Abstract
We analyzed antimicrobial use in 509 episodes of clinically significant bloodstream infection to assess the impact that microbiology laboratory reporting had on antimicrobial management. Most therapy interventions occurred at the time of phlebotomy and after notification of Gram stain results by telephone. Release of antimicrobial susceptibility data had the least impact on antimicrobial management.
Bloodstream infection (BSI) is an important cause of serious morbidity and mortality. Approximately 200,000 patients are diagnosed with BSI annually in the United States (9, 15-17; M. L. Towns, S. M. Quartey, M. P. Weinstein, L. G. Reimer, and L. B. Reller, Abstr. 93rd Gen. Meet. Am. Soc. Microbiol. 1993, abstr. C-232), with an estimated mortality from BSI of 22 to 29% (9, 17; D. J. Diekema, S. E. Beekmann, K. C. Chapin, E. L. Munson, and G. V. Doern, submitted for publication; Towns et al., Abstr. 93rd Gen. Meet. Am. Soc. Microbiol.).
The clinical microbiology laboratory plays a significant role in the management of patients with BSI. Culturing a pathogenic microorganism from blood is a highly specific indicator of BSI, and performance of antimicrobial susceptibility testing (AST) may assist in the appropriate use of antimicrobial therapy for patients with BSI (2, 4, 6). Furthermore, early and rapid administration of antimicrobial therapy to patients with BSI has been shown to reduce mortality (8-13).
The purpose of this study was to evaluate the association between positive blood culture reporting by the clinical microbiology laboratory and the antimicrobial management of patients with BSI. Findings from consecutive episodes of BSI at the University of Iowa Hospitals and Clinics over a 13-month period are summarized.
All patients at the University of Iowa Hospitals and Clinics from July 1999 through July 2000 with a positive signal from the BacT/Alert continuous monitoring blood culture system (bioMérieux, Marcy l'Etoile, France) were screened for inclusion in this study. An episode of BSI was considered to have begun at the time the initial positive blood culture was obtained. Referral blood cultures from outside facilities and autopsy blood cultures, as well as patients with false-positive signals (no organisms observed on Gram stain or cultivated from bottle subculture) and patients not admitted to the hospital, were excluded from the study.
Of the 509 episodes of bacteremia characterized in this study, 59% were judged to be hospital acquired; the remaining 41% were judged to be community acquired. The following percentages of bacteremic episodes occurred in the patients studied: ≤1 year of age, 8%; 1 to 15 years of age, 11%; 16 to 39 years of age, 18%; 40 to 59 years of age, 32%; 60 to 79 years of age, 28%; 80 to 101 years of age, 4%. Thirty percent of patients were hospitalized in intensive-care units at the time of the septic episode; the remaining patients were in general-care areas. The six most common causes of bacteremia in this study were Staphylococcus aureus (20% of episodes), Escherichia coli (14%), coagulase-negative staphylococci (13%), enterococci (12%), Pseudomonas aeruginosa (6%), and Klebsiella pneumoniae (5%).
Each positive blood culture was critically assessed and categorized as clinically significant or not clinically significant, taking into account clinical signs and symptoms, white blood cell count, number of blood samples culture positive out of the total number drawn, results of other cultures, pathology findings, imaging results, and clinical course. All indeterminate cases were reviewed by an infectious disease physician prior to classification. Only clinically significant positive blood cultures were included in the study.
A medical record review performed by an experienced nurse epidemiologist determined the time of blood culture phlebotomy, the time at which clinicians were notified by telephone by the clinical microbiology laboratory of Gram stain results from a positive blood culture bottle, and the time at which AST results were made available on the hospital information system. No information regarding likely AST profiles was provided at the time blood culture Gram stain results were reported by telephone.
Times of antimicrobial interventions were recorded in relation to the time points described above. The percentages of antimicrobial initiations and discontinuations that took place around these designated points in time were analyzed. The significance test of proportions (14) was used to compare cumulative percentages of antimicrobial initiations or discontinuations within a given interval. The alpha level was set at 0.05, and all reported P values are two tailed.
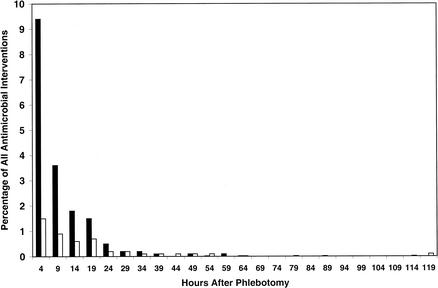
The largest proportion of therapy initiations occurred shortly after phlebotomy (Fig. 1 and 2). Half of all therapy initiations between phlebotomy and notification of positive blood culture results occurred within 4 h of the phlebotomy, and 25% took place within 2 h of the phlebotomy (data not shown).
FIG. 1.
