Abstract
There have been ample warnings that multidrug-resistant (MDR) tuberculosis (TB) will continue to emerge if countries do not strengthen their control of TB. In low-incidence European countries, however, these warnings have been substantiated mainly by outbreaks in association with human immunodeficiency virus (HIV)-positive patients. The aim of this study was to investigate an outbreak of infection with MDR and drug-resistant Mycobacterium tuberculosis that was diagnosed among 20 HIV-negative patients living in Norway. Of these, 19 were immigrants from East Africa and one was an ethnic Norwegian. We wanted to find out if transmission had taken place in Norway or abroad and to identify the genetic basis of drug resistance. The strains were analyzed by IS6110 restriction fragment length polymorphism, antibiotic susceptibility tests, spoligotyping, reverse hybridization to regions of the rpoB gene, and sequencing of the katG gene. Epidemiological links between the patients were mapped, and the strains were compared to those isolated in 36 other countries and regions. All strains were resistant to isoniazid and carried Ala234Gly, Ser315Thr, and Arg463Leu substitutions in the katG gene. Eleven strains were MDR and carried a Ser531Leu substitution in the rpoB gene. MDR was acquired in the index patient after arrival in Norway. Links were found among 14 patients. The strain was imported from Somalia but acquired MDR and was transmitted in Norway. This demonstrated that MDR strains are not necessarily imported from high-incidence countries and can be highly communicable. The outbreak underscores a deficiency in the TB control measures employed in many countries and challenges the adequacy of the policy of screening immigrants for TB only on arrival.
The incidence of tuberculosis (TB) has increased in many countries during the last decades, and more people have TB now than at any other time in history (22). Globally it is estimated that TB still kills more people than any other infection (22). The situation is made even worse by the emergence of antibiotic-resistant strains of Mycobacterium tuberculosis (6). Cases resistant to the two most important anti-TB chemicals, rifampin (RIF) and isoniazid (INH) are defined as multidrug resistant (MDR). They are especially difficult and costly to treat (6). Drug resistance in M. tuberculosis is attributable primarily to accumulation of mutations in drug target genes. Any patient with drug-resistant TB either has not been treated properly, did not take the medication as prescribed, or was infected by someone who had not been treated properly or did not take their medication as prescribed (6). In 1995, the World Health Organization estimated that 50 million people were infected with drug-resistant strains of M. tuberculosis (23), and it is estimated that 273 000 new cases of MDR TB occurred worldwide in 2000 (6). With increased international travel, TB, and inevitably MDR TB, is readily circulating throughout the world.
Norway, like many other countries in Europe, has witnessed a dramatic fall in the incidence of TB during the last century. This decline is believed to be due to a number of reasons, including improved living conditions and a well-functioning national anti-TB program. The incidence of TB among native Norwegians is 1.8/100,000 (9), and transmission is uncommon in this country (4). Norway has therefore been considered to be in the elimination phase of TB (J. D. A. van Embden and D. van Soolingen, Editorial, Int. J. Tuberc. Lung Dis. 4:285-286, 2000). However, immigrants from countries with high TB burdens who live in Norway have an incidence similar to that in their country of origin, and in 2000 they accounted for more than 70% of all TB cases in Norway (9). Obligatory TB screening of immigrants from these countries on entry into Norway has therefore been enforced for many years. The initial screening works well for people seeking political asylum, but follow-up examinations of patients with findings indicative for TB have been problematic to enforce. The TB screening of other categories of immigrants could also be improved. These problems are not unique to Norway but are also experienced in other countries (2, 8).
By January 2000, Norway had received over 6,000 refugees from Somalia. The majority of these lived in Oslo. The incidence of TB in Somalia is among the highest in the world (6, 12), and there is growing concern that MDR TB may be imported to low-incidence communities from such high-burden countries. The exact prevalence of MDR TB in Somalia is not known but has been estimated to include between 1.9 and 4.4% of new TB cases in 2000 (6). The World Health Organization and the International Union Against Tuberculosis and Lung Disease warned in 2000 that if countries do not act quickly to strengthen their control of TB, MDR strains of M. tuberculosis will continue to emerge. This will seriously hamper national and global efforts to control TB (24). As a consequence of this development, the world is facing a much more serious TB situation today than in the mid-1950s.
We describe here the first outbreak of MDR and drug-resistant TB in Norway. It included 20 human immunodeficiency virus (HIV)-negative patients. Of these, 19 were African immigrants and one was a native Norwegian. We aimed to find out whether transmission had occurred before or after arrival in Norway and to identify the genetic mechanisms of antibiotic resistance. International collaboration made it possible to compare IS6110 restriction fragment length polymorphism (RFLP) patterns in 36 other countries and regions. Such multinational studies may bring valuable information on how M. tuberculosis spreads from high-burden countries to other parts of the world.
