Abstract
Clostridium difficile, the most common cause of antibiotic-associated diarrhea, is occasionally isolated from extraintestinal sites and is usually found as part of a polymicrobial flora. We report a case of brain empyema that occurred after the recurrent intestinal carriage of a nontoxigenic strain of C. difficile. Brain abscess cultures contained both toxigenic and nontoxigenic isolates. Pulsed-field gel electrophoresis showed that nontoxigenic isolates from the intestine and from the brain were identical.
CASE REPORT
In February 1999, a 48-year-old man, with a history of chronic alcohol addiction, underwent surgery to drain a subdural hematoma that involved the motor sequelae (left hemiparesis and right hemiplegia) and had caused several epilepsy attacks. During the postoperative hospitalization period, he suffered from several infectious complications, including septic shock secondary to a severe pneumococcal pneumonia (October 1999) and acute gangrenous appendicitis of unknown origin (May 2000). On 22 November 2000, he had two epileptic fits that required treatment with gabapentine, as well as phenobarbital and dihydantoin. Three days later, fever and symptoms of meningitis appeared, leading to cerebrospinal fluid (CSF) analysis. The CSF sample contained 6,600 white blood cells/mm3, including 95% polymorphonuclear leukocytes. The white blood cell count was 17.5 × 109/liter, and the C reactive protein concentration was 129 mg/liter. Antibiotic therapy with ceftriaxone (4 g/day), vancomycin (50 mg/kg of body weight/day), amoxicillin, and gentamicin was started immediately, and the patient was transferred to the intensive care unit. Bacteriological cultures of CSF, blood, and urine were negative 48 h later, but the patient remained febrile and the meningitis-like symptoms persisted. Amoxicillin was stopped. Cerebral computed tomography showed evidence of brain empyema in the right frontal subdural region. On 29 November, gentamicin was stopped and the patient was admitted to the neurosurgery unit of our hospital for emergency surgical drainage. Two different samples were collected in the operating room for bacteriological analysis. The CSF culture and the two brain abscess cultures were positive for an anaerobic, gram-positive rod that smelled like horse manure and was identified as Clostridium difficile by RapID 32 A (BioMérieux, Marcy l'Etoile, France). This was confirmed by the National Center for Anaerobic Bacteria (Pasteur Institute, Paris, France). Two typical colonies of different sizes were found in each brain specimen: colony a (smaller colonies) and colony b (larger colonies). Antibiotic susceptibility was tested by the MIC method with the E-test (on Columbia agar supplemented with 5% sheep blood) (11). Interestingly, the MIC of metronidazole was 2 mg/liter for colony a, 3 mg/ml for the CSF strain, and 0.125 mg/liter for colony b. Given these results, the antibiotic treatment was modified as follows: ceftriaxone was replaced by ornidazole (1 g twice daily intravenously) and vancomycin was maintained at the same dose (3 g per day). Despite the lack of intestinal symptoms, a stool sample was collected. A C. difficile strain with the same antibiotic susceptibility pattern as that of brain colony a and CSF strains was isolated from this sample. The cytotoxicity assay for the C. difficile toxin using MRC-5 monolayer cells and the immunologic toxin A assay (Toxin Detection Kits; Oxoid) were negative for the stool specimen and for the brain colony a and CSF strains, but the immunologic toxin A assay was positive for the colony type b strain (Table 1) (1). PCR showed that the toxin A and B genes were missing from brain strain type a and present in the colony type b strain (National Center for Anaerobic Bacteria, Pasteur Institute). We digested DNA from the brain, CSF, and stool isolates with SmaI and subjected the digested DNA to pulsed-field gel electrophoresis (PFGE) (12) by using the interpretation criteria described by Tenover et al. (13). This analysis confirmed that two different types of C. difficile strain were involved: (i) a toxigenic colony b brain strain and (ii) CSF, stool, and nontoxigenic colony a brain strains. The CSF, stool, and nontoxigenic brain strains were genotypically indistinguishable (Fig. 1). The patient's postsurgical recovery was satisfactory from a neurological point of view in spite of several hemorrhagic episodes (otorrhagia, subcutaneous hematoma, and recurrent epistaxis) that could be explained by hemostasis disorders. Thirteen days after admission to our hospital, the patient was discharged back to the hospital in which he was initially hospitalized.
TABLE 1.
