Abstract
α-Factor receptors from Saccharomyces cerevisiae are G-protein-coupled receptors containing seven transmembrane segments. Receptors solubilized with the detergent n-dodecyl β-d-maltoside were found to sediment as a single 8S species in glycerol density gradients. When the membranes from cells coexpressing two differentially tagged receptors were solubilized with detergent and subjected to immunoprecipitation, we found that the antibodies specific for either epitope tag resulted in precipitation of both tagged species. Coprecipitation was not a consequence of incomplete detergent extraction because the abundant plasma membrane protein Pma1 did not coprecipitate with the receptors. Moreover, the receptor complexes were present prior to detergent extraction because coimmunoprecipitation was not observed when cells expressing the single tagged species were mixed prior to membrane preparation. Treatment of cultures with α-factor had little effect on the extent of oligomerization as judged by the sedimentation behavior of the receptor complexes and by the efficiency of coimmunoprecipitation. The ability of receptor complexes to undergo ligand-mediated endocytosis was evaluated by using membrane fractionation and fluorescence microscopy. Mutant receptors that fail to bind α-factor (Ste2-S184R) or lack the endocytosis signal (Ste2-T326) became competent for ligand-mediated endocytosis when they were expressed in cells containing wild-type receptors. Coimmunoprecipitation experiments indicated that the C-terminal cytoplasmic domain and intermolecular disulfide bonds were unnecessary for oligomer formation. We conclude that α-factor receptors form homo-oligomers and that these complexes are subject to ligand-mediated endocytosis. Furthermore, we show for the first time that unoccupied receptors participate in these endocytosis-competent complexes.
INTRODUCTION
The G-protein-coupled receptors (GPCRs) comprise the largest and most diverse superfamily of cell-surface receptors (Hebert and Bouvier, 1998; Bockaert and Pin, 1999). GPCRs mediate responses to a variety of extracellular stimuli such as light, odorants, calcium, hormones, and neurotransmitters. They contain a central core of seven putative transmembrane domains, and signal transduction is mediated by a heterotrimeric G-protein. Until recently, GPCRs have been assumed to function as monomers. Examples of early observations suggesting oligomerization of GPCRs include allelic complementation of coexpressed mutant receptors (Konopka and Jenness, 1991), and the presence of large SDS-resistant receptor–protein aggregates on SDS-PAGE gels (Herberg et al., 1984; Blumer et al., 1988; Konopka et al., 1988; Ng et al., 1993). More direct evidence was obtained recently by using cross-linking and coimmunoprecipitation approaches, suggesting oligomerization of the β2-adrenergic (Hebert et al., 1996), δ-opioid (Cvejic and Devi, 1997), D2 and D3 dopamine (Ng et al., 1996; Nimchinsky et al., 1997), m3 muscarinic (Zeng and Wess, 1999), metabotropic glutamate (Romano et al., 1996), and Ca2+-sensing receptors (Bai et al., 1998).
Although oligomerization is emerging as a common theme for GPCRs, our understanding of this phenomenon is limited. The influence of agonists on receptor oligomerization and the structural determinants of the receptor that are important for oligomerization have been shown to vary among the GPCRs investigated. For example, agonists appear to stabilize dimers of β2-adrenergic receptors (Hebert et al., 1996), whereas agonist binding favors the monomeric state of δ-opioid receptors (Cvejic and Devi, 1997) and fails to alter the oligomeric state m3 muscarinic receptors (Zeng and Wess, 1999). Transmembrane region VI is thought to mediate the association of β2-adrenergic receptors because peptides containing this sequence interfere with the recovery of receptor dimers and interfere with signaling (Hebert et al., 1996). In contrast, 15 amino acids in the C-terminal tail of δ-opioid receptor are associated with dimerization (Cvejic and Devi, 1997), and the Ca2+-sensing receptor and metabotropic glutamate receptor 5 oligomerize through disulfide bonds (Romano et al., 1996; Bai et al., 1998).
Functional significance of GPCR oligomerization is currently unclear. Functional interactions between oligomerized receptors have been inferred from the cooperative binding of subtype-specific ligands to receptor heterodimers containing δ- and κ-opioid receptors (Jordan and Devi, 1999). The relationship between oligomerization and signal transduction is controversial. Although the transmembrane VI peptide from the β2-adrenergic receptor interferes with detection of both receptor dimers and receptor-signaling activity (Hebert et al., 1996), this region of the D1 dopamine receptor inhibits receptor function without affecting oligomerization (George et al., 1998). For the δ-opioid receptor, ligand-induced dissociation of receptor oligomers is found to precede ligand-mediated endocytosis (Cvejic and Devi, 1997). This correlation suggests that the dissociation of oligomers may be an essential step in the endocytic pathway.
The α-factor pheromone receptor Ste2p is a GPCR that is present on the surface of yeast haploid cells of the a mating type (a cells). It binds the α-factor pheromone secreted by haploid α cells during mating of a cells and α cells. After α-factor binding, the receptor undergoes a conformational change (Bukusoglu and Jenness, 1996), resulting in the activation of a heterotrimeric G-protein and a protein kinase cascade. These intracellular signals inhibit cell division and promote transcription of mating-specific genes. α-Factor receptors are subject to ligand-mediated endocytosis and degradation in the vacuole (Jenness and Spatrick, 1986; Schandel and Jenness, 1994; Hicke, 1997; Mulholland et al., 1999); endocytosis is associated with phosphorylation and ubiquitination of the cytoplasmic C-terminal domain of the receptor (Hicke and Riezman, 1996; Hicke et al., 1998). Oligomerization of α-factor receptors in the plasma membrane was first proposed by Jenness and Spatrick (1986) because receptors were found to be internalized more rapidly than bound α-factor at subsaturating concentrations. We sought to determine whether yeast α-factor receptors form oligomers and whether oligomers are subject to ligand-mediated endocytosis.