MATERIALS AND METHODS
As part of the national TB surveillance program in Norway, 93% of all culture-positive cases during 1994 to 2000 were analyzed by IS6110 RFLP. The species identification of the isolates was based on a 16S rRNA gene hybridization technique (AccuProbe; GenProbe Inc., San Diego, Calif.) and conventional biochemical tests (nitrate reduction and niacin accumulation tests). The DNA fingerprints were made according to internationally agreed guidelines (19). Briefly, for isolation of genomic DNA, M. tuberculosis strains were grown on Löwenstein-Jensen slants for 3 to 5 weeks. All bacterial cells from one slant were transferred in 400 μl of TE buffer (0.01 M Tris-HCl, 0.001 M EDTA, pH 8), and the solution was heated at 80°C for 20 min to kill the bacteria. Fifty microliters of lysozyme (10 mg/ml) was added, and the tube was incubated for 1 h at 37°C. Seventy microliters of sodium dodecyl sulfate (10%) and 5 μl of proteinase K (10 mg/ml) were added, and the mixture was incubated for 10 min at 65°C. A 100-μl volume of 5 M NaCl and an N-cetyl-N,N,N-trimethylammonium bromide (CTAB)-NaCl solution (4.1 g of NaCl and 10 g of CTAB per 100 ml) was added and incubated for 10 min at 65°C. An equal volume of chloroform-isoamylalcohol (24:1) was added, the mixture was centrifuged for 5 min at 13,000 × g, and the aqueous supernatant was transferred to a fresh tube. The total DNA was precipitated with isopropanol and redissolved in an appropriate volume of double-distilled water. The DNA was digested with the restriction endonuclease PvuII prior to IS6110 RFLP. The digested DNA was separated by using horizontal 0.8% agarose gels in Tris-acetate buffer and vacuum blotted onto a nylon membrane. For the IS6110 RFLP, the DNA was hybridized to the 254-bp internal PCR fragment of IS6110 and visualized by use of a digoxigenin-dUTP labeling and detection kit (Roche Diagnostics GmbH, Mannheim, Germany). Twenty strains that carried the same DNA IS6110 fingerprints were subjected to further analyses and are presented in this report.
These 20 isolates were also analyzed by direct repeat (DR)-RFLP (4) and spoligotyping as described by Kamerbeek et al. (10). In brief, for the DR RFLP, AluI-digested DNA was hybridized to a 36-bp synthetic oligonucleotide directed against the directly repeated sequences of 36 bp which are clustered in one region of the M. tuberculosis genome, which was used as probe. The DR probe was labeled and visualized by use of an enhanced chemiluminescence kit (ECL; Amersham, Little Chalfont, Buckinghamshire, United Kingdom). Spoligotyping is based on the in vitro amplification of the DNA of the highly polymorphic DR genomic locus present in the M. tuberculosis chromosome. The reverse primer was biotinylated at the 5′ end. A set of 43 oligonucleotides (10), each corresponding to one of the unique spacer DNA sequences within the DR locus, were covalently bound to a commercially available membrane (Immunetics, Cambridge, Mass.). For hybridization, 20 μl of the PCR product was diluted and heat denatured. The samples were pipetted into parallel channels of a miniblotter (Immunetics) in such a way that they were perpendicular to the rows of oligonucleotides. The hybridization was performed for 60 min at 60°C. The membranes were incubated with streptavidin-peroxidase conjugate (Boehringer Mannheim Biochemicals, East Sussex, United Kingdom) for 60 min at 42°C. The patterns were visualized by use of the ECL liquid (Amersham) and by exposure of the membrane to X-ray film (Hyperfilm; Amersham) for 5 min.
Susceptibility tests were performed with a radiometric respirometry system (BACTEC 460) as described by Siddiqi et al. (17). The anti-TB drugs tested and the concentrations used were as follows: INH, 0.2 μg/ml; RIF, 2.0 μg/ml; ethambutol, 7.5 μg/ml; streptomycin, 6.0 μg/ml; pyrazinamide, 100 μg/ml; amikacin, 4 μg/ml; ciprofloxacin, 2 μg/ml; clarithromycin, 2 μg/ml; clofazimin, 2 μg/ml; rifabutin, 2 μg/ml; azithromycin, 2 μg/ml; para-aminosalicylic acid, 4 μg/ml; cycloserine, 50 μg/ml; capreomycin, 10 μg/ml; ethionamide, 5 μg/ml; and thiacetazone, 2 μg/ml. The MIC of INH was determined twice on different days for all strains as described by Abate et al. (1). The additional concentrations of INH tested were 1.0, 2.0, 4.0, and 8.0 μg/ml. Strains resistant to both RIF and INH are designated MDR, while strains resistant to INH without being MDR are referred to as drug resistant, in this article.