Chronology of C. difficile carriage and infection in our patient and characteristics of the isolates (toxigenicity, susceptibility to metronidazole, and PFGE restriction pattern)
| Date (mo-day-yr) | Sample type | Presence of C. difficile in culture | Assay result for toxin A (sample/isolate) | PCR result for toxins A and B | Metronidazole MIC (mg/liter) | PFGE restriction pattern |
|---|---|---|---|---|---|---|
| 10-13-99 | Stool | + | −/− | ND | 2 | A |
| 11-19-99 | Stool | + | −/− | ND | 0.75 | A |
| 12-6-99 | Stool | − | −/NDa | |||
| 4-29-00 | Stool | − | −/ND | |||
| 6-23-00 | Stool | + | −/− | ND | 2 | A |
| 7-18-00 | Stool | − | −/ND | |||
| 8-1-00 | Stool | − | −/ND | |||
| 8-11-00 | Stool | − | −/ND | |||
| 8-30-00 | Stool | − | −/ND | |||
| 11-25-00 | CSF | + | ND/− | − | 3 | A |
| 11-29-00 | Brain empyema (type a colony) | + | ND/− | − | 2 | A |
| 11-29-00 | Brain empyema (type b colony) | + | ND/+ | + | 0.125 | B |
| 12-1-00 | Stool | + | −/− | ND | 4 | A |
ND, not determined.
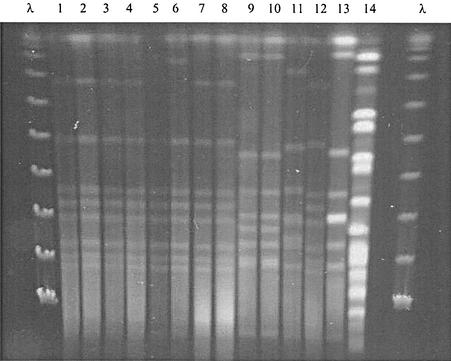
FIG. 1.
PFGE patterns after SmaI restriction of chromosomal DNA from C. difficile isolates. Lanes 1 and 2, strains isolated from stools in December 2000; lanes 3 and 4, brain empyema type a strain; lanes 5, 6, and 8, strains isolated from stool samples in November 1999, June 2000, and October 1999, respectively; lane 7, CSF strain; lanes 9 and 10, brain empyema type b strain; and lanes 11 through 14, four distinct, epidemiologically unrelated, C. difficile clinical isolates. λ, molecular weight markers.
C. difficile is an anaerobic, spore-forming, gram-positive rod that is nearly exclusively located in the gastrointestinal tract in humans. Toxigenic strains cause antibiotic-associated diarrhea, but only a few cases of extraintestinal infection have been reported (3, 6, 7). Garcia-Lechuz et al. found that there were four cases of extraintestinal illness caused by C. difficile per 100,000 admissions in their hospital in their 10-year study period (7). These extraintestinal infections were frequently polymicrobial infections in fluids or structures that are anatomically close to the colon (6, 7). Central nervous system infections due to Clostridium spp. are also uncommon, Clostridium perfringens (5) and Clostridium septicum (4) being the most frequently recovered species. Garcia-Lechuz et al. reported the first case of a brain abscess due to C. difficile in association with other microorganisms. Thus, we describe the second case of a severe brain infection caused by this organism, but this is the first time that C. difficile has been implicated in a monomicrobial culture in a human without a recent history of diarrhea. It was previously shown that extraintestinal infections frequently occur without concomitant diarrhea and are not always caused by toxigenic isolates (7). This suggests that their low intestinal virulence allows prolonged carriage followed by opportunistic infections.
Superinfection of a chronic subdural hematoma occurred in our patient, who had previously been colonized with a nontoxigenic C. difficile strain for a long time. It appears that the patient's digestive tract was discontinuously colonized because several stool specimen cultures were negative (Table 1). Indeed, before admission to our hospital, this bacterium had been found in three stool specimens from this patient (Table 1) and was first isolated 13 months before the appearance of the empyema. These three stool isolates were genotypically indistinguishable according to the criteria of Tenover et al. (13) (Fig. 1). They were also similar to those obtained from the nontoxigenic brain, CSF, and stool (collected at the time of empyema) strains. PFGE is usually used for epidemiological investigations during nosocomial outbreaks of diarrhea (12), but it has also been used to detect prolonged carriage with the same strain in a few healthy carriers (9).
The nontoxigenic strain of C. difficile that caused the brain infection was probably disseminated in the blood from the digestive tract. This dissemination is linked to the strong intestinal tropism of the bacterium and the long-lasting colonization of this site with the same nontoxigenic strain, as demonstrated by PFGE. These intestinal recurrences were probably promoted by repeated antibiotic treatments for infectious complications arising at that time. Moreover, the carriage of C. difficile in the portal system may have been facilitated by the fact that the patient had chronic alcoholism and secondary liver disease. The source of the toxigenic isolate remains undetermined, as this strain showed a radically different PFGE pattern from the others and was not found in the stool specimen.
