Abstract
Nocardia africana was isolated from a subcutaneous nodule of a cat. The isolate developed orange, wrinkled colonies. The bacteria were rod shaped to coccoid (1 by 5 μm) and gram positive. Analysis of the 16S ribosomal DNAs of the isolate and a reference strain of N. africana showed 100% homology.
CASE REPORT
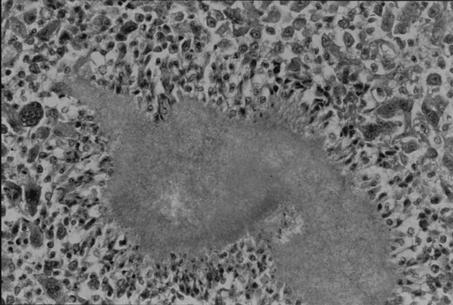
A 5-year-old male American shorthaired cat was examined at the Veterinary Pathology Department of Gifu University. Gross examination revealed subcutaneous nodules at the base of the tail and on the abdomen. This cat had a history of subcutaneous nodules, which were diagnosed as pyogranulomatous inflammation on the basis of histologic analysis of a biopsy specimen. The animal had been treated with enrofloxacin (5 mg/kg/day) for 1 month and a trimethoprim-sulfadimethoxine combination (30 mg/kg/day) for 1 month, but the lesions did not respond to the treatment. The nodules were round, white-yellow, and 1 by 1 to 3 by 3 cm in size and drained to the skin. Histopathological analysis of nodules in the subcutis, liver, and spleen disclosed granulomatous inflammation and many gram-positive bacilli in the center of the lesion (Fig. 1). The bacilli were positive with acid-fast staining.
FIG. 1.
Hematoxylin-and-eosin staining of subcutaneous nodules. Granulomatous inflammation is visible in the center of the lesion.
A clinical isolate was obtained from an abdominal nodule after inoculation and subsequent growth on Sabouraud's dextrose agar (5) at 24°C for 2 weeks. The colony formed by the clinical isolate was orange with a wrinkled surface (Fig. 2). The clinical isolate was an aerobic, gram-positive, rod-shaped to coccoid bacterium approximately 1 by 5 μm in size. Physiological tests revealed that the clinical isolate was identical to a reference strain of N. africana (Table 1).
FIG. 2.
A colony of the clinical isolate from the specimen developed on Sabouraud's dextrose agar at 24°C for 2 weeks.
TABLE 1.
Comparison of the physiological properties of the clinical isolate of N. africana and other Nocardia species
| Characteristic | Isolate | N. asteroides | N. brasiliensis | N. vaccinii | N. africana |
|---|---|---|---|---|---|
| Decomposition of: | |||||
| Caseine | + | − | + | − | + |
| Hypoxanthine | − | − | + | − | − |
| Xanthine | − | − | − | − | − |
| Tyrosine | − | − | + | − | − |
| Assimilation of (concn [%], wt/vol) | |||||
| l-Rhamnose (1.0) | − | − | − | − | − |
| d-Sorbitol (1.0) | − | − | − | − | − |
| Sodium acetate (0.2) | + | + | + | − | + |
| Sodium citrate (0.2) | − | + | + | − | − |
DNA was extracted from an isolated colony as follows. Samples were lysed with 10 μg of proteinase K per ml in a lysis buffer containing 0.1 mM EDTA, 1% sodium dodecyl sulfate, and 10 mM Tris hydrochloride (pH 8.0) at 37°C for 3 h. High-molecular-weight DNA was obtained from these samples by phenol and chloroform extraction and then precipitated with ethanol.