A line probe assay kit, INNO-LiPA Rif. TB (Innogenetics, Ghent, Belgium), was used according to the manufacturer's instructions for rapid identification of mutations in the RIF resistance-determining region of the rpoB gene. The entire katG gene was amplified by use of PCR (primers P1 and P10), and the PCR products were sequenced with five sets of overlapping primers as described by Marttila et al. (15). Sequencing was performed as described by Maiden et al. (14), and the results were compared to the completed genome sequence of M. tuberculosis (3). All mutations, as well as the wild-type part of the gene, were confirmed by the presence of complementary bases on the complementary DNA strand.
Municipal health authorities carried out contact investigations. The information was collected at the Norwegian Institute of Public Health. Additional information was collected through direct communication with local health services. For some patients, the results of the chest radiographs taken on arrival to Norway were available at the reception center for political asylum seekers in Oslo.
The IS6110 RFLP patterns were compared to those available at the internet site of the Concerted Action on Tuberculosis (CAonTB) (http://www.caontb.rivm.nl). The CAonTB is a project within the framework of the European Union BIOMED II program and includes IS6110 RFLP patterns of M. tuberculosis isolates from Argentina, Austria, Bolivia, Brazil, Chile, China, Comores, Czech Republic, Denmark, Ecuador, Ethiopia, France, Greenland, Guinea, Honduras, India, Iran, Malaysia, Mongolia, The Netherlands, the Philippines, Russia, Rwanda, South Africa, South Korea, Spain, Switzerland, Tahiti, Tanzania, Thailand, Tunisia, the United States, Venezuela, Vietnam, and Zambia. In addition, the IS6110 RFLP patterns were compared to those of M. tuberculosis strains isolated in London during 1995 to 1997 (13).
RESULTS
From 1994 to 2000 there were 1,647 cases of TB in Norway. Of these, 1,139 (69%) were culture positive, and isolates from 13 (1.1%) patients were MDR TB. The M. tuberculosis isolates from 1,064 of these patients (including all MDR strains) were fingerprinted by IS6110 RFLP. The results showed that transmission of TB in Norway was uncommon (4). The largest outbreak included 20 cases that were resistant to INH, 11 of which were MDR TB (Table 1).
TABLE 1.
Data on patients infected with the same strain of M. tuberculosis in Oslo, Norway
| Patient | Yr of diagnosis | Yr of birth | Sex | Country of birth | Residence permit (mo.yr)a | Type of TB | Sputum smear | Date (mo.yr) of chest X ray (result) | Drug resistanceb | Epidemiological link (group) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1994 | 1967 | Male | Somalia | 11.88 | Pulmonary | Positive | ?.92 (negative) | INH | Lived with patient 7 (1) |
| 1995 | Pulmonary | Positive | MDR | |||||||
| 2 | 1994 | 1972 | Male | Somalia | 08.88 | Pulmonary | Negative | 08.88 (negative) | INH | Friend of patient 6 (2) |
| 3 | 1996 | 1975 | Male | Somalia | 03.92 | Pulmonary | NAc | ?.92 (negative) | MDR | Lived with patient 7 (1) |
| 4 | 1996 | 1974 | Male | Somalia | 01.91 | Pulmonary | NA | 01.91 (negative) | INH | Lived with patient 7 (1) |
| 5 | 1996 | 1979 | Male | Somalia | 09.89 | Pulmonary | Positive | NA | MDR | None found (none) |
| 6 | 1996 | 1972 | Male | Somalia | 05.88 | Pulmonary | Positive | NA | INH | Friend of patient 2 (2) |
| 7 | 1997 | 1972 | Male | Somalia | 02.92 | Pulmonary | NA | NA | MDR | Lived with patients 1, 3, 4 (1) |
| 8 | 1997 | 1971 | Male | Somalia | 11.88 | Lymphnode | NA | INH | Friend of patient 4 (1) | |
| 9 | 1997 | 1962 | Male | Somalia | 07.87 | Bone-joint | NA | MDR | None found (none) | |
| 10 | 1997 | 1969 | Male | Somalia | 09.88 | Pulmonary | NA | NA | INH | Lived with patient 8 (1) |
| 11 | 1997 | 1965 | Male | Ethiopia | 04.90 | Pulmonary | NA | NA | INH | None found (none) |
| 12 | 1997 | 1975 | Male | Somalia | 01.91 | Pulmonary | Positive | NA | INH | None found (none) |
| 13 | 1997 | 1996 | Male | Norway | CNSd | NA | INH | None found (none) | ||
| 14 | 1997 | 1967 | Male | Ethiopia | 11.89 | Pulmonary | NA | NA | MDR | Lived with patient 1 (1) |
| 15 | 1998 | 1975 | Male | Somalia | 09.90 | Bone-joint | 10.88 (negative) | INH | Friend of patients 4, 7 (1) | |