The patient's medical chart indicated that, during the 13-month period before the onset of empyema, the patient had been prescribed metronidazole and then vancomycin for C. difficile colitis, even though the toxin was not detected in the stool specimen (Table 1). Indeed, it is questionable whether the patient really suffered from postantibiotic C. difficile colitis, as the 10 stool specimens and the isolates obtained from four of them were negative for C. difficile toxin A (C. difficile toxin A assay; Vidas, ROCHE). The cytotoxicity assay was also negative for the four isolates.
The long period of time during which the patient had been colonized with a nontoxigenic strain of C. difficile may have partially resulted from low metronidazole sensitivity. Indeed, the MICs of this antibiotic, as assessed by the E-test method, were between 0.75 and 4 mg/liter for all the nontoxigenic isolates (Table 1). Although these isolates are susceptible according to the NCCLS guidelines (10), their MICs are relatively high compared to those of wild-type strains, all the more as the metronidazole MICs for C. difficile usually seem to be lower when assessed by this method than when assessed by the reference agar dilution method (2). Furthermore, the intraluminal concentration of metronidazole may have been inadequate because of intestinal absorption and this antibiotic has been found to be ineffective for eradicating carriage in asymptomatic patients (8). These considerations may explain why C. difficile colonization continued despite antibiotic treatment with metronidazole. Alternatively, the patient may have been colonized from an environmental source.
In conclusion, C. difficile is rarely isolated from extraintestinal sites and, as shown here, is not always associated with a recent history of diarrhea. In some cases, this organism appears to behave in an opportunistic manner in hospitalized patients.
Acknowledgments
We thank Bernard Clair (Intensive Care Unit, Raymond Poincarré Hospital) and Guillaume Lot (Neurosurgical Unit, Lariboisière Hospital) for their collaboration and for providing the clinical information.
REFERENCES
- 1.Barbut, F., C. Kajzer, N. Planas, and J.-C. Petit. 1993. Comparison of three enzyme immunoassays, a cytotoxicity assay, and toxigenic culture for diagnosis of Clostridium difficile-associated diarrhea. J. Clin. Microbiol. 31:963-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbut, F., D. Decré, B. Burghoffer, D. Lesage, F. Delisle, V. Lalande, M. Delmée, V. Avesani, N. Sano, C. Coudert, and J.-C. Petit. 1999. Antimicrobial susceptibilities and serogroups of clinical strains of Clostridium difficile isolated in France in 1991 and 1997. Antimicrob. Agents Chemother. 43:2607-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byl, B., F. Jacobs, M. J. Struelens, and J. P. Thys. 1996. Extraintestinal Clostridium difficile infections. Clin. Infect. Dis. 22:712.. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, Y. T., C. T. Huang, H. S. Leu, J. S. Cheng, and M. C. Kiu. 1997. Central nervous system infection due to Clostridium septicum: a case report and review of the literature. Infection 25:171-174. [DOI] [PubMed] [Google Scholar]
- 5.Domingo, Z. 1994. Clostridial brain abscesses. Br. J. Neurosurg. 8:691-694. [DOI] [PubMed] [Google Scholar]
- 6.Feldman, R. J., M. Kallich, and M. P. Weinstein. 1995. Bacteremia due to Clostridium difficile: case report and review of extraintestinal C. difficile infections. Clin. Infect. Dis. 20:1560-1562. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Lechuz, J. M., S. Hernangomez, R. San Juan, T. Pelaez, L. Alcala, and E. Bouza. 2001. Extra-intestinal infections caused by Clostridium difficile. Clin. Microbiol. Infect. 7:453-457. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, S., S. R. Homann, K. M. Bettin, J. N. Quick, C. R. Clabots, L. R. Peterson, and D. N. Gerding. 1992. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann. Intern. Med. 117:297-302. [DOI] [PubMed] [Google Scholar]
- 9.Kato, H., H. Kita, T. Karasawa, T. Maegawa, Y. Koino, H. Takakuwa, T. Saikai, K. Kobayashi, T. Yamagishi, and S. Nakamura. 2001. Colonisation and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J. Med. Microbiol. 50:720-727. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2001. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 5th ed. Approved standard M11-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa..
- 11.Poilane, I., P. Cruaud, J. G. Rousseau, J. C. Torlotin, and A. Collignon. 1999. Susceptibility of Clostridium difficile to metronidazole using the E-test: effect of the culture medium. Pathol. Biol. 47:515-518. [PubMed] [Google Scholar]
- 12.Talon, D., P. Bailly, M. Delmée, M. Thouverez, B. Mulin, M. Iehl-Robert, V. Cailleaux, and Y. Michel-Briand. 1995. Use of pulsed-field gel electrophoresis for investigation of an outbreak of Clostridium difficile infection among geriatric patients. Eur. J. Clin. Microbiol. Infect. Dis. 14:987-993. [DOI] [PubMed] [Google Scholar]
- 13.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]