Abstract
OBJECTIVE
To determine how functional status and walking ability are related to both severity of lower extremity peripheral arterial disease (PAD) and PAD-related leg symptoms.
DESIGN
Cross-sectional study.
SETTING
Academic medical center.
PARTICIPANTS
Patients aged 55 years and older diagnosed with PAD in a blood flow laboratory or general medicine practice (n=147). Randomly selected control patients without PAD were identified in a general medicine practice (n=67).
MEASUREMENTS
Severity of PAD was measured with the ankle-brachial index (ABI). All patients were categorized according to whether they had (1) no exertional leg symptoms; (2) classic intermittent claudication; (3) exertional leg symptoms that also begin at rest (pain at rest), or (4) exertional leg symptoms other than intermittent claudication or pain at rest (atypical exertional leg symptoms). Participants completed the 36-Item Short-Form Health Survey (SF-36) and the Walking Impairment Questionnaire (WIQ). The WIQ quantifies patient-reported walking speed, walking distance, and stair-climbing ability, respectively, on a scale of 0 to 100 (100 = best).
MAIN RESULTS
In multivariate analyses patients with atypical exertional leg symptoms, intermittent claudication, and pain at rest, respectively, had progressively poorer scores for walking distance, walking speed, and stair climbing. The ABI was measurably and independently associated with walking distance (regression coefficient = 2.87/0.1 ABI unit, p = .002) and walking speed (regression coefficient = 2.09/0.1 ABI unit, p = .015) scores. Among PAD patients only, pain at rest was associated independently with all WIQ scores and six SF-36 domains, while ABI was an independent predictor of WIQ distance score.
CONCLUSIONS
Both PAD-related leg symptoms and ABI predict patient-perceived walking ability in PAD.
Keywords: peripheral arterial disease, functioning, walking impairment, leg pain
Clinicians traditionally associate lower extremity peripheral arterial disease (PAD) with intermittent claudication.1 However, epidemiologic studies document a 12% to 18% prevalence of PAD when the ankle-brachial index (ABI) is used as a noninvasive screening tool among community-dwelling older men and women, and most of these men and women with PAD do not have symptoms of classic intermittent claudication.2–4
Although the effect of cardiovascular disease on functioning has been studied among patients with coronary artery disease, congestive heart failure, and stroke, the effect of PAD and PAD-related leg symptoms on functioning during daily activities is not well defined. This study addresses two questions regarding functioning in patients with PAD. First, do leg symptoms associated with PAD predict walking ability and functional status, independently of disease severity as measured by ABI? Second, does ABI predict functioning independently of leg symptoms? Patient-reported walking ability was assessed with the Walking Impairment Questionnaire (WIQ), and functional status was assessed with the 36-Item Short-Form Health Survey (SF-36).5–7 Identifying characteristics associated with poorer functioning will facilitate development of targeted interventions to maintain functioning and prevent mobility loss in patients with PAD.
METHODS
Recruitment
The study was approved by Northwestern University Medical School’s Institutional Review Board, and all participants gave informed consent. During 1996 consecutive patients aged 55 years and older with abnormal lower extremity arterial studies documented in Northwestern Memorial Hospital’s noninvasive vascular laboratory were identified using the hospital’s computerized record system. For a short time patients with PAD were recruited by randomly selecting patients in the hospital’s vascular surgery practice with an International Classification of Diseases, North Revision (ICD-9) code consistent with lower extremity arterial disease. Additional patients were recruited from patients aged 55 years and older with scheduled appointments in the Division of General Internal Medicine at Northwestern Medical Faculty Foundation. Identified patients received a letter notifying them of the study and were telephoned and invited to participate.
Exclusion Criteria
Patients with severe functional limitations or limb-threatening ischemia, as evidenced by wheelchair dependence, foot or lower extremity amputations, predicted life expectancy less than 6 months, nursing home residence, or lower extremity ulcers were excluded. Because of communication difficulty, non-English-speaking participants were excluded, and patients with a Mini-Mental Status Examination score less than 18 out of a possible 30 points were excluded.8
Measurements
The WIQ and SF-36 were mailed to participants for completion prior to their study visit. At the study visit participants were administered a medical history questionnaire and the San Diego intermittent claudication questionnaire.9 All participants underwent ABI measurement.