| 16 | 1999 | 1960 | Male | Somalia | 01.87 | Bone-joint | 09.90 (nonactive TB) | MDR | Friend of patients 4, 7 (1) | |
| 17 | 1999 | 1975 | Female | Somalia | 08.87 | Pulmonary | Positive | NA | MDR | Aunt of patient 18, sister of patient 19 (3) |
| 18 | 2000 | 1997 | Female | Norway | Pulmonary | Negative | NA | MDR | Niece of patient 17, daughter of patient 19 (3) | |
| 19 | 2000 | 1979 | Female | Somalia | ?.85 | Pulmonary | Positive | NA | MDR | Sister of patient 17, mother of patient 18 (3) |
| 20 | 2000 | 1959 | Male | Somalia | 01.89 | Pulmonary | Negative | 01.89 (negative) | MDR | None found (none) |
Date that application for residence permit was received at the Norwegian Directorate of Immigration.
INH, resistant to INH, clarithromycin, azithromycin, para-aminosalicylic acid, cycloserine, and thiacetazon; MDR, resistant to INH, RIF, clarithromycin, rifabutin, azithromycin, para-aminosalicylic acid, cycloserine and thiacetazone.
NA, not available.
CNS, central nervous system.
Patients.
All 20 patients were HIV negative. One patient was in jail while treated, but all others lived outside institutions in the Oslo region. The first patient (patient 1) (Table 1) diagnosed with this outbreak strain of M. tuberculosis was born in Somalia in 1967. In 1992 a chest radiograph had been taken and evaluated as negative, but a tuberculin skin test had not been performed. In 1994 he was diagnosed with pulmonary TB (Table 1). The patient had formerly abused narcotics; he claimed that he had not been treated for TB before. Anti-TB treatment with INH, RIF, and pyrazinamid was started in 1994. His intake of antibiotics was not given under direct observation, however, and he did not appear for check-up appointments. It was demonstrated that the M. tuberculosis strain was resistant to INH. The patient appeared 1 year later and was hospitalized with smear-positive pulmonary TB. It was demonstrated by IS6110 RFLP that the M. tuberculosis strain isolated was identical to the strain isolated a year previously. One important difference, however, was that the strain now was resistant to RIF and he had therefore acquired MDR TB. Another Somali immigrant (patient 2) (Table 1) was also diagnosed with a strain of M. tuberculosis that carried the same RFLP in 1994. This strain was also INH resistant but was susceptible to RIF, and the patient was treated successfully.
In the following 6 years, 18 other patients (patients 3 to 20) (Table 1) were diagnosed with a strain of M. tuberculosis that carried the same IS6110 fingerprint pattern. Data on all 20 patients are presented in Table 1.
The median age of the patients was 24 years (range, 1 to 48); 17 patients were male and 3 were female. Sixteen patients were immigrants from Somalia, two were immigrants from Ethiopia, one was born in Norway to Somali parents, and one was born in Norway to Norwegian parents. All of the immigrants had applied for residence permits between 1985 and 1992. Information on previous chest radiographs taken in Norway was available for seven of the patients (Table 1). Six of these had no radiographic findings indicative of TB (patients 1 to 4, 14, and 20), and one had a pattern described as nonactive TB (patient 15).
Epidemiological links were found within three groups of the patients (Table 1). The largest group consisted of one Ethiopian man and eight Somali men who had shared accommodation for some time or were close friends. The second group consisted of two Somali men who were close friends. The third group consisted of two Somali sisters and the daughter of one of them. These women had been in contact with a friend in London who had TB. No epidemiological link was found between these three groups or between the remaining six patients: four Somali men, a boy with Norwegian parents, and an Ethiopian man.
One patient died of reasons other than TB before the treatment was completed. The other patients were cured, except for the 1-year-old Norwegian child (patient 13), who died after treatment due to severe cerebral TB sequelae. No patient was diagnosed with this strain during 2001 and the first half of 2002, indicating that the outbreak had finally been arrested.