Percentage of all antimicrobial interventions occurring within the first 8 h (initiations, solid bars; discontinuations, open bars) after each event of interest. *, P < 0.001 for differences noted in therapy initiations. †, P < 0.05 for differences noted in therapy discontinuations.
FIG. 2.
Histograms representing percentages of all analyzed antimicrobial regimens that were initiated (solid bars) or discontinued (open bars) from the time of phlebotomy to the time of notification by telephone of a positive blood culture Gram stain result.
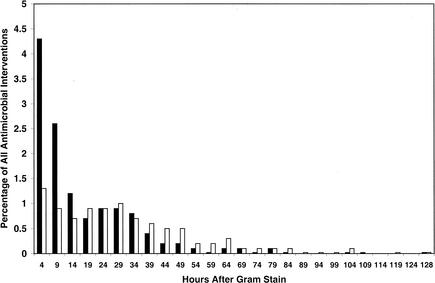
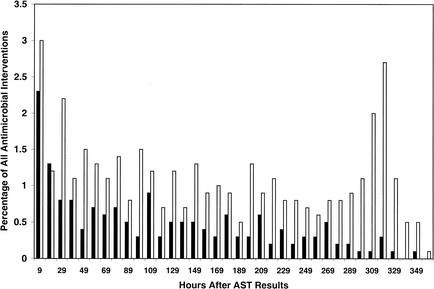
A smaller proportion of therapy initiations occurred shortly after notification of Gram stain results (Fig. 1 and 3). Similar to the pattern noted after phlebotomy, most therapeutic interventions occurred very early after notification of positive blood cultures. Therapy initiation and discontinuation events following release of AST results were spread over a much more protracted time period and exhibited a more random distribution (Fig. 4). In particular, a disproportionate percentage of discontinuation events appeared to occur >10 days following provision of AST results. It is likely that these represented patients in whom therapy was discontinued as a result of completion of a defined course of therapy, rather than as a consequence of the availability of AST results.
FIG. 3.
Histograms representing percentages of all analyzed antimicrobial regimens that were initiated (solid bars) or discontinued (open bars) from the time of notification by telephone of Gram stain morphology to the time of release of AST data.
FIG. 4.
Histograms representing percentages of all analyzed antimicrobial regimens that were initiated (solid bars) or discontinued (open bars) after release of AST results.
Statistically significantly more therapeutic interventions occurred in the time period immediately following collection of blood cultures than during any other time period. The hours leading up to phlebotomy (data not illustrated) and the hours just following release of susceptibility test results were associated with the fewest antimicrobial interventions.
Clinically significant BSI remains an important cause of mortality. Reports have suggested the necessity of prompt medical intervention in the early hours of BSI. Kreger et al. (8) demonstrated that 60% of the fatalities in a study of 612 patients with Gram-negative bacteremia occurred within the first 48 h of infection. Another study (13) noted a mortality rate of 50% in granulocytopenic patients within the first 72 h of P. aeruginosa bacteremia. Since many studies have documented the importance of appropriate antimicrobial therapy in the clinical outcome of BSI (1, 3, 5, 7, 8, 10, 11), we sought to determine which, if any, service offered by the clinical microbiology laboratory had the most impact upon the management of clinically significant BSI.
Many patients had already been started on antimicrobial therapy before the index positive blood culture was obtained, and there was no particular pattern of use noted, except for a slight increase in discontinuation of therapy up until about 10 h prior to phlebotomy (data not illustrated). These data suggest that a fair number of regimens may be stopped in anticipation of blood culturing in an effort to increase assay sensitivity.
The hours immediately following phlebotomy were a time of tremendous activity with respect to antimicrobial management of patients with BSI. Greater than 50% and 30% of antimicrobial regimen initiations and discontinuations, respectively, that occurred between phlebotomy and reporting of Gram stain morphology took place within the first 4-h interval after phlebotomy (data not illustrated). These findings corroborate current clinical practice, which recommends blood culturing and concomitant initiation of appropriate empirical therapy in the face of suspected sepsis.
Two time points in this study represent action by the clinical microbiology laboratory. Of the two, notification by telephone of a positive blood culture and Gram stain results appeared to have a much greater impact on antimicrobial usage than did provision of AST results. Of the antimicrobial regimens initiated between notification by telephone of Gram stain morphology and release of AST results, 75% were initiated within 24 h of the telephone call. Of the antimicrobial regimens initiated after AST reporting, 75% were initiated within 177 h of the report. Similarly, 75% of antimicrobial discontinuations within the second temporal interval occurred approximately 39 h after notification of Gram stain results and 75% of stoppages after AST reporting occurred within 264 h of the report (data not illustrated). The effect of notification of Gram stain morphology by telephone on antimicrobial usage was shown to be statistically significantly greater than that of AST result availability. However, both time points were associated with fewer interventions in antimicrobial therapy than the time around blood culture phlebotomy.