MATERIALS AND METHODS
Plasmids
pJBK008 is a yeast centromeric plasmid that contains the STE2 and URA3 genes (Konopka et al., 1988). pYe(CEN3)30 is a yeast centromeric plasmid that contains the TRP1 gene (Fitzgerald-Hayes et al., 1982). Plasmid pNED1(-Cys) (provided by Pam Torrance and Jeremy Thorner, University of California, Berkeley, Berkeley, CA) is a derivative of plasmid pNED1 (David et al., 1997); it encodes a modified Ste2p that contains the Flag epitope and the 6His tags at the C terminus, lacks Cys residues 59 and 252, and is expressed from the TDH3 promoter. pDJ123 (provided by Kim Schandel, University of Massachusetts, Worcester, MA) was constructed by cloning the 4.3-kilobase (kb) BamHI fragment that contains the STE2 gene into the BamHI site of plasmid vector pYe(CEN3)30. pDJ323 (provided by Gul Bukusoglu, University of Massachusetts, Worcester, MA) contains ste2-S184R and was created by hydroxylamine mutagenesis of plasmid pJBK008; the STE2-coding region was confirmed by DNA sequencing. The yeast integrating plasmid pDJ320 contains the URA3 gene, and it directs synthesis of a fusion protein that contains the α-factor receptor and the green fluorescent protein (GFP) under the control of the STE2 promoter (Li et al., 1999). Integrating plasmid pDJ379 contains the URA3 gene and the STE2 codons 302–431 fused to the coding sequence for GFP (Li et al., 1999). Cleavage of pDJ379 with PstI followed by integration at the chromosomal STE2 locus results in production of full-length Ste2p tagged at the C terminus with GFP. Integrating plasmid pDJ466 contains the TRP1 gene and STE2 codons 302–431 fused to the coding sequence for the triple influenza hemagglutinin (HA) epitope. Cleavage of pDJ466 with PstI followed by integration at the chromosomal STE2 locus results in production of full-length Ste2p tagged at the C terminus with the triple HA epitope. pDJ466 was constructed in two steps: first, the 0.4-kb NsiI/SacII fragment from pDJ320 (containing STE2 codons 302–431) was subcloned into plasmid vector pRS304 (Sikorski and Hieter, 1989) that had been cut with PstI and SacII; and second, the resulting plasmid was digested with SacI and SacII and ligated with the SacI/SacII-digested product of a polymerase chain reaction (PCR) that contains the triple HA epitope DNA (from Mike Tyers, Mount Sinai Hospital, Toronto, ONT) as template and oligonucleotide primers PO-140 (CGTGCCGAGCTCCCATGGTCAAGCAGCGTAATCTGGAACGTCATA) and PO-177 (GGCTCCCCGCGGTCTTTTACCCATACGATGTTCCTGAC-TAT). Integrating plasmid pDJ467 contains the URA3 gene and STE2 codons 156–326 fused to the GFP-coding sequence. Cleavage of pDJ467 with ClaI followed by integration at the chromosomal STE2 locus results in production of Ste2-T326 tagged at the C terminus with GFP. pDJ467 was constructed by ligating the 4.5-kb NsiI/SacII fragment (lacking STE2) from pDJ320 with the PstI/SacII-digested product of a PCR reaction that contained STE2 DNA (pDJ320) as template and oligonucleotide primers PO-141 (GCGAAACTGCAGGGCGACAACTTCAAAAGGATAGGTTT) and PO-147 (CCACACCTACGAGTTCAA). pDJ469 (provided by Padhma Radhakrishnan, University of Massachusetts, Worcester, MA) is a yeast centromere plasmid that contains URA3 and directs synthesis of Ste2-T326 tagged at the C terminus with GFP. pDJ469 was created by cloning the ClaI-XbaI fragment (containing STE2 codons 259–326 and GFP) from pDJ467 into ClaI-SpeI sites in plasmid pDB02 (Dube and Konopka, 1998). Integrating plasmid pDJ470 contains TRP1 and STE2 codons 156–326 fused to the triple HA-coding sequence. Cleavage of pDJ470 with Eco47III followed by integration at the chromosomal STE2 locus results in production of Ste2-T326 tagged at the C terminus with the triple HA epitope. pDJ470 was created in two steps. In the first step, primers PO-177 and PO-186 (GCGAAAGGTACCGGCGACAACTTCAAAAGGATAGGTTT) were used to amplify DNA encoding the triple HA epitope, and the PCR product was cloned between the SacII and XbaI sites of plasmid pDJ467, replacing the GFP gene. In the second step, a 0.6-kb sequence in this plasmid (containing STE2 codons 156–326 and the triple HA epitope) was PCR-amplified with oligonucleotide primers PO-140 and PO-141, and the PstI/SacI-digested product was cloned between the PstI and SacI sites of pRS304.
Yeast Strains
Yeast strains listed in Table 1 are congenic to strain 381G. Strains DJ1400-A, DJ1403-A, DJ1404-A, DJ1405-A, DJ1406-A, DJ1408-A, DJ1414-A to DJ1417-A were derived from DJ211-5-3; strains DJ1402-A, DJ1407-A, and DJ1418-A were derived from AY1; strains DJ1410-A, DJ1411-A, and DJ1413-A were derived from strain DJ1205-6-3 by transformation with the plasmids indicated in Table 1. Plasmids pDJ379 and pDJ466 were digested with PstI and plasmids pDJ467 and pDJ470 were digested with ClaI and Eco47III, respectively, prior to transformation to target the integration at the STE2 locus. pDJ320 was digested with StuI to target the integration at the URA3 locus. Production of relevant proteins was confirmed by Western blotting. Yeast strains were transformed with plasmids by using standard techniques (Soni et al., 1993). Strain AY1 (provided by Amy Yang, University of Massachusetts, Worcester, MA) was constructed by subcloning the ste2-S184R allele into integrating plasmid pDJ251 and then introducing it into the chromosomal locus of strain DJ211-5-3 by using the two-step gene replacement described previously (Schandel and Jenness, 1994).
Table 1.
Yeast strains used in this study
| Straina | Genotypeb |
|---|---|
| 381G | MATa cry1 ade2-1 his4-580 lys2 trp1 tyr1 SUP4-3ts |
| DJ211-5-3 | 381G leu2 ura3 bar1-1 |
| AY1 | DJ211-5-3 ste2-S184R |
| DJ1205-6-3 | 381G ADE2+HIS4+LYS2+TYR1+ura3 bar1-1 |
| DJ1400-A | DJ211-5-3 STE2∷pDJ466 |
| DJ1402-A | DJ211-5-3 ste2-S184R∷pDJ466 containing plasmid pJBK008 |
| DJ1403-A | DJ211-5-3 STE2∷pDJ466 containing plasmid pDJ323 |
| DJ1404-A | DJ211-5-3 STE2∷pDJ379 |
| DJ1406-A | DJ211-5-3 STE2∷pDJ379 containing plasmid pDJ123 |
| DJ1407-A | DJ211-5-3 ste2-S184R∷pDJ379 containing plasmid pYe(CEN3)30 |
| DJ1408-A | DJ211-5-3 STE2∷pDJ466 containing plasmid pYe(CEN3)30 |
| DJ1410-A | DJ1205-6-3 STE2∷pDJ467 containing plasmid pYe(CEN3)30 |
| DJ1411-A | DJ1205-6-3 STE2∷pDJ467 containing plasmid pDJ123 |
| DJ1413-A | DJ1205-6-3 STE2∷pDJ379 containing plasmid pYe(CEN3)30 |
| DJ1414-A | DJ211-5-3 ura3∷pDJ320 STE2∷pDJ466 |
| DJ1417-A | DJ211-5-3 STE2∷pDJ470 containing plasmid pDJ469 |
| DJ1418-A | DJ211-5-3 ste2-S184R∷pDJ466 containing plasmid pDJ323 |
| DJ1419-A | DJ211-5-3 STE2∷pDJ466 containing plasmid pJBK008 |
All strains are congenic with strain 381G (Hartwell, 1967).
Mutation bar1-1 inhibits α-factor degradation (Sprague and Herskowitz, 1981). Temperature-sensitive mutation SUP4-3 suppresses amber mutations his4-580 and trp1 at 22°C. STE2∷pDJ466, ste2-S184R∷pDJ466, STE2∷pDJ379, ste2-S184R∷pDJ379, STE2∷pDJ467, STE2∷pDJ470, and ura3∷pDJ320 result in the production of Ste2-HA, Ste2-S184R-HA, Ste2-GFP, Ste2-S184R-GFP, Ste2-T326-GFP, Ste2-T326-HA, and Ste2-GFP, respectively. The CEN plasmids pJBK008, pDJ323, pDJ123, and pDJ469 result in the production of Ste2, Ste2-S184R, Ste2, and Ste2-T326-GFP, respectively. pYe(CEN3)30 is a control plasmid that contains a TRP1 marker.
Media
Liquid and solid media were prepared as previously described (Jenness et al., 1997). YM-1 is a rich liquid medium (Hartwell, 1967). Minimal selective media lacking uracil (−Ura + CAA) or lacking tryptophan (−Trp + CAA) are described elsewhere (Hirschman et al., 1997).
Antisera and Reagents
Rabbit polyclonal antisera were specific for GFP (Seedorf et al., 1999), for Escherichia coli aspartate transcarbamoylase (ATCase) (from Y.R. Yang and H.K. Schachman, University of California, Berkeley, Berkeley, CA) or for the carboxy-terminal portion of the α-factor receptor Ste2 (Konopka et al., 1988). Mouse monoclonal antibodies that recognize the yeast plasma membrane ATPase (Pma1) were from clone C56 (Aris and Blobel, 1988; Schandel and Jenness, 1994). Mouse monoclonal antibodies that recognize the influenza HA epitope (HA.11) were from Babco, Berkeley Antibody, Richmond, CA. Peroxidase-conjugated goat anti-rabbit secondary antibodies were purchased from Life Technologies, Baltimore, MD. Peroxidase-conjugated goat anti-mouse secondary antibodies, purified mouse immunoglobulin and n-dodecyl β-d-maltoside were from Sigma Chemical, St. Louis, MO. Purified bovine serum albumin (BSA) was purchased from Boehringer Mannheim, Indianapolis, IN. Peroxidase-conjugated goat anti-mouse and goat anti-rabbit F(ab′)2 fragment specific IgG were purchased from Jackson Immunoresearch, West Grove, PA. The chemiluminescence kit Super Signal and Ultralink Immobilized Protein A beads were from Pierce Chemical, Rockford, IL.
