Abstract
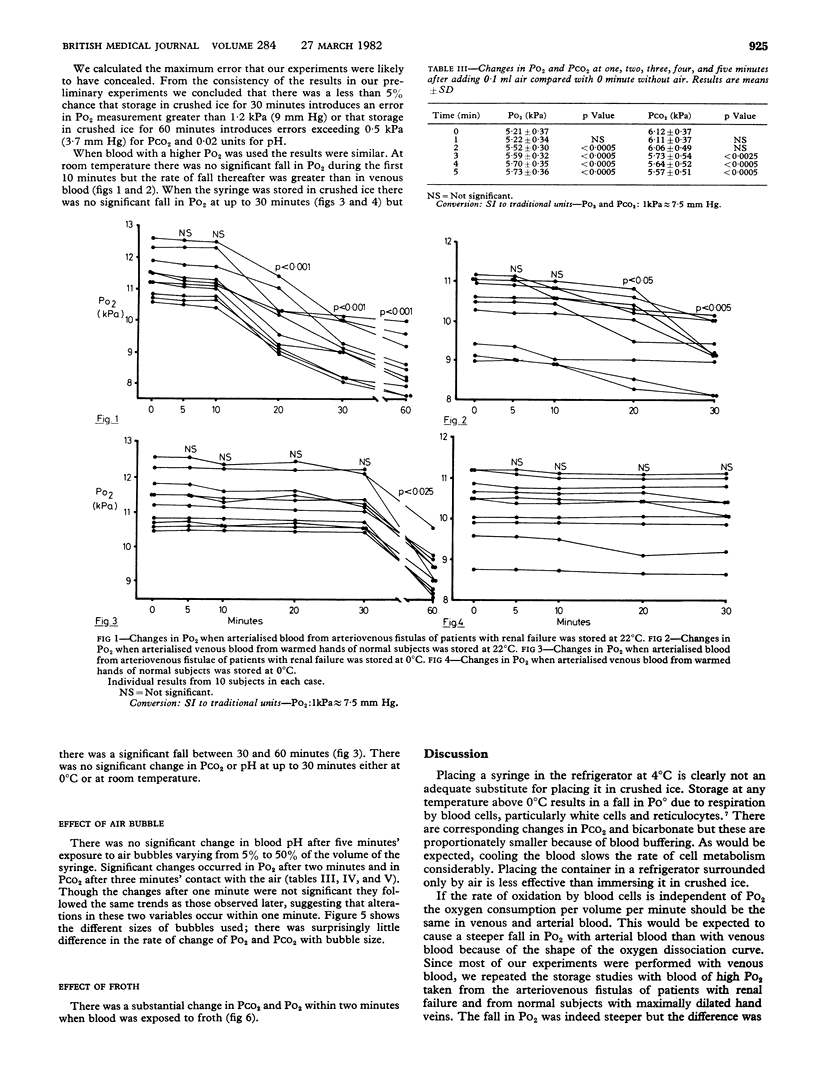
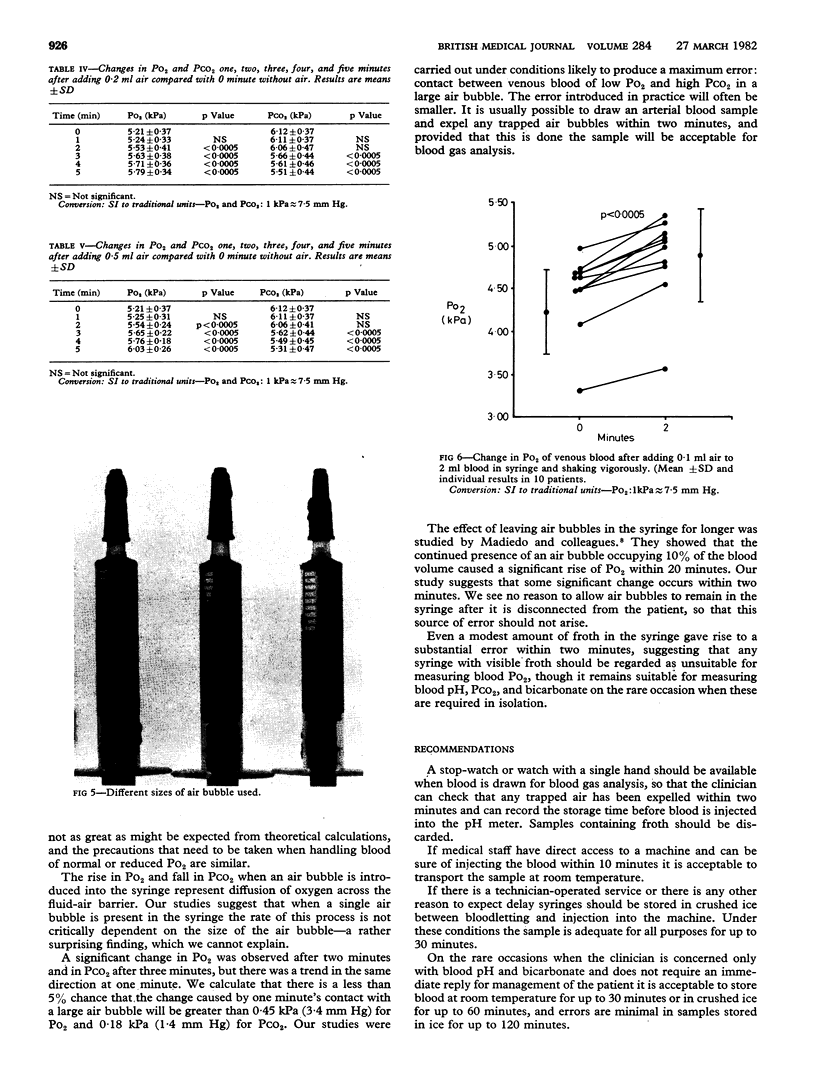
Two common sources of error in blood pH and blood gas analysis were studied. The effect of delay in estimation was studied in 10 volunteers and 40 patients. Syringes were stored at 0 degree C, (crushed ice), 4 degrees C (refrigerator) and 22 degrees C (room temperature). The pressure of oxygen (PO2) fell significantly by 20 minutes at 4 degrees C and 22 degrees C but did not change significantly at 0 degree C for up to 30 minutes. Blood pH, pressure of carbon dioxide (PCO2), and base excess did not change significantly for up to 30 minutes at 4 degrees C and 22 degrees C and up to 60 minutes at 0 degrees C. The effect of air bubbles in the syringe was studied by leaving a single bubble or froth in contact with the blood for one to five minutes in 40 patients. Po2 rose significantly after two minutes' contact with froth and two minutes' contact with the air bubble, and PCO2 fell significantly after three minutes' contact with the air bubble. Size of the bubble had little effect on rates of change. Blood pH, bicarbonate, TCO2, and base excess did not change significantly after up to five minutes' contact. For accurate estimation of PO2 and PCO2 it is necessary to avoid frothing, to expel all air bubbles within two minutes, and to inject the sample into the machine within 10 minutes or store the syringe in crushed ice. The requirements for blood pH and base excess measurement are less exacting.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN O. S. Sampling and storing of blood for determination of acid-base status. Scand J Clin Lab Invest. 1961;13:196–204. [PubMed] [Google Scholar]