Ankle-Brachial Index Measurement
The ABI measurement was performed in accordance with previously accepted methods.10, 11 Prior to beginning data collection, we determined that the ABI would be calculated for each leg by dividing the lowest of the posterior tibial and dorsalis pedis pressures by the brachial systolic pressure. This method was chosen because we believed the vessel with poorest perfusion would have the greatest effect on lower extremity functioning. Participants judged to have PAD were those patients with ABI <0.90, based on previous studies.4, 12 Control participants were patients identified from the Division of General Internal Medicine with an ABI ≥0.90 and <1.50. The investigator measuring ABI was blinded to total WIQ and SF-36 scores.
Leg Symptom Definitions
Leg symptoms were ascertained using the San Diego intermittent claudication questionnaire, which assesses the presence, location, and character of symptoms in each leg.9 Patients were classified into the following four symptom categories 9: absence of exertional leg symptoms; classic Rose intermittent claudication (Rose claudication); exertional leg symptoms, which also occur at rest (pain at rest); and exertional leg symptoms not consistent with either Rose claudication or pain at rest (atypical exertional leg pain). Classic Rose intermittent claudication is exertional calf pain that resolves within 10 minutes of rest and does not begin at rest.1
Medical Outcomes Study 36-Item Short Form
The SF-36 measures multiple functioning domains, scored on a scale of 0 to 100 (100 = best).7The SF-36 has been standardized, validated, and used successfully in a variety of patient populations with varying sociodemographic characteristics, diagnoses, and illness severity.7
Walking Impairment Questionnaire
The WIQ measures walking distance, walking speed, and stair climbing in the community.5 For walking distance the participant ranks his or her degree of difficulty walking specific distances on a 0-to-4 Likert scale, where 0 represents inability to walk the distance and 4 represents no difficulty. Distances range from walking indoors around the home to walking five blocks (1,500 ft). For walking speed the participant ranks the degree of difficulty walking slowly, at average speed, quickly, or running or jogging one block. In the stair-climbing component, patients rank their ability to walk up and down one, two, or three flights of stairs, respectively.
Scoring the Walking Impairment Questionnaire
In the WIQ walking distance component, each distance, expressed as feet, is multiplied by the Likert score selected for that distance. Products are summed and divided by the maximum possible score to obtain the percentage score. Similar percentage scores are obtained for the walking speed and stair-climbing WIQ components. For the walking speed component, each speed is given a “weight,” ranging from 1 mile per hour to 5 miles per hour, which is multiplied by the Likert scale response. In the stair-climbing component, each stair-climbing category is weighted according to the number of stairs in each category.