Renografin Density Gradients
Cultures were grown in −Ura + CAA or in −Trp + CAA medium according to the plasmid markers used. Membranes were resolved in Renografin density gradients as previously described (Schandel and Jenness, 1994).
Immunoblotting, Quantitation, and Immunoprecipitation
Western blotting procedures and quantitation were carried out as previously described (Hirschman et al., 1997). Cell lysates were prepared as described previously (Schandel and Jenness, 1994). Membranes were collected by centrifugation (Beckman airfuge for 20 min, or SW50 rotor at 40,000 krpm for 90 min). The pellet containing the membranes was suspended in ice-cold IP buffer (50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 2 mg/ml n-dodecyl β-d-maltoside, 10% glycerol, 100 μg/ml phenylmethylsulfonyl fluoride, and 10 μg/ml pepstatin A) and incubated on ice for 2 h with occasional mixing. The solution was then centrifuged at 13,000 × g for 5 min to remove insoluble material. In each experiment, an equivalent number of cells was processed for each immunoprecipitation reaction; however, for the experiments in which the cultures had been treated with α-factor; the extracts were corrected for protein concentration. Bicinchoninic acid protein assay (Pierce Chemical) was used according to manufacturer's instructions. Precipitating antibodies were added to the supernatant, and the mixture was incubated at 4°C with gentle agitation for 2 h. Protein A beads were added, and the mixture was incubated for 2 h at 4°C. The beads were allowed to settle for 5 min, and then collected by centrifugation 1100 × g for 5 s. Beads were washed four times with IP buffer and extracted with concentrated SDS sample buffer for 10 min at 37°C. Samples were centrifuged at 13,000 × g for 5 min. The proteins were resolved on 10% SDS-PAGE gels and detected by using immunoblotting methods. Peroxidase-conjugated goat anti-mouse or goat anti-rabbit F(ab′)2 fragment was used as the secondary reagent. The results were quantified by using laser-scanning densitometry (Molecular Dynamics, Sunnyvale, CA).
Glycerol Gradient Sedimentation
Cells (2 × 109) were collected from exponentially growing cultures that had been untreated or treated with 10−7 M α-factor for 5 min. Crude membranes were extracted with n-dodecyl β-d-maltoside as described in the previous section. The extract was cleared by centrifugation for 15 min at 13,000 × g and mixed with 4.5 μg of BSA (4.3S), 5 μg of mouse IgG (7S), and 10 μg of ATCase (11.7S) as internal marker proteins. The mixture was applied to an 8–30% glycerol gradient in IP buffer and centrifuged in an SW50 rotor at 40,000 rpm for 14 h at 4°C. Fourteen 350-μl fractions were collected assayed for the presence of the tagged receptors and the marker proteins by using SDS-PAGE and immunoblotting methods.
Fluorescence Microscopy
Cultures were grown overnight at 34°C to a density of 2 × 106 cells/ml in −Trp + CAA medium. These conditions provided selection of the plasmids bearing the TRP1 gene. Cells were collected and resuspended in the same volume of YM-1 medium, and then cultured at 30°C to a density of 107 cells/ml. Cultures received cycloheximide (10 μg/ml) for 5 min and were then incubated at 30°C for 15 min in the presence or in the absence of α-factor (10−7 M). Endocytosis was terminated by chilling the cells and adding the metabolic poisons, NaN3 (10 mM) and KF (10 mM). Cells were collected by centrifugation, washed with ice-cold phosphate-buffered saline, and suspended in 1/10 volume of phosphate-buffered saline. Epifluorescent images were obtained with a Nikon microscope equipped with a cooled charge-coupled device camera.
RESULTS
Immunoprecipitation of Differentially-tagged Receptors
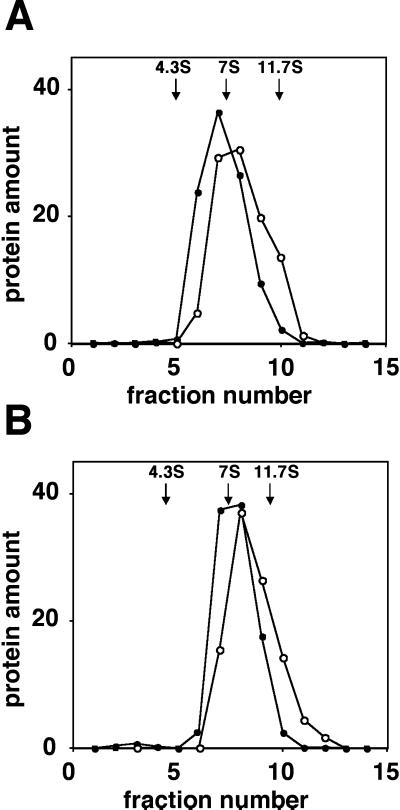
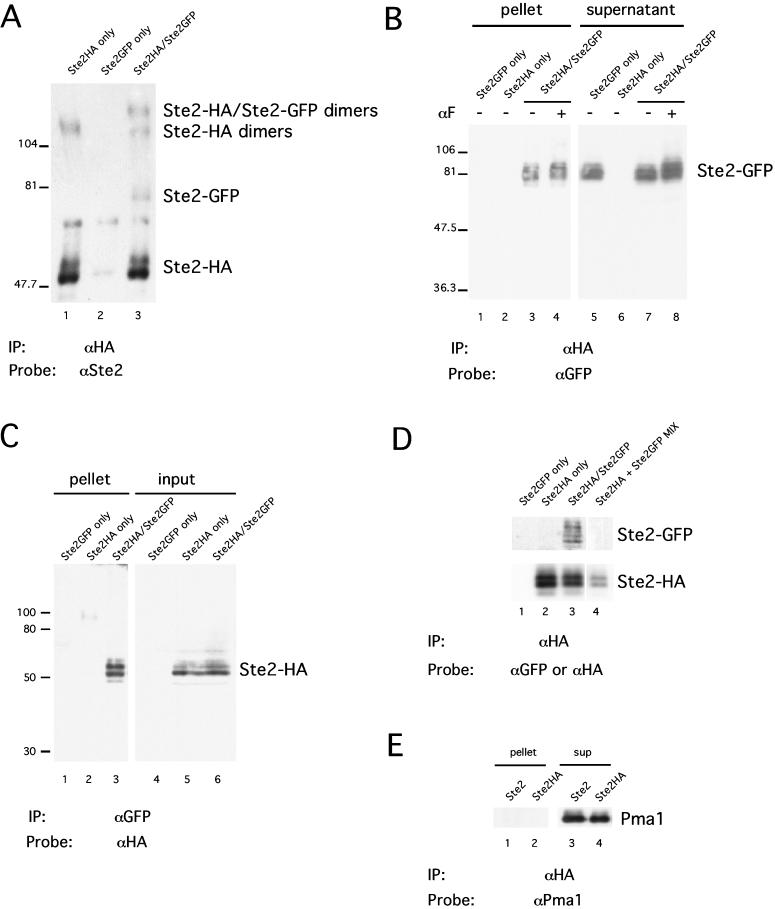
Interactions between α-factor receptors were evaluated by performing coimmunoprecipitation experiments with differentially tagged receptors that had been solubilized with nondenaturing detergent n-dodecyl β-d-maltoside. We examined cells that express α-factor receptors tagged with the influenza HA epitope (Ste2-HA) as well as α-factor receptors tagged with the GFP (Ste2-GFP). Both epitope tags were fused to the receptor after the C-terminal residue. The genes encoding the two fusion proteins were present in single copy and used the native STE2 promoter. Crude membranes were prepared and extracted with the detergent. Glycerol gradients were performed to evaluate the size and the homogeneity of the complexes containing receptors. Both Ste2-HA and Ste2-GFP sedimented as a single peak with sedimentation coefficient of roughly 8S in glycerol density gradients (Figure 1A), although Ste2-HA sedimented slightly faster than Ste2-GFP. The relatively small differences in the molecular weights of the two tagged species are not expected to influence the sedimentation rate. Similar results were obtained when the cultures had been exposed to α-factor (Figure 1B). Both Ste2-HA and Ste2-GFP were found to coimmunoprecipitate, when the receptors in the detergent extract were precipitated with anti-HA antibodies and analyzed with immunoblotting methods (Figure 2A, lane 3). The two tagged species were resolved on the blot because Ste2-GFP is significantly larger than Ste2-HA (80 vs. 55 kDa, respectively). No precipitation of Ste2-GFP was detected in the analysis of the control cells that expressed only Ste2-HA or only Ste2-GFP (Figure 2A, lanes 1 and 2, respectively). Similar results were obtained by using two other detergents, 1% Triton X-100 and 0.1% C12E8, suggesting that the interactions detected between the receptors are not specific to n-dodecyl β-d-maltoside (our unpublished results).