In conclusion, the results of this study suggest that empirical therapy was usually administered early in the clinical course of BSI at our tertiary-care facility, prior to reporting of positive blood culture results. With respect to antimicrobial management, the most important information provided by the clinical microbiology laboratory appeared to be the first telephone call reporting positive blood culture and Gram stain results. Release of AST data did not appear to have a comparatively important impact on antimicrobial management among patients with BSI.
It is clear that current antimicrobial practice tends toward the use of broad-spectrum agents for empirical coverage of serious infections. In part, this is driven by increasing rates of antimicrobial resistance. Combined with a reluctance of clinicians to narrow antimicrobial coverage in the setting of a clinical improvement, AST results appeared to play a relatively minor role in the fine tuning of antimicrobial therapy in our institution. It must be recognized that our observations are predicated on a study conducted in a single, large, tertiary-care teaching medical center with extremely high patient acuity. Further studies of different patient populations are needed to address the impact of provision of AST results on antimicrobial drug administration to bacteremia patients in other care settings.
REFERENCES
- 1.Abboud, F. M., and B. A. Waisbren. 1959. Correlation between results of the tube dilution method for determining bacterial sensitivity to antibiotics and the results of the administration of these antibiotics to patients with staphylococcic bacteremia. Antibiot. Ann. 1958-1959:748-756. [PubMed] [Google Scholar]
- 2.Bohte, R., R. van Furth, and P. J. van den Broek. 1995. Aetiology of community-acquired pneumonia: a prospective study among adults requiring admission to hospital. Thorax 50:543-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant, R. E., A. F. Hood, C. E. Hood, and M. G. Koenig. 1971. Factors affecting mortality of gram-negative rod bacteremia. Arch. Intern. Med. 127:120-128. [PubMed] [Google Scholar]
- 4.Chalasani, N. P., M. A. L. Valdecanas, A. K. Gopal, J. E. McGowan, Jr., and R. L. Jurado. 1995. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 108:932-936. [DOI] [PubMed] [Google Scholar]
- 5.DuPont, H. L., and W. W. Spink. 1969. Infections due to gram-negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center 1958-1966. Medicine 48:307-332. [DOI] [PubMed] [Google Scholar]
- 6.Fine, M. J., M. A. Smith, C. A. Carson, S. S. Mutha, S. S. Sankey, L. A. Weissfeld, and W. N. Kapoor. 1996. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA 275:134-141. [PubMed] [Google Scholar]
- 7.Freid, M. A., and K. L. Vosti. 1968. The importance of underlying disease in patients with gram-negative bacteremia. Arch. Intern. Med. 121:418-423. [PubMed] [Google Scholar]
- 8.Kreger, B. E., D. E. Craven, and W. R. McCabe. 1980. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am. J. Med. 68:344-355. [DOI] [PubMed] [Google Scholar]
- 9.Leibovici, L., H. Konisberger, and S. D. Pitlik. 1992. Bacteremia and fungemia of unknown origin in adults. Clin. Infect. Dis. 14:436-439. [DOI] [PubMed] [Google Scholar]
- 10.McCabe, W. R., and G. G. Jackson. 1962. Gram-negative bacteremia. I. Etiology and ecology. Arch. Intern. Med. 127:847-855. [Google Scholar]
- 11.McCabe, W. R., and G. G. Jackson. 1962. Gram-negative bacteremia. II. Clinical, laboratory and therapeutic observations. Arch. Intern. Med. 127:856-869. [Google Scholar]
- 12.McGowan, J. E., Jr., M. W. Barnes, and M. Finland. 1975. Bacteremia at Boston City Hospital: occurrence and mortality during 12 selected years (1935-1972) with special reference to hospital-acquired cases. J. Infect. Dis. 132:316-335. [DOI] [PubMed] [Google Scholar]
- 13.Schimpff, S., W. Satterlee, V. M. Young, and A. Serpick. 1971. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N. Engl. J. Med. 284:1061-1065. [DOI] [PubMed] [Google Scholar]
- 14.Triola, M. F. 1992. Elementary statistics, 5th ed., p. 544-577. Addison-Wesley, Boston, Mass.
- 15.Washington, J. A., II, and D. M. Ilstrup. 1986. Blood cultures: issues and controversies. Rev. Infect. Dis. 8:792-802. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein, M. P., L. B. Reller, J. R. Murphy, and K. A. Lichtenstein. 1983. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev. Infect. Dis. 5:35-53. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein, M. P., M. L. Towns, S. M. Quartey, S. Mirrett, L. G. Reimer, G. Parmigiani, and L. B. Reller. 1997. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin. Infect. Dis. 24:584-602. [DOI] [PubMed] [Google Scholar]