Comorbidity Ascertainment
We used methods derived from the Women’s Health and Aging Study disease ascertainment algorithms to document and verify comorbid disease.13 These algorithms combine data from medical record review, a primary care physician questionnaire, patient report, and medications.13 Comorbidities assessed were previous myocardial infarction, heart failure, knee or hip arthritis, diabetes mellitus, lumbar disk disease, spinal stenosis, angina, stroke, previous hip fracture, chronic pulmonary disease, and Parkinson’s disease. These comorbidities have been previously shown to affect functioning among older persons.14–18
Statistical Analyses
Differences in categorical variables between PAD and control patients were compared by χ2analyses. Two-sample Student’s t tests compared differences in continuous variables. Analysis of covariance was performed to compare WIQ and SF-36 scores by ABI category and patient-reported leg symptoms, adjusting for leg symptoms, ABI category, and age. Linear regression analyses were used to identify independent predictors of WIQ and SF-36 scores, controlling for age, sex, race, comorbidities, and history of prior revascularization. The lowest leg ABI was used in all analyses. Dummy variables were created for each leg symptom category. Atypical exertional leg symptoms, Rose claudication, and pain at rest were each compared with the reference category of patients without exertional leg pain. Diagnostic collinearity showed no collinearity between the ABI and leg symptom categories.19
RESULTS
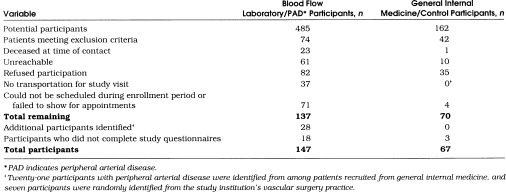
Table 1 summarizes the recruitment process. We obtained further data from our noninvasive vascular laboratory to describe potentially eligible nonparticipants. The mean age ± SD of vascular laboratory nonparticipants was 74.2 ± 8.7 years and 52% were female as compared with 71.5 ± 9.8 years and 45% female among vascular laboratory participants, respectively. The mean ABI level of potentially eligible nonparticipants was 0.60 as compared with 0.56 among PAD study participants identified from the noninvasive vascular laboratory.
Table 1.
Summary of Recruitment Process for Patients with Peripheral Arterial Disease and Control Patients Aged 55 Years and Older Identified from an Academic Medical Center
Patient Characteristics
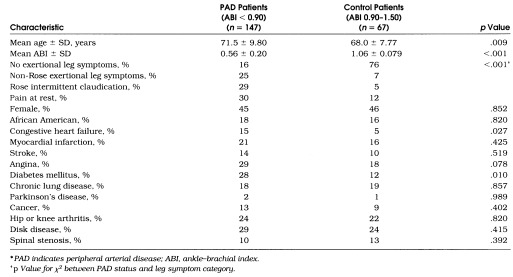
Characteristics of patients in the PAD and control groups are shown in Table 2. Fifty-five PAD participants (34%) had undergone prior revascularization. Mean ABI levels ± SD were 0.63 ± 0.13 for PAD participants with no exertional leg symptoms, 0.57 ± 0.18 for PAD participants with non-Rose exertional leg symptoms, 0.47 ± 0.22 for PAD participants with Rose claudication, and 0.61 ± 0.21 for PAD participants with pain at rest. Participants with PAD who had pain at