Figure 1.
Glycerol gradient sedimentation. Membrane proteins from strain DJ1414-A were solubilized in IP buffer containing n-dodecyl β-d-maltoside and then resolved on an 8–30% glycerol gradient. Cultures had been untreated (A) or treated with 10−7 M α-factor for 5 min (B). Fractions were assayed for Ste2-HA (○) and Ste2-GFP (●) and for marker proteins, BSA (4.3S), mouse IgG (7S), and ATCase (11.7S).
Figure 2.
Immunoprecipitation of differentially tagged receptors. Cells that expressed one or two tagged forms of the receptor (Ste2-HA and Ste2-GFP) were analyzed. Membrane proteins extracted with n-dodecyl β-d-maltoside were subjected to immunoprecipitation and then analyzed by using immunoblotting methods. The antibodies used for precipitation and for immunoblotting are denoted below each panel (“IP” and “Probe,” respectively). As indicated above each lane, the cells analyzed expressed Ste2-HA, Ste2-GFP, or both. (A) Anti-HA antibody precipitates Ste2-HA, Ste2-GFP, and SDS-resistant dimers. (B) Efficiency of Ste2-GFP precipitation with anti-HA antibody. Lanes 1–4 contained 10% of the immunoprecipitated protein (pellet). Lanes 5–8 contained 2.5% of the unprecipitated protein (supernatant). Cells expressing both Ste2-HA and Ste2-GFP had been either untreated or treated with 10−7 M α-factor for 5 min prior to analysis. (C) Efficiency of Ste2-HA precipitation with anti-GFP antibody. Lanes 1–3 contained 17% of the immunoprecitated protein (pellet). Lanes 4–6 contained 1% of the total protein (input). (D) Coprecipitation does not result from processing of the samples after cell lysis. Lanes 1–3, processed as in B. Lane 4, cells expressing only Ste2-HA were mixed prior to lysis with cells expressing only Ste2-GFP. The same blot was sequentially probed with anti-GFP (top) and anti-HA antibody (bottom). (E) Plasma membrane ATPase is not present in immunoprecipitates containing Ste2-HA. Strains were DJ1400-A (Ste2-HA only), DJ1404-A (Ste2-GFP only), DJ1414-A (Ste2-HA/Ste2-GFP), and DJ211–5-3 (Ste2).
Two protein species containing Ste2 were detected that had molecular weights greater than 100 kDa (Figure 2A, lanes 1 and 3). Such high-molecular-weight forms, designated “SDS-resistant dimers,” are commonly observed when analyzing Ste2 and other GPCR proteins (Blumer et al., 1988; Konopka et al., 1988; Hebert et al., 1996). We found, however, that the proportion of receptors that migrated as SDS-resistant dimers was variable among different preparations. In earlier work, it has been unclear whether this high-molecular-weight species reflects the aggregation of Ste2 with itself or with other proteins, and also it has been unclear whether it represents receptor dimers present in the membrane or dimers that arise only after SDS extraction. Interestingly, we detected a single high-molecular-weight protein from cells expressing Ste2-HA alone (Figure 2A, lane 1), consistent with Ste2-HA dimers, whereas cells expressing both Ste2-HA and Ste2-GFP produced bands consistent with Ste2-HA/Ste2-GFP heterodimers in addition to Ste2-HA homodimers (Figure 2A, lane 3). The absence of Ste2-GFP homodimers is expected because the sample had been immunoprecipitated with anti-HA antibodies. These observations indicate that an SDS-resistant dimer contains more than one molecule of Ste2.
As a more defined method of evaluating coimmunoprecipitation of Ste2-HA and Ste2-GFP, we performed reciprocal immunoprecipitation experiments. Antibodies against one epitope were used to precipitate receptors from the detergent extract, and then antibodies against the second epitope were used to test for the presence of the second tagged species in the immunoprecipitate. In Figure 2B, anti-HA antibodies precipitated 11% of the Ste2-GFP from the extracts of cell expressing both receptors (compare lanes 3 and 7), whereas no detectable Ste2-GFP was precipitated from the control cells' extracts containing only Ste2-GFP or only Ste2-HA (lanes 1 and 2, respectively). Similar results were obtained when the cultures had been exposed to α-factor for 5 min (lanes 4 and 8) or 15 min (our unpublished results) prior to the analysis. Receptors are normally depleted from the plasma membrane after 20 min (Schandel and Jenness, 1994). In the reciprocal experiment (Figure 2C), anti-GFP antibodies precipitated ∼6% of the Ste2-HA from the extracts of cell expressing both receptors (compare lanes 3 and 6), and no Ste2-HA was precipitated from either control extract (lanes 1 and 2). Again, prior exposure to α-factor 5 or 15 min (our unpublished results) had no discernable effect. In both experiments, the antibody used for immunoprecipitation cleared all the antigen from the supernatant (our unpublished results).
Coprecipitation of Ste2-HA and Ste2-GFP was not a consequence of incomplete membrane solubilization or nonspecific aggregation of membrane proteins. The coprecipitated Ste2-HA and Ste2-GFP were apparently part of the 8S complex because essentially all of Ste2-HA and Ste2-GFP extracted under these conditions sediment as 8S species (Figure 1A). Moreover, when the 8S peak in Figure 1A was pooled and analyzed according to the reciprocal immunoprecipitation method, we found that 5% of Ste2-GFP was precipitated with anti-HA antibody and that 5% of Ste2-HA was precipitated with anti-GFP (our unpublished results). In addition, the protein complexes containing Ste2 do not appear to result from nonspecific aggregation of membrane proteins because the more abundant transmembrane protein, plasma membrane ATPase (Pma1p), was not found in immunoprecipitates containing Ste2-HA (Figure 2E).
We considered the possibility that receptor complexes that we observed were formed only after the proteins were extracted from the membrane with detergent. To test this possibility, we mixed cells that only expressed Ste2HA with cells that only express Ste2-GFP, and then processed the mixture for immunoprecipitation as described above (Figure 2D). We detected Ste2-GFP coprecipitate with Ste2-HA only when both receptors were expressed in the same cells (Figure 2D, lane 3) but not from the mixture of the two cultures expressing Ste2-HA and Ste2-GFP receptors individually (Figure 2D, lane 4). We conclude that α-factor receptors are present in the plasma membrane as complexes containing two or more receptor molecules. Although we were unable to detect changes in the complexes induced by α-factor pretreatment, we cannot rule out the possibility that α-factor influences higher order states of aggregation that were not stable in the solvent conditions used.
Ste2-S184R Is Internalized with Wild-Type Receptors upon α-Factor Exposure
Early work with α-factor receptor endocytosis suggested that receptors are internalized as oligomeric units (Jenness and Spatrick, 1986). When yeast cells are exposed to subsaturating concentrations of α-factor, the rate at which α-factor receptor sites are lost from the plasma membrane is greater than the rate of α-factor uptake. This observation suggests that unoccupied receptors are internalized together with the occupied receptors. Three explanations (not mutually exclusive) account for these phenomena: 1) binding of α-factor may be necessary only to initiate the events that lead to the internalization, i. e., receptor internalization may proceed even after α-factor dissociates; 2) the invaginations of the plasma membrane that occur during endocytosis may be large enough to include both occupied receptors and neighboring unoccupied receptors; and/or 3) the occupied and unoccupied receptors may exist as oligomeric units that remain coupled during endocytosis. To determine whether α-factor binding is required to initiate receptor internalization, we coexpressed wild-type Ste2 and the α-factor–binding-defective mutant Ste2-S184R. In the presence of α-factor, these two receptors represent occupied and unoccupied receptors, respectively. If the internalization of the unoccupied receptors requires prior occupancy, then endocytosis of Ste2-S184R should not be induced by α-factor because it does not bind α-factor. Conversely, if internalization of unoccupied receptors reflects cointernalization, then Ste2-S184R internalization should be stimulated by α-factor.