Molecular typing.
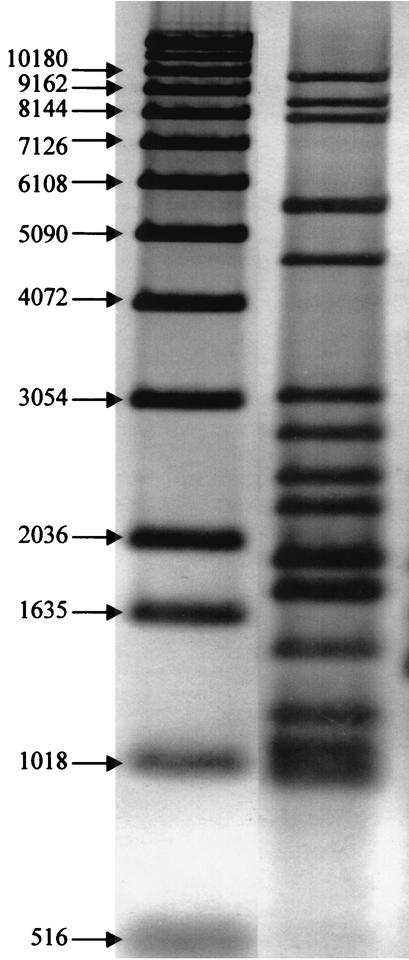
By use of IS6110 RFLP, 15 identical copies of the IS6110 were revealed in all 20 strains. The DNA fragments that carried a copy of IS6110 were between 500 and 10,200 bp long (Fig. 1). In comparisons of the IS6110 RFLP patterns of these strains to those of M. tuberculosis strains isolated in 36 other countries and regions, no other strain demonstrated the same pattern (13) (http://www.caontb.rivm.nl). It did, however, appear that the RFLP of the present outbreak strain was similar to those of a group of strains isolated mainly from Somali immigrants living in London during 1995 to 1997 (13). It was likely that they represented a common family of M. tuberculosis strains in Somalia to which the present outbreak strain belonged. Spoligotyping confirmed the similarity of the isolates from these 20 patients. All strains belonged to spoligotype 703777740203171 (hexadecimal code = 70-7F-7F-40-83-1F). DR-RFLP revealed one DNA fragment of approximately 1,500 bp that carried the DR sequence for the 20 strains. The isolate from patient 11 (Table 1) carried an additional copy of the DR sequence on a DNA fragment of approximately 1,800 bp.
FIG. 1.
Right lane, IS6110 RFLP pattern of the strain of M. tuberculosis isolated from 20 patients in Norway; left lane, molecular markers in base pairs.
The sequence of the katG gene was identical for all strains and included Ala234Gly, Ser315Thr, and Arg463Leu substitutions. The rest of the katG gene was wild type, i.e., identical to the one present in the sequenced genome of M. tuberculosis strain H37Rv (3). All of the 11 MDR strains demonstrated a Ser531Leu mutation in the rpoB gene, and the isolates from the nine RIF-susceptible cases had the wild-type rpoB gene.
Antibiotic resistance.
All 20 strains were resistant to INH, azithromycin, para-aminosalicylic acid, cycloserine, clarithromycin, and thiacetazone. Eleven isolates were also resistant to RIF (MDR TB) and rifabutin. Of all culture-positive cases in Norway during this period, only two other individual cases were MDR. We found that for 18 strains the INH MIC was 4 μg/ml, and for 2 strains (patients 10 and 11) the INH MIC was 8 μg/ml.
DISCUSSION
There have been ample warnings that MDR TB will continue to emerge if countries do not act quickly to strengthen their control of TB (24). This is the first reported outbreak of MDR TB in Norway. Many countries around the world practice the same TB control measures as Norway, and these measures have been extremely efficient. It is therefore especially noteworthy that MDR TB was acquired and allowed to spread in Norway in particular.
The outbreak that involved 20 HIV-negative patients included 11 cases of MDR TB and 9 cases of drug-resistant TB. During this period, 93% of all culture-positive cases of TB were fingerprinted by IS6110 RFLP. Thus, the isolates studied were representative of the population of M. tuberculosis present in Norway at the time. With the exception of the boy who was born in 1996 (patient 13), the present strain was not isolated from ethnic Norwegians. The majority of the patients were immigrants from Somalia, and two were immigrants from Ethiopia. For these reasons, it was reasonable to believe that the strain of M. tuberculosis responsible for this outbreak was imported from East Africa. There was little doubt, however, that most of these immigrants and the Norwegian child were infected in Norway.
