominant-negative H-Ras. However, dominant-negative R-Ras had little effect on PEA-15 activation of ERK. Activated R-Ras also did not promote ERK activation. Thus, PEA-15 represents a novel stimulator of distinct pathways that depend on activity of either H-Ras or R-Ras.
PEA-15 activation of the MAP kinase pathway is anchorage independent. PEA-15–activated ERK equally well in both suspended and adherent cells. In contrast, efficient ERK activation by Ras or serum requires cell adhesion (Lin et al., 1997; Renshaw et al., 1997). Activated ERK contributes to cell proliferation and anchorage-independent growth correlates with tumorigenicity (Freedman and Shin, 1974). Therefore, increased PEA-15 could contribute to tumorigenicity. Indeed, the conserved 3′-untranslated region of PEA-15 was identified as a transforming gene called mammary-transforming gene 1 (Bera et al., 1994). In addition, we find that PEA-15 is expressed in some metastatic breast cancer cell lines (MDA-MB-231 and BT20) at levels comparable to those in the transfected CHO cells. In contrast, PEA-15 is poorly expressed in a nonmetastatic line (MCF-7). We found that elevated expression of PEA-15 correlated with elevated activity of ERK in one of these lines (BT20) but not the other (MDA-MB-231). This suggests that other factors influence the basal level of ERK activity in addition to PEA-15. PEA-15 also is present in certain other human carcinoma lines derived from larynx, cervix, and skin (Condorelli et al., 1998). Our finding that PEA-15 activation of ERK is adhesion independent suggests that high level PEA-15 expression in these cancer cells could contribute to their pathogenic potential. We are currently exploring this hypothesis.
ACKNOWLEDGMENTS
This is publication number 11856-VB from The Scripps Research Institute. We thank our colleagues for their generosity in providing the reagents acknowledged under MATERIALS AND METHODS. We also are grateful to Dr. Sandy Shattil for critical review of the manuscript. J.W.R. and P.E.H. are fellows of the Leukemia Society of America. M.W.R. was supported by a National Research Service Award from the National Institutes of Health. This work was funded by a grant from the National Institutes of Health and by funds received from the Cancer Research Fund, under interagency agreement 97-12013 (University of California contract 98-00924V) with the Department of Health Services, Cancer Research Program.
REFERENCES
- Alig L, Edenhofer A, Hadvary P, Hurzeler M, Knopp D, Muller M, Steiner B, Trzeciak A, Weller T. Low molecular weight, non-peptide fibrinogen receptor antagonists. J Med Chem. 1992;35:4393–4407. doi: 10.1021/jm00101a017. [DOI] [PubMed] [Google Scholar]
- Araujo H, Danziger N, Cordier J, Glowinski J, Chneiweiss H. Characterization of PEA-15, a major substrate for protein kinase C in astrocytes. J Biol Chem. 1993;268:5911–5920. [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- Baker EK, Tozer EC, Pfaff M, Shattil SJ, Loftus JC, Ginsberg MH. A genetic analysis of integrin function: Glanzmann thrombasthenia in vitro. Proc Natl Acad Sci USA. 1997;94:1973–1978. doi: 10.1073/pnas.94.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera TK, Guzman RC, Miyamoto S, Panda DK, Sasaki M, Hanyu K, Enami J, Nandi S. Identification of a mammary transforming gene (MAT1) associated with mouse mammary carcinogenesis. Proc Natl Acad Sci USA. 1994;91:9789–9793. doi: 10.1073/pnas.91.21.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- Bos JL. Ras-like GTPases. Biochim Biophys Acta. 1997;1333:M19–M31. doi: 10.1016/s0304-419x(97)00015-2. [DOI] [PubMed] [Google Scholar]
- Brtva TR, Drugan JK, Ghosh S, Terrell RS, Campbell-Burk S, Bell RM, Der CJ. Two distinct Raf domains mediate interaction with Ras. J Biol Chem. 1995;270:9809–9812. doi: 10.1074/jbc.270.17.9809. [DOI] [PubMed] [Google Scholar]
- Chardin P, Cussac D, Maignan S, Ducruix A. The Grb2 adaptor. FEBS Lett. 1995;369:47–51. doi: 10.1016/0014-5793(95)00578-w. [DOI] [PubMed] [Google Scholar]
- Chen Y-P, O'Toole TE, Shipley T, Forsyth J, LaFlamme SE, Yamada KM, Shattil SJ, Ginsberg MH. “Inside-out” signal transduction inhibited by isolated integrin cytoplasmic domains. J Biol Chem. 1994;269:18307–18310. [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- Condorelli G, Vigliotta G, Cafieri A, Trencia A, Andalo P, Oriente F, Miele C, Caruso M, Formisano P, Beguinot F. PED/PEA-15: an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis. Oncogene. 1999;18:4409–4415. doi: 10.1038/sj.onc.1202831. [DOI] [PubMed] [Google Scholar]
- Condorelli G, Vigliotta G, Iavarone C, Caruso M, Tocchetti CG, Andreozzi F, Cafieri A, Tecce MF, Formisano P, Beguinot L, Beguinot F. PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J. 1998;17:3858–3866. doi: 10.1093/emboj/17.14.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Yang SS, Degenhardt KR, Bar-Sagi D. Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol Cell Biol. 1996;16:5674–5682. doi: 10.1128/mcb.16.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Danziger N, Yokoyama M, Jay T, Cordier J, Glowinski J, Chneiweiss H. Cellular expression, developmental regulation, and phylogenic conservation of PEA-15, the astrocytic major phosphoprotein and protein kinase C substrate. J Neurochem. 1995;64:1016–1025. doi: 10.1046/j.1471-4159.1995.64031016.x. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Bos JL. Minimal Ras-binding domain of Raf1. can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- Eberstadt M, Huang B, Chen Z, Meadows RP, Ng SC, Zheng L, Lenardo MJ, Fesik SW. NMR structure and mutagenesis of the FADD (Mort1) death-effector domain. Nature. 1998;392:941–945. doi: 10.1038/31972. [DOI] [PubMed] [Google Scholar]