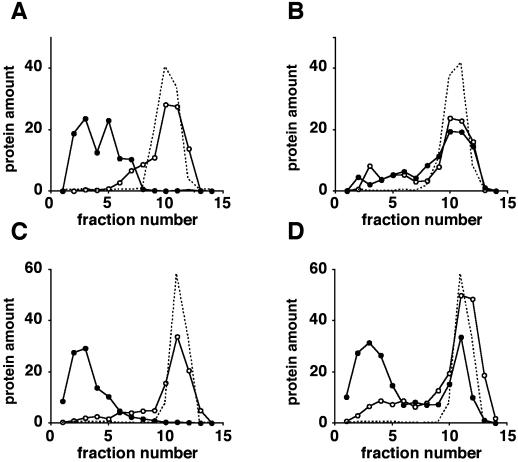
Membrane fractionation was used to evaluate ligand-induced exit of Ste2-S184R from the plasma membrane in the presence and in the absence of the wild-type receptor. We created strains that coexpress both wild-type Ste2 and mutant Ste2-S184R receptors. Genes encoding both receptors were present in a single copy and contained the native STE2 promoter. In each experiment, the chromosomal allele directed synthesis of HA-tagged receptors and a plasmid-borne, allele-directed synthesis of untagged receptors. Exponentially growing cultures were treated with cycloheximide to block new receptor synthesis, incubated further either in the presence or in the absence of α-factor, and then after 15 min, the membranes were resolved on Renografin density gradients. Because essentially all of the receptors were on the cell surface prior to treatment, receptors detected in internal membrane fractions represent molecules that have exited the plasma membrane. As shown previously (Schandel and Jenness, 1994), the cells that expressed only wild-type receptors internalized essentially all their receptors in response to α-factor, as indicated by a shift of the Ste2 protein from the denser plasma membrane fractions to the more buoyant internal membrane fractions (Figure 3A). In contrast, cells expressing only Ste2-S184R showed no α-factor–induced internalization (Figure 3B). Upon α-factor exposure, a significant fraction of the tagged Ste2-S184R receptors were internalized in cells containing untagged wild-type receptors (Figure 3D). This observation suggests that internalization of unoccupied receptors is not a consequence of prior α-factor occupancy because Ste2-S184R receptors do not bind α-factor. In the reciprocal experiment, internalization of tagged wild-type receptors was not influenced by the presence of the mutant Ste2-S184R receptors (Figure 3C).
Figure 3.
Binding-defective receptors, Ste2-S184R, undergo ligand-induced endocytosis when expressed with wild-type receptors. Each of the four strains analyzed expressed an HA-tagged receptor (Ste2-HA or Ste2-S184R-HA) encoded by a chromosomal allele and an untagged plasmid-encoded receptor (Ste2 or Ste2-S184R). Log-phase cultures in −Ura + CAA medium were treated with cycloheximide and then cultured for 15 min in the absence (○) or presence of α-factor (●). Membranes were fractionated by using Renografin density gradients. Fractions were assayed for HA-tagged receptors (○,●) and for plasma membrane ATPase (no plot symbol) by using immunoblotting methods. Plasma membrane marker is shown for α-factor-treated cultures only. Protein amount is the percentage of the total protein. (A) Cells expressing Ste2-HA and Ste2 (strain DJ1419-A). (B) Cells expressing Ste2-S184R-HA and Ste2-S184R (strain DJ1418-A). (C) Cells expressing Ste2-HA and Ste2-S184R (strain DJ1403-A). (D) Cells expressing Ste2-S184R-HA and Ste2 (strain DJ1402-A).
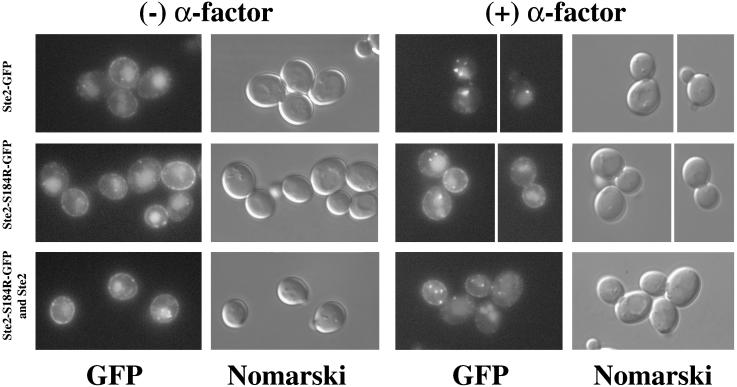
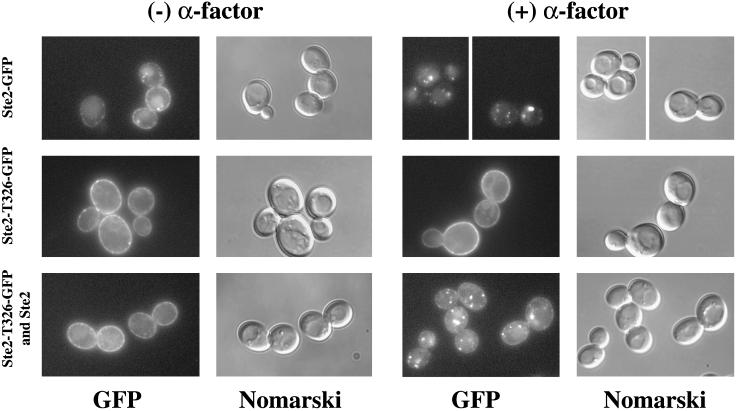
As a second assay for endocytosis of occupied and unoccupied receptors, we used fluorescence microscopy to monitor wild-type and mutant receptors that had been tagged with GFP. Ste2-S184R-GFP was coexpressed with untagged wild-type receptors to test whether internalization of the wild-type receptors would cause the internalization of the Ste2-S184R-GFP receptors. To this end, cultures that had been treated with cycloheximide were challenged with α-factor. In the absence of α-factor, the cells containing GFP-tagged mutant and wild-type receptors exhibited fluorescence both at the plasma membrane and in the vacuole (Figure 4). Our previous results (Li et al., 1999) indicate fluorescence in the vacuole reflects the free GFP that remains after the Ste2-GFP fusion protein has been endocytosed and the Ste2 portion of the protein degraded. In presence of α-factor, the wild-type Ste2-GFP was completely removed from the plasma membrane and appeared as punctate structures presumably corresponding to endocytic vesicles (Figure 4, top row), whereas cells expressing only the Ste2-S184R mutant receptors showed very little internalization of cell-surface fluorescence (Figure 4, middle row). In contrast, in cells expressing both Ste2-S184R-GFP and untagged Ste2, the plasma membrane fluorescence was significantly reduced and a greater proportion of the fluorescence appeared in internal punctate structures (Figure 4, bottom row). When analyzed quantitatively, 32% of the cells expressing both Ste2-S184R-GFP and Ste2 showed three or more fluorescent foci after α-factor treatment, whereas only 7% of the cells expressing Ste2-S184R-GFP alone showed more than three fluorescent foci (Table 2). These observations are consistent with our results from the Renografin gradients, indicating that Ste2-S184R receptors are internalized with the wild-type receptors in the presence of α-factor.
Figure 4.
Ligand-induced internalization of GFP-tagged binding-defective receptors depends on the presence of wild-type receptors. Cultures were treated with cycloheximide and α-factor as described in Figure 3. First two columns are controls lacking α-factor; the last two columns are α-factor–treated cells. GFP fluorescence images and Nomarski images are indicated below each column. Top row, cells expressing Ste2-GFP only (strain DJ1408-A). Middle row, cells expressing Ste2-S184R-GFP only (strain DJ1407-A). Bottom row, cells expressing both Ste2-S184R-GFP and untagged Ste2 (strain DJ1406-A).