rest were more often African American (29% vs 13%, p= .018) and more likely to have knee or hip arthritis (38% vs 18%, p= .008) than participants without pain at rest. Prevalences of each leg symptom category were similar between PAD participants with and those without previous revascularization. Consistent with previous study,9 no participants with bilateral exertional leg symptoms had discrepancies in the category of symptoms experienced between the right and left legs.
Table 2.
Characteristics of Patients with Peripheral Arterial Disease and Control Patients Among Men and Women Aged 55 Years and Older Identified from an Academic Medical Center*
Walking Impairment Questionnaire Results
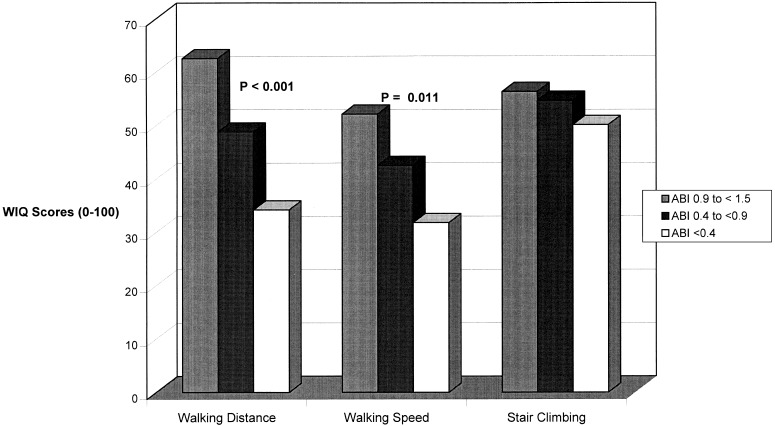
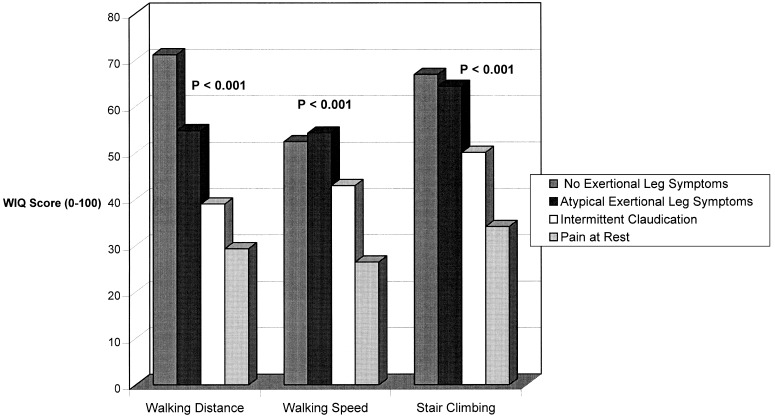
Figure 1 shows the relations between ABI and each WIQ component, adjusting for leg symptoms and age. Within each WIQ component, the percentage score increased with ABI category. Significant differences by ABI category were identified for walking distance and walking speed.Figure 2 relates leg symptoms to each WIQ score, adjusting for ABI level and age. Patients without exertional leg symptoms scored best, followed by patients with atypical exertional leg symptoms, Rose claudication, and pain at rest. Differences between leg symptom categories were highly statistically significant.
Figure 1.
Relationships between Walking Impairment Questionnaire scores and ankle–brachial index (ABI) among men and women aged 55 years and older, adjusting for age and leg symptoms.
Figure 2.
Relations between Walking Impairment Questionnaire scores and leg symptoms among men and women aged 55 years and older, adjusting for ankle–brachial index and age. Ankle–brachial index values were entered into the model as continuous variables.
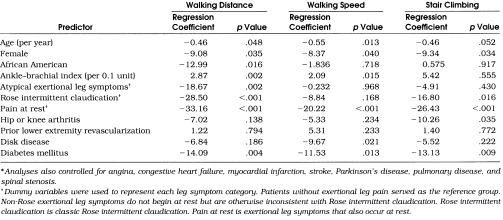
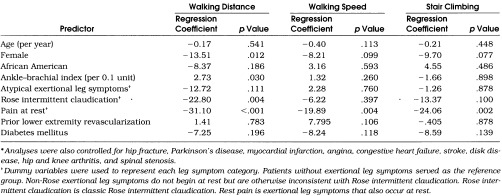
Table 3 shows linear regression analyses results for each WIQ score. Compared with participants without exertional leg symptoms, those with pain at rest had significantly poorer scores in all three WIQ components. After controlling for leg symptoms and comorbid disease, ABI remained an independent predictor of walking distance and walking speed. An increase of 0.1 in ABI was associated with an increase of 2.87 in WIQ distance, and an increase of 0.1 in ABI was associated with a 2.09-point increase in WIQ speed score.Table 4 shows linear regression analyses for each WIQ score among PAD patients only. The ABI remained an independent predictor of walking distance, controlling for leg symptoms, comorbidities, and previous revascularization.
Table 3.
Predictors of Walking Impairment Questionnaire Scores Among Patients with Peripheral Arterial Disease and Control Patients: Results of Multivariate Linear Regression Analyses (n= 214)*
Table 4.