- English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb MH. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;253:255–270. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- Esko JD, Rostand KS, Weinke JL. Tumor formation dependent on proteoglycan biosynthesis. Science. 1988;241:1092–1096. doi: 10.1126/science.3137658. [DOI] [PubMed] [Google Scholar]
- Estelles A, Yokoyama M, Nothias F, Vincent JD, Glowinski J, Vernier P, Chneiweiss H. The major astrocytic phosphoprotein PEA-15 is encoded by two mRNAs conserved on their full length in mouse and human. J Biol Chem. 1996;271:14800–14806. doi: 10.1074/jbc.271.25.14800. [DOI] [PubMed] [Google Scholar]
- Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson IA, Lerner RA, Wigler M. Purification of a RAS-responsive adenyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman VH, Shin SI. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Frelinger AL, III, Du X, Plow EF, Ginsberg MH. Monoclonal antibodies to ligand-occupied conformers of integrin αIIbβ3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J Biol Chem. 1991;266:17106–17111. [PubMed] [Google Scholar]
- Goltsev YV, Kovalenko AV, Arnold E, Varfolomeev EE, Brodianskii VM, Wallach D. CASH, a novel caspase homologue with death effector domains. J Biol Chem. 1997;272:19641–19644. doi: 10.1074/jbc.272.32.19641. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Bissonnette R, Mahboubi A, Martin S, Nishioka W, Brunner T, Baier G, Baier-Bitterlich G, Byrd C, Lang F. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995;2:341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Hsie AW, Puck TT. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3′:5′-monophosphate and testosterone. Proc Natl Acad Sci USA. 1971;68:358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Vincenz C, Buller M, Dixit VM. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- Huff SY, Quilliam LA, Cox AD, Der CJ. R-Ras is regulated by activators and effectors distinct from those that control Ras function. Oncogene. 1997;14:133–143. doi: 10.1038/sj.onc.1200815. [DOI] [PubMed] [Google Scholar]
- Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Schwartz MA, Ginsberg MH. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signaling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Kitsberg D, Formstecher E, Fauquet M, Kubes M, Cordier J, Canton B, Pan G, Rolli M, Glowinski J, Chneiweiss H. Astrocytes lacking the neural death-effector-domain protein PEA-15 show increased susceptibility to TNF alpha. J Neurosci. 1999;19:8244–8251. doi: 10.1523/JNEUROSCI.19-19-08244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme SE, Thomas LA, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois WJ, Sasaoka T, Saltiel AR, Olefsky JM. Negative feedback regulation and desensitization of ins. J Biol Chem. 1995;270:25320–25323. doi: 10.1074/jbc.270.43.25320. [DOI] [PubMed] [Google Scholar]
- Leader WM, Stopak D, Harris AK. Increased contractile strength and tightened adhesions to the substratum result from reverse transformation of CHO cells by dibutyryl cyclic adenosine monophosphate. J Cell Sci. 1983;64:1–11. doi: 10.1242/jcs.64.1.1. [DOI] [PubMed] [Google Scholar]
- Lin TH, Chen Q, Howe A, Juliano RL. Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J Biol Chem. 1997;272:8849–8852. [PubMed] [Google Scholar]
- Lowe DG, Capon DJ, Delwart E, Sakaguchi AY, Naylor SL, Goeddel DV. Structure of the human and murine R-Ras genes, novel genes closely related to ras proto-oncogenes. Cell. 1987;48:137–146. doi: 10.1016/0092-8674(87)90364-3. [DOI] [PubMed] [Google Scholar]
- Marais R, Light Y, Mason C, Paterson H, Olson MF, Marshall CJ. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Newton K, Harris AW, Bath ML, Smith KC, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura RN, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- Ramos JW, Kojima TK, Hughes PE, Fenczik CA, Ginsberg MH. The death effector domain of PEA-15 is involved in its regulation of integrin activation. J Biol Chem. 1998;273:33897–33900. doi: 10.1074/jbc.273.51.33897. [DOI] [PubMed] [Google Scholar]
- Renshaw MW, Lea-Chou E, Wang JYJ. Rac is required for v-Abl tyrosine kinase to activate mitogenesis. Curr Biol. 1996;6:76–83. doi: 10.1016/s0960-9822(02)00424-4. [DOI] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schievella AR, Chen JH, Graham JR, Lin LL. MADD, a novel death domain protein that interacts with the type 1 tumor necrosis factor receptor and activates mitogen-activated protein kinase. J Biol Chem. 1997;272:12069–12075. doi: 10.1074/jbc.272.18.12069. [DOI] [PubMed] [Google Scholar]
- Sethi T, Ginsberg MH, Downward J, Hughes PE. The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway. Mol Biol Cell. 1999;10:1799–1809. doi: 10.1091/mbc.10.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb-IIIa Complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- Simon JA, Schreiber SL. Grb2 SH3 binding to peptides from Sos: evaluation of a general model for SH3-ligand interactions. Chem Biol. 1995;2:53–60. doi: 10.1016/1074-5521(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Walsh CM, Wen BG, Chinnaiyan AM, O'Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- Xie Y, Pendergast AM, Hung MC. Dominant-negative mutants of Grb2 induced reversal of the transformed phenotypes caused by the point mutation-activated Rat HER-2/Neu. J Biol Chem. 1995;270:30717–30724. doi: 10.1074/jbc.270.51.30717. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Wang H-G, Reed JC, Ruoslahti E. Integrin activation by R-Ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]