RFLP analysis with the IS6110 element as a probe is an internationally established method well suited to study the epidemiology and transmission of TB. Identical IS6110 RFLP patterns are generally considered to indicate transmission chains of TB. The cases that follow the index case are considered to have recently transmitted TB (4, 12, 19). Transmission of M. tuberculosis is uncommon in Norway, and outbreaks have as a rule consisted of small clusters due to drug-susceptible isolates (4). It has recently been suggested that drug-resistant strains are less transmissible and/or less pathogenic than drug-susceptible strains (5, 20). It was therefore unexpected that that the present strain of M. tuberculosis contributed to the largest outbreak of TB identified by DNA fingerprinting in Norway.
It was not possible to establish epidemiological links between all the patients in order to confirm the results of the DNA fingerprinting. This has also been the case in many previous studies where molecular methods could not be corroborated by conventional contact tracing (5, 12, 13, 20). Patients 1 and 2 could have been infected from a common source earlier, but DNA fingerprinting was first started in Norway in 1994. It was likely that patient 1 was the source of infection to the other eight patients in epidemiological group 1 (Table 1), since he was diagnosed first and it had also been established that he acquired MDR TB in Norway. This patient was infectious for a long period of time, and it was probable that he transferred drug-resistant TB and MDR TB to other patients in the group. It was also likely that patient 2 had infected patient 6 and that patient 17 was the infectious source for patients 18 and 19 (Table 1). These three groups and the remaining six patients, however, could not be linked epidemiologically, but most of the patients were immigrants from Somalia, and all were living in Oslo, which is a small city with 500.000 inhabitants.
Clustering may be identified by DNA fingerprinting of isolates from individuals who have no recent link but for whom there was a link in the past and in whom reactivation of old infections occurs at about the same time. This is especially likely if a strain predominates over a long period of time (12). Since the present strain was not observed in other countries that also received Somali immigrants during the period, it was believed that such a common link existed in the past in Norway and that the cases represented new transmission. This was unlike the situation in London, where most immigrants had endogenous reactivation of TB (13). Many immigrants stay privately while their application for residence permit is pending, and others are not registered or stay illegally in Norway. TB screening is therefore not carried out on all new arrivals, and it is possible that people with TB enter and leave the country without being diagnosed. However, since both patients with drug-resistant TB and MDR TB could not be linked to the others, these missing links must have been connected to patient 1 (Table 1) or two unidentified patients. It is likely that patient 1 (Table 1) represents this common link but that language barriers, cultural differences, fear of deportation, or other factors left some immigrants reluctant to release information on their contacts in Norway. Due to the design of interviews and questionnaires applied in the contact tracing work, links from the past may be overlooked. These investigations are employed for prophylactic reasons and are designed to identify infected people among the current or recent contacts in the community around a newly diagnosed patient. They are not retrospective, and since health personnel cannot reveal the identities of previous patients, direct questioning about these is not possible. It is possible that a cafe in Oslo where the Somali population meets is the place of transmission for the unlinked immigrants. Transmission of TB by casual contact is uncommon, but cafes and bars are often poorly ventilated and crowded, and transmission of TB in these public places has been described. In a recent study, Klovdahl et al. demonstrated that such locations often represent the common link between patients with newly transmitted TB (11).
The parents of the 1-year-old ethnic Norwegian (patient 13) indicated that contact with Somali immigrants had taken place at a day care center. No other person at this day care was infected, according to the investigations employed by the municipal health authorities. It was possible that one of the previous patients had visited the day care center before he had been diagnosed with TB and had transmitted the disease to the boy. This could not be confirmed, and casual contact elsewhere may also explain how this child acquired the drug-resistant strain of M. tuberculosis.
Nitta et al. describe limited transmission of MDR TB in Los Angeles, Calif. (16). Those authors concluded that a possible reason for this limited transmission could be the excellent TB control measures in Los Angeles. It may also be that MDR strains of M. tuberculosis are less transmissible or less pathogenic than drug-susceptible strains (5, 20). Transmission of drug-susceptible strains of M. tuberculosis in Norway is uncommon (4), and our well-established TB control measures are believed to be an important reason for this. The present outbreak strain of M. tuberculosis from patients living in Norway suggested that MDR TB strains need not be less transmissible and/or less pathogenic than drug-susceptible strains. The potent transmissibility of the present strain is exemplified by the fact that patient 13 (Table 1) contracted the disease without any known connection to any of the other patients and that the strain may have been transmitted within a public place to some of the other patients.