Table 2.
Quantitation of fluorescent foci from cells expressing Ste2 and Ste2-S184R
| Ste2 forms expressedb | α-Factor | Percentage
of cellsa
|
|
|---|---|---|---|
| n ≥ 1 | n ≥ 3 | ||
| Ste2-GFP | − | 31 ± 3 | 7 ± 2 |
| Ste2-GFP | + | 94 ± 2 | 44 ± 3 |
| Ste2-S184R-GFP | − | 31 ± 3 | 6 ± 1 |
| Ste2-S184R-GFP | + | 57 ± 3 | 7 ± 2 |
| Ste2-S184R-GFP and Ste2 | − | 33 ± 3 | 7 ± 2 |
| Ste2-S184R-GFP and Ste2 | + | 72 ± 2 | 32 ± 2 |
Fluorescent foci were counted from GFP images of cells treated with cycloheximide and α-factor as in Fig. 3. The percentage of cells with one or more fluorescent foci (n ≥ 1) and the percentage of cells with three or more fluorescent foci (n ≥ 3) are indicated for the cells that had been cultured in the presence or absence of α-factor. More than 200 cells were examined for each entry.
The forms of Ste2 expressed in each strain are shown. Strains used are DJ1408-A (rows 1 and 2), DJ1407-A (rows 3 and 4), and DJ1406-A (rows 5 and 6).
Two criteria were used to judge whether endocytosis of occupied receptors results in the internalization of a significant portion of surrounding plasma membrane and membrane proteins. First, essentially all of the abundant plasma membrane protein ATPase Pma1 remained at the plasma membrane when the cells containing wild-type receptors were treated with α-factor (Figure 3). Second, the bulk endocytosis of plasma membranes marked with the vital stain FM4-64 (Vida and Emr, 1995) was not influenced by α-factor (our unpublished results). A significant portion of the FM4-64 was internalized after only 2 min; however, the staining pattern was indistinguishable for the cells that were untreated or treated with α-factor. These results suggest that the endocytosis of unoccupied receptors does not simply reflect an increased rate of plasma membrane internalization in response to α-factor, and they are consistent with the hypothesis that unoccupied receptors are endocytosed because they form oligomeric complexes with occupied receptors.
Wild-Type Ste2 Causes Internalization of Endocytosis-defective Receptors in the Presence of α-Factor
The C-terminal cytoplasmic tail of Ste2 contains sequence elements that are essential for both basal and ligand-induced endocytosis (Rohrer et al., 1993; Schandel and Jenness, 1994). The truncated receptor Ste2-T326 binds α-factor normally, even though it lacks most of the C-terminal tail (Konopka et al., 1988). This domain contains the well-characterized endocytosis motif DAKSS (Rohrer et al., 1993). We wished to determine whether the severe endocytosis defect associated with this mutant receptor could be overcome by forming oligomers with wild-type receptors. Coimmunoprecipitation of Ste2-HA and Ste2-T326 was observed (our unpublished results) when detergent extracts were analyzed according to the methods depicted in Figure 2. Renografin density gradients and fluorescence microscopy were used to monitor endocytosis of Ste2-T326 tagged with GFP. Table 3 summarizes α-factor–induced internalization of receptors, as judged by Renografin density gradients. Consistent with previous results (Schandel and Jenness, 1994), Ste2-T326-GFP showed no net internalization when the mutant cells were treated with α-factor in the absence of protein synthesis. In Table 3 and in the previous study (Schandel and Jenness, 1994), a small number of the truncated receptors (17.6%) cofractionated with the internal membranes, and this quantity decreased slightly (to 13.5%) in the cells that had been exposed to α-factor. However, under these conditions, α-factor resulted in a nearly fivefold increase in the accumulation of Ste2-T326-GFP in the internal membrane fraction when Ste2-T326-GFP and wild-type Ste2 were coexpressed. This result is consistent with the endocytosis of oligomeric complexes containing truncated and wild-type receptors. It is currently unclear why some of the truncated receptors accumulate in the internal membrane pool, and why this quantity decreases when truncated and wild-type receptors are coexpressed. As has been proposed for CCR5 receptors (Benkirane et al., 1997), it is possible that the truncated α-factor receptors are partially retained in the endoplasmic reticulum and that the defect is overcome by forming oligomers with the wild-type receptors.
Table 3.
Wild-type receptors cause truncated receptors to be internalized in presence of α-factor
| Strain | Ste2 forms expressedb | Percentage of
receptors in internal membranesa
|
|
|---|---|---|---|
| (−) α-F | (+) α-F | ||
| DJ1413-A | Ste2-GFP | 9.7 ± 2.2 | 86.2 ± 2.7 |
| DJ1410-A | Ste2-T326-GFP | 17.6 ± 6.7 | 13.5 ± 5.7 |
| DJ1411-A | Ste2-T326-GFP and Ste2 | 4.7 ± 2.1 | 23.0 ± 2.1 |
| DJ1411-A | Ste2-T326-GFP and Ste2 | 24.7 ± 10.7 | 73.2 ± 8.1 |
Cells were processed as in Fig. 3. The percentage of receptors in internal membranes is the amount of receptor detected in Renografin gradient fractions 1–7 divided by the total amount of receptor assayed. Entries are the mean ± SE for three independent experiments.
The Ste2 forms expressed in each strain are shown. For each entry, the Ste2 form that was assayed is marked in bold type and underlined.
Internalization of Ste2-T326-GFP also was monitored by using fluorescence microscopy. In the control cells exposed to α-factor, wild-type Ste2-GFP was depleted from the plasma membrane (Figure 5, top row), and all of the cells examined contained fluorescent foci (Table 4). When expressed alone, the majority of Ste2-T326-GFP was at the cell surface at all times, consistent with the endocytosis defect of this mutant (Figure 5, middle row; Table 4). However, when cells coexpressing both Ste2-T326-GFP and untagged wild-type receptors were exposed to α-factor, plasma membrane fluorescence was diminished, accompanied by intracellular accumulation of fluorescent foci (Figure 5, bottom row; Table 4). These results are in agreement with the Renografin density gradient experiments (Table 3) and suggest that oligomeric complexes containing internalization-defective truncated receptors and wild-type receptors are internalized in an α-factor–dependent manner.
Figure 5.
Ligand-induced internalization of GFP-tagged endocytosis-defective receptors depends on the presence of wild-type receptors. Cultures were treated with cycloheximide and α-factor as described in Figure 3. First two columns are controls lacking α-factor; the last two columns are α-factor–treated cells. GFP fluorescence images and Nomarski images are indicated below each column. Top row, cells expressing Ste2-GFP only (strain DJ1413-A). Middle row, cells expressing Ste2-T326-GFP only (strain DJ1410-A). Bottom row, cells expressing both Ste2-T326-GFP and untagged Ste2 (strain DJ1411-A).
Table 4.
Quantitation of fluorescent foci from cells expressing Ste2 and Ste2-T326
| Ste2 forms expressedb | α-Factor | Percentage
of cellsa
|
|
|---|---|---|---|
| n ≥ 1 | n ≥ 3 | ||
| Ste2-GFP | − | 42 ± 9 | 9 ± 5 |
| Ste2-GFP | + | 100 | 71 ± 11 |
| Ste2-T326-GFP | − | 5 ± 3 | 5 ± 3 |
| Ste2-T326-GFP | + | 5 ± 3 | 2 ± 2 |
| Ste2-T326-GFP and Ste2 | − | 10 ± 5 | 7 ± 5 |
| Ste2-T326-GFP and Ste2 | + | 74 ± 4 | 36 ± 5 |
Fluorescent foci were counted from GFP images of cells treated with cycloheximide and α-factor as in Fig. 3. The percentage of cells with one or more fluorescent foci (n ≥ 1) and the percentage of cells with three or more fluorescent foci (n ≥ 3) are shown in the presence or absence of α-factor.
The forms of Ste2 expressed in each strain are shown on the leftmost column. Strains used are DJ1413-A (rows 1 and 2), DJ1410-A (rows 3 and 4), and DJ1411-A (rows 5 and 6).