Genomic DNA (10 ng) was amplified by PCR in a reaction mixture (30 μl) containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin, 200 mM deoxynucleoside triphosphate, 1.0 U of Taq polymerase (Takara, Kyoto, Japan), and 0.5 μg of primer pairs. The sequences of the 16S ribosomal DNA primers were based upon previously reported sequences (1): forward primer p27f, 5′-AGA GTT TGA TCM TGG CTC AG-3′ (M = A or C); reverse primer P1525R, 5′-AAG GAG GTG WTC CAR CC-3′ (W = A or T, R = A or G). With these primers, a 1,450-bp fragment containing the coding sequence of the 16S ribosomal DNA was expected to be amplified. Amplification yielded fragments of about 1,450 bp, consistent with the reported sizes of Nocardia species 16S ribosomal DNA (1).
PCR amplification was carried out for 35 cycles consisting of template denaturation (1 min at 94°C), primer annealing (2 min at 55°C), and polymerization (2 min at 72°C). The PCR products were stained with ethidium bromide. The PCR products were then analyzed three times by the dideoxy-chain termination method with an ABI Prism 310 Genetic Analyzer (Perkin-Elmer, Foster City, Calif.).
The homology relationships between the 16S ribosomal DNAs of reference strains of Nocardia species and the clinical isolate were analyzed via the FASTA database of the DNA Data Bank of Japan. The nucleotide sequence of the 16Sribosomal DNA of the clinical isolate and that of a reference strain of N. africana (DDBJ accession no. AF302232) showed 99% homology.
Nocardiae cause a variety of suppurative infections in humans and animals (5) that may be distinguished clinically as cutaneous nocardiosis, pulmonary nocardiosis, solitary extrapulmonary nodules, and disseminated nocardiosis. Such infections are uncommon in dogs and even rare in cats (3). Nocardia asteroides is the most common species isolated, and the other Nocardia species are very infrequently isolated.
N. asteroides is the species most commonly isolated from infected animals, and there have been very few reports of infections of cats by other species (3).
In recent years, molecular techniques with which to identify Nocardia species have been introduced. Restriction endonuclease analysis and 16S rRNA analysis have been used for the identification of these species (1, 2, 6). This is due, in part, to the slow growth of aerobic actinomycetes and the inconsistent results of the growth-based morphological and biochemical tests used to identify them.
N. africana was first isolated from a human patient as a causative agent of pulmonary nocardiosis by Hamid et al. (4). This new species was classified by both physiological tests and 16S ribosomal DNA analysis (4). Animal infection with this species has not, to date, been reported. The present study describes the first isolation of N. africana from a cat with systemic nocardiosis. The clinical isolate was also identified by physiological tests and 16S ribosomal DNA analysis.
This is the first report of N. africana as an agent of pyogranulomatous inflammation in a cat. On the basis of our observations and the fact that there are several documented cases of cutaneous nocardiosis transmission from cats to humans by bites or scratches from clinically healthy animals (3), care should be taken when handling exudates from infected animals to prevent zoonotic transmission to humans.
REFERENCES
- 1.Chun, J., and M. Goodfellow. 1995. A phylogenetic analysis of the genus Nocardia with 16S rRNA gene sequences. Int. J. Syst. Bacteriol. 45:240-245. [DOI] [PubMed] [Google Scholar]
- 2.Conville, P. S., S. H. Fischer, C. P. Cartwright, and F. G. Witebsky. 2000. Identification of Nocardia species by restriction endonuclease analysisof an amplified protein of the 16S rRNA gene. J. Clin. Microbiol. 38:158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edward, D. F. 1998. Actinomycosis and nocardiosis, p. 308-313. In C. E. Greene (ed.), Infectious diseases of the dog and cat, 3rd ed. W. B. Saunders, Philadelphia, Pa.
- 4.Hamid, M. E., L. Maldonado, G. S. S. Eldin, M. F. Mohamed, N. G. Saeed, and M. Goodfellow. 2001. Nocardia africana sp. nov., a new pathogen isolated from patients with pulmonary infections. J. Clin. Microbiol. 39:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon-Chung, J. K., and J. E. Bennett. 1992. Medical mycology, p. 560-573, 816-826. Lea & Febiger, Philadelphia, Pa.
- 6.Laurent, F. J., F. Provost, and P. Boiron. 1999. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J. Clin. Microbiol. 37:99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]