- Adams A. P., Morgan-Hughes J. O., Sykes M. K. pH and blood-gas analysis. Methods of measurement and sources of error using electrode systems. Anaesthesia. 1967 Oct;22(4):575–597. doi: 10.1111/j.1365-2044.1967.tb10156.x. [DOI] [PubMed] [Google Scholar]
- Bradley J. G. Errors in the measurement of blood P CO2 due to dilution of the sample with heparin solution. Br J Anaesth. 1972 Feb;44(2):231–232. doi: 10.1093/bja/44.2.231. [DOI] [PubMed] [Google Scholar]
- Ishikawa S., Fornier A., Borst C., Segal M. S. The effects of air bubbles and time delay on blood gas analysis. Ann Allergy. 1974 Aug;33(2):72–77. [PubMed] [Google Scholar]
- Kelman G. R., Nunn J. F. Nomograms for correction of blood Po2, Pco2, pH, and base excess for time and temperature. J Appl Physiol. 1966 Sep;21(5):1484–1490. doi: 10.1152/jappl.1966.21.5.1484. [DOI] [PubMed] [Google Scholar]
- Madiedo G., Sciacca R., Hause L. Air bubbles and temperature effect on blood gas analysis. J Clin Pathol. 1980 Sep;33(9):864–867. doi: 10.1136/jcp.33.9.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty B. D., Nunn J. F. Regional quality control survey of blood-gas analysis. Ann Clin Biochem. 1977 Sep;14(5):245–253. doi: 10.1177/000456327701400168. [DOI] [PubMed] [Google Scholar]
- Siggaard-Andersen O. Titratable acid or base of body fluids. Ann N Y Acad Sci. 1966 Apr 1;133(1):41–58. doi: 10.1111/j.1749-6632.1966.tb50707.x. [DOI] [PubMed] [Google Scholar]