C-Terminal Cytoplasmic Tail and Cysteine Residues of Ste2 Are Not Required for Oligomerization
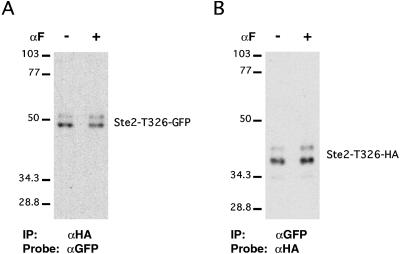
Coimmunoprecipitation experiments were used to test whether specific structural features of the receptor play an essential role in the formation of oligomers. To test whether the C-terminal cytoplasmic tail of the receptor is dispensable for oligomerization, we coexpressed two truncated forms of the receptor that were tagged differentially and performed coimmunoprecipitation tests on the detergent-solubilized receptors as described in Figure 1. We found coprecipitation of Ste2–T326-GFP and Ste2–T326-HA when the precipitating antibodies were either anti-GFP or anti-HA (Figure 6). This result suggests that the C-terminal cytoplasmic tail of the receptor is not required for oligomerization. This efficiency of precipitation was unaltered when the cells were cultured for 5 min in α-factor prior to analysis. We also considered whether either of the two cysteine residues in the receptor was essential for oligomer formation. The Ca2+-sensing and metabotropic glutamate receptors are proposed to dimerize through disulfide bonds (Romano et al., 1996; Bai et al., 1998). Two differentially tagged forms of the receptor were coexpressed under the direction of the strong TDH3 promoter. Plasmid pNED1(-Cys) encodes a mutant form the receptor (Ste2-C59S, C252A-Flag-His6) that lacks both cysteines and contains the Flag epitope, and this plasmid was introduced into strain 440-A that directs synthesis of wild-type receptors containing the T7 epitope. We were able to precipitate Ste2-C59S, C252A-Flag-His6 with the anti-T7 antibodies (our unpublished results), indicating the presence oligomers even though one of the receptors lacked cysteine residues. We conclude that the C-terminal cytoplasmic domain and interchain disulfide bonds are unnecessary for the formation of receptor oligomers.
Figure 6.
C-terminal tail of Ste2 is not required for coprecipitation. Cells expressing both Ste2-T326-HA and Ste2–T326-GFP (strain DJ1417-A) were treated with α-factor and processed for immunoprecipitation as in Figure 2. (A) Anti-HA antibody precipitates Ste2-T236-GFP. (B) Anti-GFP antibody precipitates Ste2-T326-HA.
DISCUSSION
In this study, we present evidence indicating that the α-factor receptors from S. cerevisiae form oligomeric complexes in the plasma membrane. Protein complexes containing the receptor were efficiently solubilized with the nondenaturing detergent n-dodecyl β-d-maltoside, and, on glycerol density gradients, they sedimented as a monodisperse species with a sedimentation coefficient of ∼8S. When the complexes containing differentially tagged receptors were solubilized under these conditions and subjected to immunoprecipitation, both tagged species were precipitated with antibodies specific for either of the two tags. The efficiency of coprecipitation was not influenced by the presence of α-factor in the culture prior to extraction. Membrane fractionation and fluorescence microscopy indicated that oligomeric receptor complexes were subject to endocytosis and that unoccupied receptors could participate in these complexes. First, tagged mutant receptors that lacked the DAKSS endocytosis signal and were unable to undergo constitutive and ligand-induced endocytosis became competent for endocytosis when they were coexpressed with untagged wild-type receptors. Second, unoccupied receptors were able to enter these endocytosis-competent complexes because tagged mutant receptors that were unable to bind α-factor also showed ligand-dependent endocytosis when they were coexpressed with untagged wild-type receptors. A recently published independent study also reports evidence for oligomerization of α-factor receptors (Overton and Blumer, 2000). These authors used fluorescence resonance energy transfer between differentially tagged receptors in whole cells as an indicator for oligomerization. They also showed that tagged receptors lacking the DAKSS endocytosis signal were endocytosed when coexpressed with wild-type receptors; however, they did not explore the endocytosis of unoccupied receptors, and they did not identify complexes in a membrane-free detergent-soluble system.
Little is known about the size and the structure of the oligomeric complexes that contain GPCRs. Although detergent-solubilized α-factor receptors (48 kDa) sedimented faster than the IgG marker protein (160 kDa), we do not know the extent to which detergent, shape, hydration, and other proteins contribute to the sedimentation rate. Two observations indicate that at least a portion of the α-factor–receptor complexes contain more than one receptor molecule. First, the HA-tagged receptors sediment slightly faster than the GFP-tagged receptors even though the GFP-tagged receptors are larger. One interpretation is that oligomers containing Ste2-HA are more stable than those containing Ste2-GFP; however, we have no direct corroborating evidence for a difference in affinity. Second, 6–11% coprecipitation of the differentially tagged receptors indicates that a minimum of 12–22% of these complexes are in oligomers (assuming two receptors per complex). This value is likely to be an underestimate because some complexes may disaggregate during analysis and because tighter associating forms (i.e., Ste2-HA) may tend to reassociate into relatively stable homo-oligomers leaving the weaker interacting species (i.e., Ste2-GFP) to form less stable oligomers. Based on the extensive cointernalization of mutant and wild-type receptors, it is likely that most of the receptors are in the oligomeric form in vivo. The structural determinants that bind α-factor receptors together are also unclear. Although metabotropic glutamate and Ca2+-sensing receptors require the disulfide bonds of cysteines for oligomerization and δ-opioid receptors oligomerize through sequences in the C-terminal domain, we find that neither of these structural features play an essential role in the oligomerization of α-factor receptors. Although transmembrane segment VI of β2-adrenergic receptors and D1 dopamine receptors are thought to play an essential role in aggregation, this possibility has not yet been explored for the α-factor receptor. Our data do not exclude the possibility that a bridging protein mediates the association of receptor molecules.
Functional consequences of GPCR oligomerization are not well understood. The ability of one receptor to influence the activity of another receptor in the same oligomeric complex has been inferred from the cooperative binding of type-specific agonists to cells that coexpress δ- and κ-opioid receptors. Although the hypersensitivity phenotype of truncated α-factor receptors is reversed when they are coexpressed with wild-type receptors (Konopka et al., 1988; Reneke et al., 1988), this phenomenon is most readily explained by competition of the mutant and wild-type receptors for a common pool of G-proteins (Dosil et al., 2000) rather than by direct aggregation of the two receptor forms. The functional consequences of disrupting GPCR oligomerization in vivo have been investigated by exposing cells to peptides corresponding to single transmembrane segments of the receptor. A peptide that comprises transmembrane VI of β2-adrenergic receptors inhibits both oligomerization and signal transduction activities of these receptors, suggesting that oligomerization may be essential for signaling (Hebert et al., 1996). However, the significance of this argument has recently been called into question because, for D1 dopamine receptors, a peptide containing transmembrane segment VI inhibits signaling without inhibiting oligomerization (George et al., 1998). It is possible that variations in the amount of oligomerized GPCRs mediate the response to agonists because the oligomeric state of some GPCRs is either increased or decreased by ligand binding (Hebert et al., 1996; Cvejic and Devi, 1997). For the δ-opioid receptor, the dimer-to-monomer transition has been associated with endocytosis because the natural agonists induce monomers prior to endocytosis and because morphine does not induce receptor internalization and does not alter the oligomeric state (Cvejic and Devi, 1997). Disaggregation of α-factor receptors does not appear to control ligand-mediated endocytosis because the aggregation state is unaffected by the α-factor and because endocytosis-defective mutant receptors are internalized when they are associated with wild-type receptors. α-Factor receptors provide a genetically tractable model to study the role that oligomerization plays in the GPCR function.
ACKNOWLEDGMENTS
We thank Gul Bukusoglu, Amy Yang, Kimberly Schandel, Padhma Radhakrishnan, Tara Pellegrino, Pam Torrance, Jeremy Thorner, and Mike Tyers for providing plasmids and strains, and Pam Silver, Y.R. Yang, and H.K. Schachman for providing antibodies. We also thank Kimberly Schandel and Ching-Hung Shen for comments on the manuscript. This work was supported by Public Health Service research grant GM34719 from the National Institute of General Medical Sciences.