A few genotypes of M. tuberculosis have demonstrated high abilities to be transmitted and are distributed throughout the world (such as the Beijing and Haarlem genotype families). Outbreaks of MDR TB are often associated with strains of the Beijing genotype. It was therefore noteworthy that the present outbreak strain did not belong to any of the known M. tuberculosis families with high transmission rates and that it was not observed outside Norway. It was also unusual that all 20 patients were HIV negative.
Molecular techniques hold great promise for the rapid detection of resistance to antimicrobial agents in M. tuberculosis. It should be kept in mind, however, that isolates that are susceptible to antibiotics according to molecular assays might contain mechanisms of resistance other than those assayed for. In addition, the effect on drug susceptibility of different mutations and combinations of mutations is not always known. Therefore, standard microbiological susceptibility test methods such as the BACTEC method remain the “gold standard.” The katG gene is commonly mutated in strains that are resistant to INH (7, 17), and all isolates from the present outbreak carried three mutations at the katG gene. It has been demonstrated that the susceptibility to INH in M. tuberculosis is not associated with the Arg463Leu mutation in katG (18). The mutation at codon 315, however, is commonly found in strains that are transmitted successfully and is an important indicator for INH resistance and for MDR among isolates of M. tuberculosis (7, 15, 17, 18, 21). The effect that the Ala234Gly mutation in katG has on the susceptibility to INH remains to be examined. It is clear that all of these mutations are important epidemiological markers for the present isolates, corroborating the IS6110 RFLP results. The two isolates (from patients 10 and 11 [Table 1]) for which the MICs were higher than those for the other strains might carry mutations in other INH regulatory genes, such as the inhA, oxyR, or aphC gene. Eleven strains carried an Arg531Leu mutation in the rpoB gene. Mutations in rpoB are less likely to occur than mutations in katG, but resistance to RIF is strongly associated with mutations within this gene (7). It was clear that the Arg531Leu mutation gave rise to resistance to RIF in the present 11 MDR strains.
The enormous TB problem in many parts of the world and the global emergence of MDR TB are important matters to consider when revising national control programs for TB. MDR TB, even in a nonoutbreak setting, remains a devastating illness. The strain of this first outbreak of MDR TB in Norway originated from abroad, most likely from Somalia, but acquired MDR and spread further within the immigrant population in Norway. This was likely due to deficiencies in the Norwegian TB control system when applied to a population with a different social and ethnic background. This kind of program has very efficiently reduced the incidence of TB in Norway and many other countries. However, the present outbreak demonstrated that these routines were not sufficient to prevent an outbreak of drug-resistant and MDR TB among the immigrant population in Oslo. Also, as pointed out by Klovdahl et al. (11), programs to reduce TB incidence that focus on household members and other close contacts require reconsideration. Modern, multicultural societies encompass different ways of living and different social patterns among groups of people. Therefore, different definitions of close contacts need to be applied for different groups. The incidence of TB also varies greatly between different groups within a society. It appears that contact tracing and TB prevention work may be more efficient if applied differently to these different groups. The present outbreak demonstrated the emergence of MDR TB in a country that has been said to be in the elimination phase of TB. It underlines that as long as TB remains a major health problem in some parts of the world, no nation can expect to eliminate this disease.
Acknowledgments
We thank the staff of the Tuberculosis Laboratories at Ullevaal University Hospital, the Norwegian Institute of Public Health, and the municipal health authorities for help in data collection and technical assistance. We are grateful to Jeremy Dale (University of Surrey, Surrey, United Kingdom) and Troels Lillebaek (Statens Serum Institute, Copenhagen, Denmark) for comparing DNA fingerprints from Norway with those from London and Denmark. We are grateful to the staff at the RIVM in The Netherlands for making the CAonTB database available on the internet and to all of the scientists around the world who have contributed to this database.