Predictors of Walking Impairment Questionnaire Scores Among Patients with Peripheral Arterial Disease: Results of Multiple Linear Regression Analyses (n= 147)*
Results of 36-Item Short Form
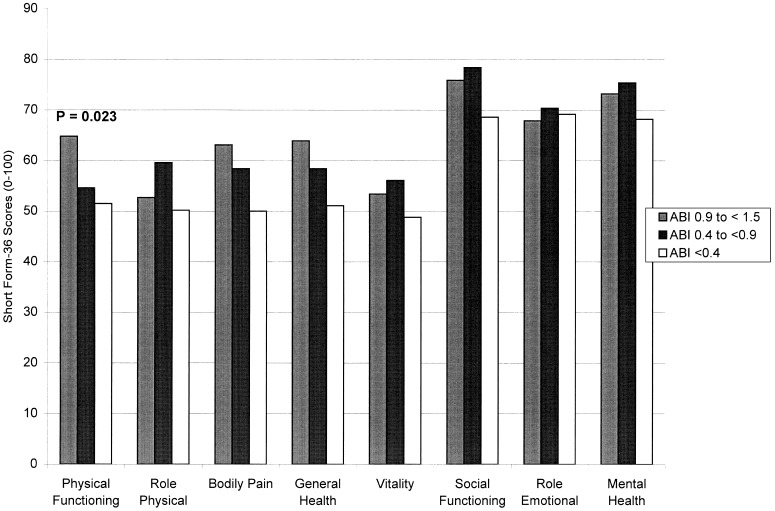
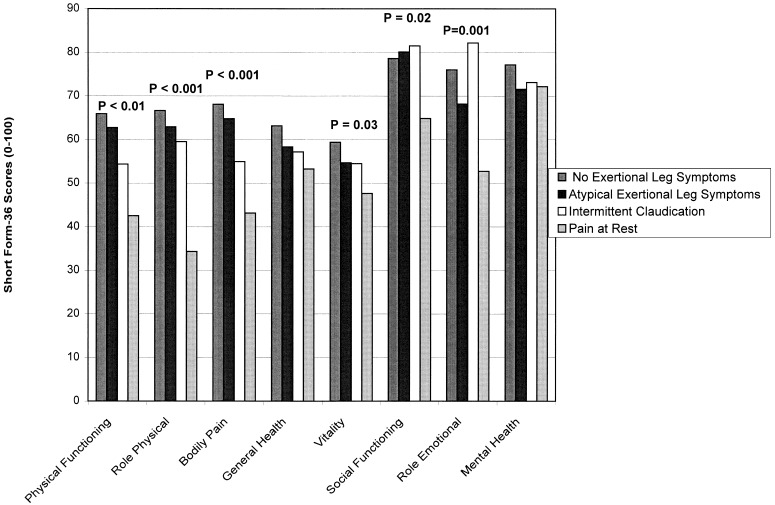
Figure 3 relates ABI categories to SF-36 scores, adjusting for leg symptoms and age. The ABI categories were significantly associated with physical functioning score.Figure 4 relates leg symptoms to SF-36 scores, controlling for ABI category and age. Patients with pain at rest scored more poorly than other PAD participants.
Figure 3.
Relations between SF-3 6 scores and ankle–brachial index (ABI) among men and women aged 55 years and older, adjusting for leg symptoms and age.
Figure 4.
Relations between SF-36 scores and leg symptoms among men and women aged 55 years and older, adjusting for ankle–brachial index (entered into the model as continuous variables) and age.
In multivariate analyses including PAD participants only, pain at rest was associated independently with lower SF-36 scores in all domains except mental health and role limitations due to emotional problems (data not shown). Rose claudication was associated independently with poorer bodily pain scores (β coefficient = −17.13, p= .004). The ABI was not associated independently with SF-36 scores after controlling for leg symptoms and comorbid diseases.
DISCUSSION
Our results suggest that clinicians should consider both leg symptoms and ABI when assessing functioning among patients with PAD. In our study PAD patients with no exertional leg symptoms, atypical exertional leg symptoms, Rose claudication, and pain at rest had progressively poorer functioning, respectively, for most outcomes. Patients with PAD who have pain at rest and a low ABI are likely to have particularly poor functional ability and may especially benefit from interventions to improve arterial perfusion.