REFERENCES
- Aris JP, Blobel G. Identification and characterization of a yeast nucleolar protein that is similar to a rat liver nucleolar protein. J Cell Biol. 1988;107:17–31. doi: 10.1083/jcb.107.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Trivedi S, Brown EM. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J Biol Chem. 1998;273:23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT. Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5Δ32. J Biol Chem. 1997;272:30603–30606. doi: 10.1074/jbc.272.49.30603. [DOI] [PubMed] [Google Scholar]
- Blumer KJ, Reneke JE, Thorner J. The STE2 gene product is the ligand-binding component of the α-factor receptor of Saccharomyces cerevisiae. J Biol Chem. 1988;263:10836–10842. [PubMed] [Google Scholar]
- Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukusoglu G, Jenness DD. Agonist-specific conformational changes in the yeast α-factor pheromone receptor. Mol Cell Biol. 1996;16:4818–4823. doi: 10.1128/mcb.16.9.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvejic S, Devi LA. Dimerization of the δ opioid receptor: implication for a role in receptor internalization. J Biol Chem. 1997;272:26959–26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- David NE, Gee M, Andersen B, Naider F, Thorner J, Stevens RC. Expression and purification of the Saccharomyces cerevisiae α-factor receptor (Ste2p), a 7-transmembrane-segment G protein-coupled receptor. J Biol Chem. 1997;272:15553–15561. doi: 10.1074/jbc.272.24.15553. [DOI] [PubMed] [Google Scholar]
- Dosil M, Schandel KA, Gupta E, Jenness DD, Konopka JB. The C terminus of the saccharomyces cerevisiae α-factor receptor contributes to the formation of preactivation complexes with its cognate G protein. Mol Cell Biol. 2000;20:5321–5329. doi: 10.1128/mcb.20.14.5321-5329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube P, Konopka JB. Identification of a polar region in transmembrane domain 6 that regulates the function of the G protein-coupled α-factor receptor. Mol Cell Biol. 1998;18:7205–7215. doi: 10.1128/mcb.18.12.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Hayes M, Clarke L, Carbon J. Nucleotide sequence comparisons and functional analysis of yeast centromere DNAs. Cell. 1982;29:235–244. doi: 10.1016/0092-8674(82)90108-8. [DOI] [PubMed] [Google Scholar]
- George SR, Lee SP, Varghese G, Zeman PR, Seeman P, Ng GY, O'Dowd BF. A transmembrane domain-derived peptide inhibits D1 dopamine receptor function without affecting receptor oligomerization. J Biol Chem. 1998;273:30244–30248. doi: 10.1074/jbc.273.46.30244. [DOI] [PubMed] [Google Scholar]
- Hartwell LH. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967;93:1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert TE, Bouvier M. Structural and functional aspects of G protein-coupled receptor oligomerization. Biochem Cell Biol. 1998;76:1–11. doi: 10.1139/bcb-76-1-1. [DOI] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M. A peptide derived from a β2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- Herberg JT, Codina J, Rich KA, Rojas FJ, Iyengar R. The hepatic glucagon receptor. Solubilization, characterization, and development of an affinity adsorption assay for the soluble receptor. J Biol Chem. 1984;259:9285–9294. [PubMed] [Google Scholar]
- Hicke L. Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J. 1997;11:1215–1226. doi: 10.1096/fasebj.11.14.9409540. [DOI] [PubMed] [Google Scholar]
- Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Hicke L, Zanolari B, Riezman H. Cytoplasmic tail phosphorylation of the α-factor receptor is required for its ubiquitination and internalization. J Biol Chem. 1998;141:349–358. doi: 10.1083/jcb.141.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman JE, De Zutter GS, Simonds WF, Jenness DD. The Gβγ complex of the yeast pheromone response pathway. Subcellular fractionation and protein-protein interactions. J Biol Chem. 1997;272:240–248. doi: 10.1074/jbc.272.1.240. [DOI] [PubMed] [Google Scholar]
- Jenness DD, Li Y, Tipper C, Spatrick P. Elimination of defective α-factor pheromone receptors. Mol Cell Biol. 1997;17:6236–6245. doi: 10.1128/mcb.17.11.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness DD, Spatrick P. Down regulation of the α-factor pheromone receptor in S. cerevisiae. Cell. 1986;46:345–353. doi: 10.1016/0092-8674(86)90655-0. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka JB, Jenness DD. Genetic fine-structural analysis of the Saccharomyces cerevisiae α-pheromone receptor. Cell Regul. 1991;2:439–452. doi: 10.1091/mbc.2.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka JB, Jenness DD, Hartwell LH. The C-terminus of the S. cerevisiae α-pheromone receptor mediates an adaptive response to pheromone. Cell. 1988;54:609–620. doi: 10.1016/s0092-8674(88)80005-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Kane T, Tipper C, Spatrick P, Jenness DD. Yeast mutants affecting possible quality control of plasma membrane proteins. Mol Cell Biol. 1999;19:3588–3599. doi: 10.1128/mcb.19.5.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J, Konopka J, Singer-Kruger B, Zerial M, Botstein D. Visualization of receptor-mediated endocytosis in yeast. Mol Biol Cell. 1999;10:799–817. doi: 10.1091/mbc.10.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng GY, George SR, Zastawny RL, Caron M, Bouvier M, Dennis M, O'Dowd BF. Human serotonin1B receptor expression in Sf9 cells: phosphorylation, palmitoylation, and adenylyl cyclase inhibition. Biochemistry. 1993;32:11727–33. doi: 10.1021/bi00094a032. [DOI] [PubMed] [Google Scholar]
- Ng GY, O'Dowd BF, Lee SP, Chung HT, Brann MR, Seeman P, George SR. Dopamine D2 receptor dimers and receptor-blocking peptides. Biochem Biophys Res Commun. 1996;227:200–204. doi: 10.1006/bbrc.1996.1489. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Hof PR, Janssen WGM, Morrison JH, Schmauss C. Expression of dopamine D3 receptor dimers and tetramers in brain and in transfected cells. J Biol Chem. 1997;272:29229–29237. doi: 10.1074/jbc.272.46.29229. [DOI] [PubMed] [Google Scholar]
- Overton MC, Blumer KJ. G-protein-coupled receptors function as oligomers in vivo. Curr Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- Reneke JE, Blumer KJ, Courchesne WE, Thorner J. The carboxy-terminal segment of the yeast α-factor receptor is a regulatory domain. Cell. 1988;55:221–234. doi: 10.1016/0092-8674(88)90045-1. [DOI] [PubMed] [Google Scholar]
- Rohrer J, Benedetti H, Zanolari B, Riezman H. Identification of a novel sequence mediating regulated endocytosis of the G protein-coupled α-pheromone receptor in yeast. Mol Biol Cell. 1993;4:511–521. doi: 10.1091/mbc.4.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Yang WL, O'Malley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J Biol Chem. 1996;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- Schandel KA, Jenness DD. Direct evidence for ligand-induced internalization of the yeast α-factor pheromone receptor. Mol Cell Biol. 1994;14:7245–7255. doi: 10.1128/mcb.14.11.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedorf M, Damelin M, Kahana J, Taura T, Silver PA. Interactions between a nuclear transporter and a subset of nuclear pore complex proteins depend on Ran GTPase. Mol Cell Biol. 1999;19:1547–1557. doi: 10.1128/mcb.19.2.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni R, Carmichael JP, Murray JA. Parameters affecting lithium acetate-mediated transformation of Saccharomyces cerevisiae and development of a rapid and simplified procedure. Curr Genet. 1993;24:455–459. doi: 10.1007/BF00351857. [DOI] [PubMed] [Google Scholar]
- Sprague GF, Jr, Herskowitz I. Control of yeast cell type by the mating type locus. I. Identification and control of expression of the a-specific gene. BAR1 J. Mol Biol. 1981;153:305–21. doi: 10.1016/0022-2836(81)90280-1. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FY, Wess J. Identification and molecular characterization of m3 muscarinic receptor dimers. J Biol Chem. 1999;274:19487–19497. doi: 10.1074/jbc.274.27.19487. [DOI] [PubMed] [Google Scholar]