The CAonTB database is a project within the framework of the EU BIOMED II program (financed by European Community grant no. QLK2-CT-2000-00630)
REFERENCES
- 1.Abate, G., S. Hoffner, V. Ostergaard, and H. Miörner. 2001. Characterization of isoniazid-resistant strains of Mycobacterium tuberculosis on the basis of phenotypic properties and mutations in katG. Eur. J. Clin. Microbiol. Infect. Dis. 20:329-333. [DOI] [PubMed] [Google Scholar]
- 2.Callister, M. E. J., J. Barringer, S. T. Thanabalasingam, R. Gair, and R. N. Davidson. 2002. Pulmonary tuberculosis among political asylum seekers screened at Heathrow Airport, London, 1995-9. Thorax 57:152-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 4.Dahle, U. R., P. Sandven, E. Heldal, and D. A. Caugant. 2001. Molecular epidemiology of Mycobacterium tuberculosis in Norway. J. Clin. Microbiol. 39:1802-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daley, C. L. 2002. Transmission of multidrug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 165:742-743. [DOI] [PubMed] [Google Scholar]
- 6.Dye, C., M. A. Espinal, C. J. Watt, C. Mbiaga, and B. G. Williams. 2002. Worldwide incidence of multidrug-resistant tuberculosis. J. Infect. Dis. 185:1197-1202. [DOI] [PubMed] [Google Scholar]
- 7.Fluit, A. C., M. R. Visser, and F.-J. Schmitz. 2001. Molecular detection of antimicrobial resistance. Clin. Microbiol. Rev. 14:836-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hargreaves, S. 2000. System to detect tuberculosis in new arrivals to UK must be improved. Br. Med. J. 320:870.. [PMC free article] [PubMed] [Google Scholar]
- 9.Heldal, E. 2001. Tuberculosis in Norway 2000. Rep. Syst. Communic. Dis. 29:18. [Google Scholar]
- 10.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klovdahl, A. S., E. A. Graviss, A. Yaganehdoost, M. W. Ross, A. Wagner, G. J. Adams, and J. M. Musser. 2001. Networks and tuberculosis: an undetected community outbreak involving public places. Soc. Sci. Med. 52:681-694. [DOI] [PubMed] [Google Scholar]
- 12.Lillebaek, T., Å. Andresen, J. Bauer, A. Dirksen, S. Glismann, P. de Haas, and A. Kok-Jensen. 2001. Risk of Mycobacterium tuberculosis transmission in a low-incidence country due to immigration from high-incidence areas. J. Clin. Microbiol. 39:855-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maguire, H., J. W. Dale, T. D. McHugh, P. D. Butcher, S. H. Gillespie, A. Costetsos, H. Al-Ghusein, R. Holland, A. Dickens, L. Marston, P. Wilson, R. Pitman, D. Strachan, F. A. Drobniewski, and D. K. Banerjee. 2002. Molecular epidemiology of tuberculosis in London 1995 to1997 showing low rate of active transmission. Thorax 57:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russel, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marttila, H. J., H. Soini, P. Huovinen, and M. K. Viljanen. 1996. katG mutations in isoniazid-resistant Mycobacterium tuberculosis isolates recovered from Finnish patients. Antimicrob. Agents Chemother. 40:2187-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitta, A. T., L. S. Knowles, J. Kim, E. L. Lehnkering, L. A. Borenstein, P. T. Davidson, S. M. Harvey, and M. L. De Koning. 2002. Limited transmission of multidrug-resistant tuberculosis despite a high proportion of infectious cases in Los Angeles county, California. Am. J. Respir. Crit. Care Med. 165:812-817. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqi, S. H., J. P. Libonati, and G. Middlebrook. 1981. Evaluation of a rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 13:908-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Doorn, H. R., E. J. Kuijper, A. van der Ende, A. G. A. Welten, D. van Soolingen, P. E. W. de Haas, and J. Dankert. 2001. The susceptibility of Mycobacterium tuberculosis to isoniazid and the Arg→Leu mutation at codon 463 of katG are not associated. J. Clin. Microbiol. 39:1591-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Soolingen, D., M. W. Borgdorff, P. E. de Haas, M. M. Sebek, J. Veen, M. Dessens, K. Kremer, and J. D. van Embden. 1999. Molecular epidemiology of tuberculosis in The Netherlands: a nationwide study from 1993 through 1997. J. Infect. Dis. 180:726-736. [DOI] [PubMed] [Google Scholar]
- 21.van Soolingen, D., P. E. de Haas, H. R. van Doorn, E. Kuijper, H. Rinder, and M. W. Borgdorff. 2000. Mutations at amino acid position 315 of the katG gene are associated with high-level resistance to isoniazid, other drug resistance, and successful transmission of Mycobacterium tuberculosis in The Netherlands. J. Infect. Dis. 182:1788-1790. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. 1994. Tuberculosis—a global emergency. W. H. O. report on the TB epidemic. World Health Organization, Geneva, Switzerland.
- 23.World Health Organization. 1997. W. H. O. report on the tuberculosis epidemic. World Health Organization, Geneva, Switzerland.
- 24.World Health Organization. 2000. Anti-tuberculosis drug resistance in the world, report no. 2. The W. H. O./IUATLD global project on anti-tuberculosis drug resistance surveillance 1997-2000. World Health Organization, Geneva, Switzerland.