Previous studies of functioning in patients with PAD have almost all been limited to patients with intermittent claudication. To our knowledge WIQ and SF-36 questionnaires have not been assessed previously in a heterogeneous cohort of PAD patients, including patients with and without intermittent claudication. A major contribution of this study to previous work is the finding that ABI adds to leg symptom data, with lower ABI being associated with inability to walk longer distances. Our data suggest that asymptomatic PAD patients with a low ABI have poorer walking ability than asymptomatic patients with a normal ABI.
Controlling for leg symptoms and age, ABI was less predictive of SF-36 scores than WIQ scores. Within the SF-36 only the physical functioning domain includes questions specific to lower extremity function. The paucity of questions specific to leg functioning most likely accounts for the poorer correlation between ABI and SF-36 scores.
The WIQ has been previously validated with treadmill testing among intermittent claudication patients. Regensteiner et al. assessed the correlation between WIQ walking distance and walking speed scores and treadmill walking time among 26 patients with intermittent claudication.5 Peak treadmill walking time correlated with WIQ walking distance (Pearson coefficient = 0.58, p < .05) and WIQ walking speed (Pearson coefficient = 0.67, p < .05).
Previous studies of patients with intermittent claudication have shown inconsistent results regarding the ability of ABI to predict functioning. In a study of 555 claudication patients, both the WIQ distance score (regression coefficient = 0.33, p < .0001) and the physical functioning component of the SF-36 (regression coefficient = 18.8, p= .001) correlated with ABI, controlling for patient demographics and disease comorbidities.20 In other studies of patients with intermittent claudication, ABI has been less predictive of leg functioning. For example, among 14 claudication patients, changes in WIQ distance scores after revascularization were not significantly correlated with changes in ABI.21
Pain at Rest
We defined pain at rest as exertional leg symptoms that also occur at rest. This definition should be distinguished from “rest pain” due to limb-threatening ischemia. Limb-threatening rest pain typically worsens with limb elevation and improves with dependency. Most patients with PAD who had pain at rest in our study were unlikely to have had limb-threatening ischemia because patients with gangrene or lower extremity ulcers were excluded. Importantly, half of the non-PAD participants with exertional leg symptoms had pain at rest, consistent with our assertion that pain at rest is probably not indicative of limb-threatening ischemia.
Arthritis and Pain at Rest in Patients with Peripheral Arterial Disease
Participants with PAD who had pain at rest had a higher prevalence of knee and hip arthritis than PAD participants without pain at rest. Although this observed association may have contributed to the deleterious effect of pain at rest on functioning, the relation between pain at rest and functioning was maintained after controlling for arthritis and other comorbid diseases. We suspect that leg symptoms reported by PAD patients may lead to an evaluation for and diagnosis of knee and hip arthritis. Because arthritis is common in the elderly, poorly defined leg symptoms in PAD patients may lead to knee or hip x-rays revealing arthritis that may or may not be the cause of leg symptom complaints.
Peripheral Neuropathy and Prior Revascularization
We did not collect data on peripheral neuropathy at the baseline study visit. Therefore, we cannot determine whether peripheral neuropathy was responsible for exertional leg symptoms or functional impairment. Although many PAD participants had undergone prior revascularization, revascularization was not associated independently with patient-reported functioning. Our findings suggest that leg symptoms and ABI are more useful indicators of functioning than prior revascularization.
Generalizability of Findings
There are several limitations to the generalizability of our findings. First, many potentially eligible patients did not participate. Although we cannot exclude the possibility that our results would have differed among nonparticipants, there is no reason to believe that relationships reported here between ABI, leg symptoms, and functioning would differ in other settings. Second, our results are generalizable to ambulatory patients without severe disability or critical leg ischemia. Because most PAD patients will never develop critical leg ischemia, our findings should be applicable to most PAD patients. Finally, we cannot be certain that the relations reported here would be the same if the questionnaires were not self-administered.
Conclusions
In conclusion, our data show that leg symptoms and ABI each have a measurable, independent association with walking ability in patients with PAD. Further study is necessary to identify predictors of functional decline in a diverse group of PAD patients. Further study is also necessary to determine whether preventing development of intermittent claudication or pain at rest protects against functional disability among men and women with PAD.
Acknowledgments
The authors thank Dr. Cynthia Mulrow for her helpful review of the manuscript. This work was supported in part by a grant-in-aid from the American Heart Association of Metropolitan Chicago and in part by grant RR-00048 from the National Center for Research Resources, National Institutes of Health.
References
- 1.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull WHO. 1962;27:645–58. [PMC free article] [PubMed] [Google Scholar]
- 2.Leng GC, Fowkes FGR, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ. 1996;313:1440–4. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criqui MH, Fronek A, Barrett-Connor EB, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–5. doi: 10.1161/01.cir.71.3.510. [DOI] [PubMed] [Google Scholar]
- 4.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation. 1993;88:837–45. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 5.Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–52. [Google Scholar]
- 6.Hiatt WR, Hirsch AT, Regensteiner JG, Brass EP. Clinical trials for claudication: assessment of exercise performance, functional status, and clinical end points. Circulation. 1995;92:614–21. doi: 10.1161/01.cir.92.3.614. [DOI] [PubMed] [Google Scholar]
- 7.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, Mass: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 8.Guralnik JM, Fried LP, Simonsick EM, et al. Screening the community-dwelling population for disability. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, Md: National Institute on Aging; 1995. pp. 9–18. NIH publication 95-4009. [Google Scholar]
- 9.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 10.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. Circulation. 1995;91:1472–9. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- 11.Fiegelson HS, Criqui MH, Fronek A, Langer RD, Molgaard CA. Diagnosing peripheral arterial disease: the sensitivity, specificity, and predictive value of non-invasive tests in a defined population. Am J Epidemiol. 1994;140:518–25. doi: 10.1093/oxfordjournals.aje.a117279. [DOI] [PubMed] [Google Scholar]
- 12.Newman AB, Sutton-Tyrrell K, Vogt MT. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270:487–9. [PubMed] [Google Scholar]
- 13.Fried LP, Kaspar JD, Williamson JD, et al. Disease ascertainment algorithms. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, Md: National Institute on Aging; 1995. Appendix E. NIH publication 95-4009. [Google Scholar]
- 14.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ettinger WH, Fried LP, Harris T, Shemanski L, Schulz R, Robbins J. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. J Am Geriatr Soc. 1994;42:1035–44. doi: 10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 16.Boult C, Kane RL, Louis TA, Boult L, McCaffrey D. Chronic conditions that lead to functional limitation in the elderly. J Gerontol Med Sci. 1994;49:M28–36. doi: 10.1093/geronj/49.1.m28. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson C, Peto V, Fitzpatrick R, Greenhall R, Hyman N. Self-reported functioning and well-being in patients with Parkinson’s disease: comparison of the short-form health survey (SF-36) and the Parkinson’s disease questionnaire (PDQ-39) Age Aging. 1995;24:505–9. doi: 10.1093/ageing/24.6.505. [DOI] [PubMed] [Google Scholar]
- 18.Cole SA, Woddard JL, Juncos JL, Kogos JL, Youngstrom EA, Watts RL. Depression and disability in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1996;8(1):20–5. doi: 10.1176/jnp.8.1.20. [DOI] [PubMed] [Google Scholar]
- 19.Belsley DA, Kuh E, Welsch RE, editors. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. New York, NY: John Wiley and Sons; 1980. pp. 85–191. [Google Scholar]
- 20.Feinglass J, McCarthy WJ, Slavensky R, Manheim L, Martin GJ. The effect of lower extremity blood pressure on physical functioning for patients who have intermittent claudication. J Vasc Surg. 1996;24:503–12. doi: 10.1016/s0741-5214(96)70066-6. [DOI] [PubMed] [Google Scholar]
- 21.Regensteiner JG, Hargarten ME, Ruthorford RB, Hiatt WR. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology. 1993;44:1–10. doi: 10.1177/000331979304400101. [DOI] [PubMed] [Google Scholar]